User login
Epithelioma Cuniculatum (Plantar Verrucous Carcinoma): A Systematic Review of Treatment Options
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).
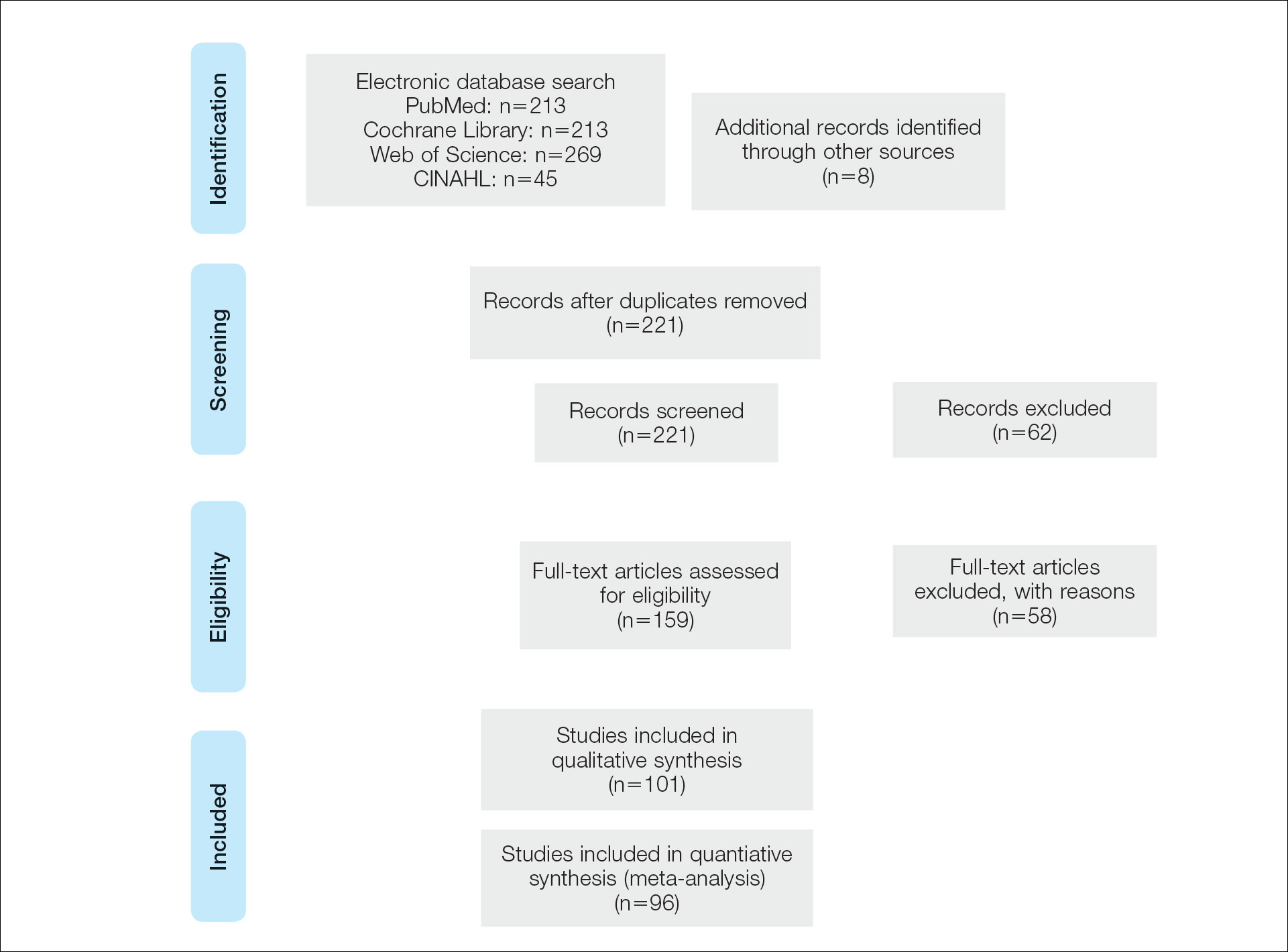
A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.
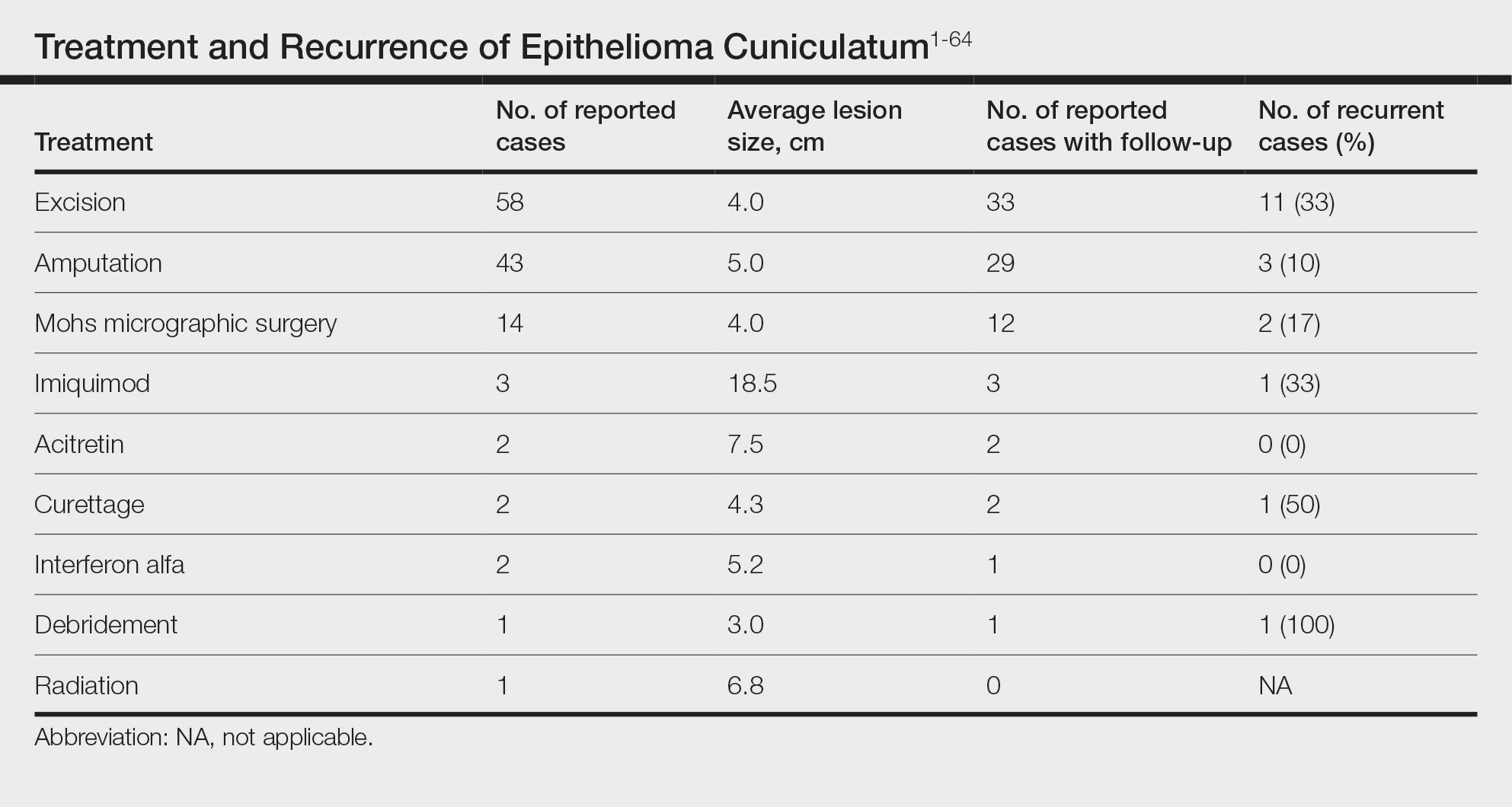
Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Practice Points
- Because of its slow-growing nature and propensity for local invasion and recurrence, diagnosis of epithelioma cuniculatum (EC) often is delayed and therefore can be associated with notable morbidity.
- Wide local excision with 5-mm to 1-cm margins is considered standard of care and is the most commonly reported treatment of EC. Amputation may be required in cases with extensive local destruction.
- Mohs micrographic surgery is a viable option for treatment of EC, with more recent cases suggesting favorable outcomes regarding recurrence rates.
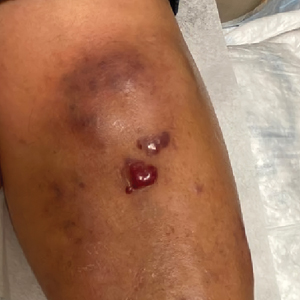
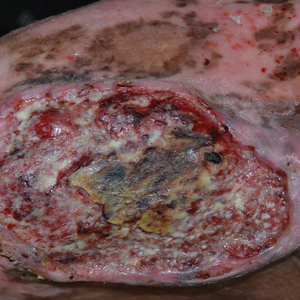
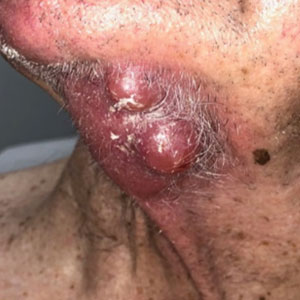
Violaceous Nodules on the Leg in a Patient with HIV
The Diagnosis: Plasmablastic Lymphoma
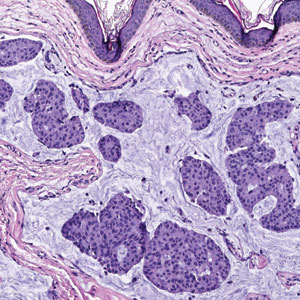
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).
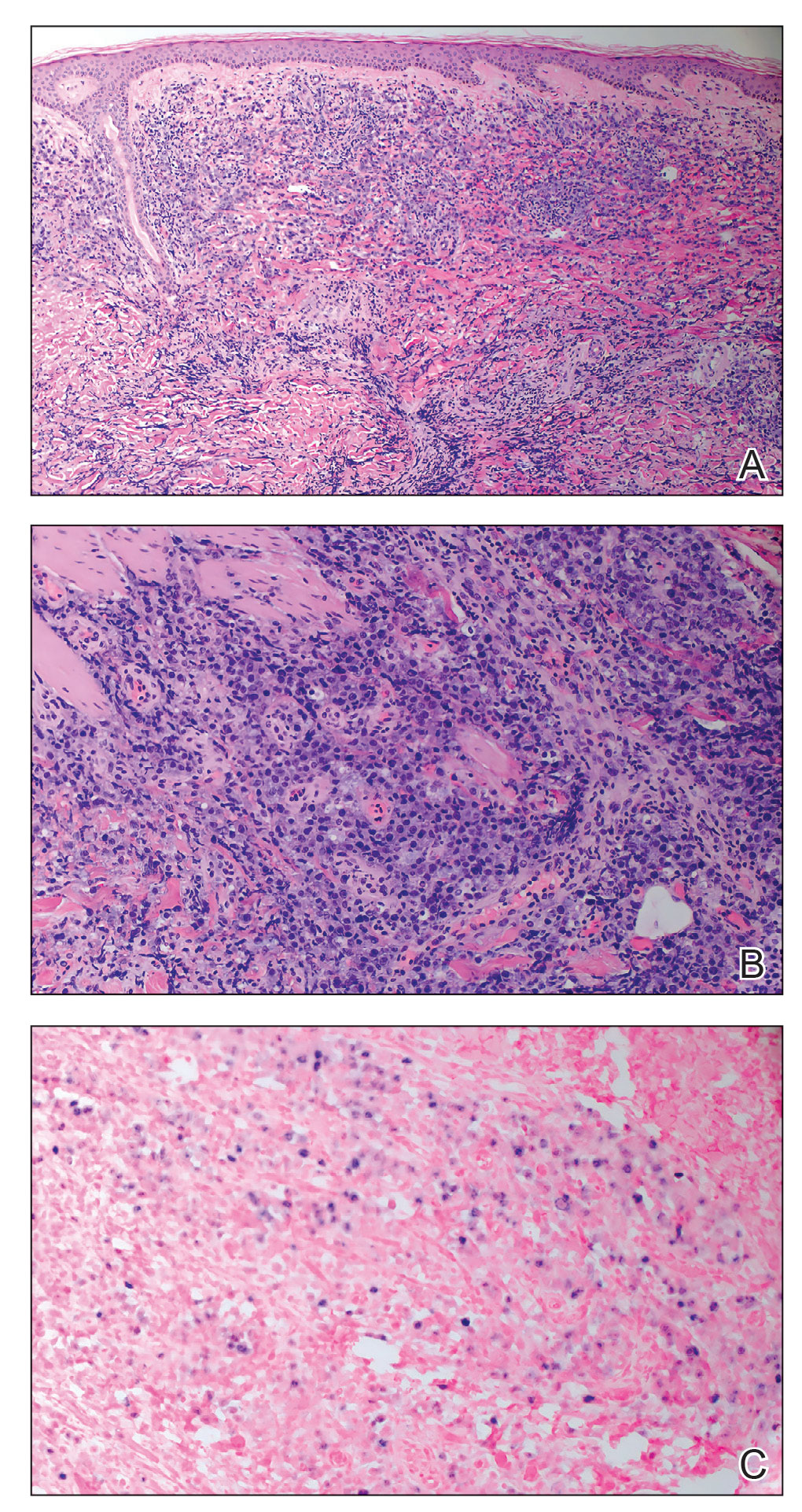
A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
A 67-year-old man with long-standing hepatitis B virus and HIV managed with chronic antiretroviral therapy presented to an urgent care facility with worsening erythema and edema of the legs of 2 weeks’ duration. He was prescribed a 7-day course of cephalexin for presumed cellulitis. Two months later, he developed nodules on the lower extremities. He was seen by podiatry and prescribed a course of amoxicillin–clavulanic acid for presumed infection. Despite 2 courses of antibiotics, his symptoms progressed. The nodules expanded in number and some developed ulceration. Three months into his clinical course, he presented to our dermatology clinic. Physical examination revealed two 2- to 3-cm, violaceous, exophytic, tender nodules. He reported tactile allodynia of the lower extremities and denied fever, chills, night sweats, or weight loss. He also denied exposure to infectious or chemical agents and reported no recent travel. The patient was chronically taking lisinopril/hydrochlorothiazide, escitalopram, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, bupropion, and aspirin with no recent changes. A complete hematologic and biochemical survey largely was unremarkable. His HIV viral load was undetectable with a CD4 count greater than 400/mm3 (reference range, 490–1436/mm3). Lactate dehydrogenase was elevated at 568 IU/L (reference range, 135–225 IU/L). The lower leg lesions were biopsied for confirmatory diagnosis.
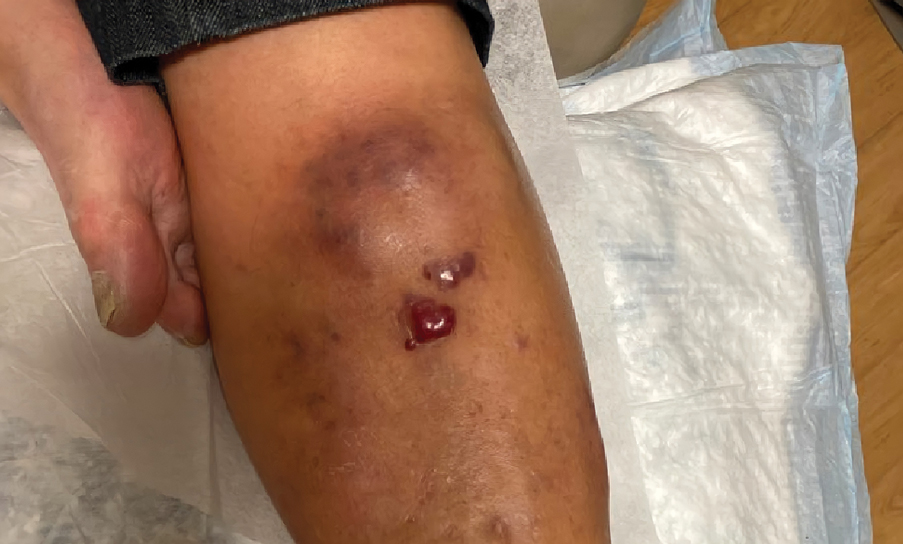
Optimal management of dysplastic nevi continues to evolve
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
AT MELANOMA 2023
Dermoscopy, other modalities for improving melanoma diagnoses reviewed
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
AT MELANOMA 2023
Dome-Shaped Periorbital Papule
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
A 76-year-old woman presented with a slowly growing, asymptomatic, 5-mm, pink-brown, dome-shaped papule adjacent to the left lateral canthus of several years’ duration. Dermoscopic examination revealed fine linear peripheral blood vessels. The lesional cells were positive with cytokeratin 7, estrogen receptor, progesterone receptor, chromogranin, synaptophysin, and neuron-specific enolase. Cytokeratin 20 and p63 were negative, and the Ki-67 proliferative index was less than 5%.
Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
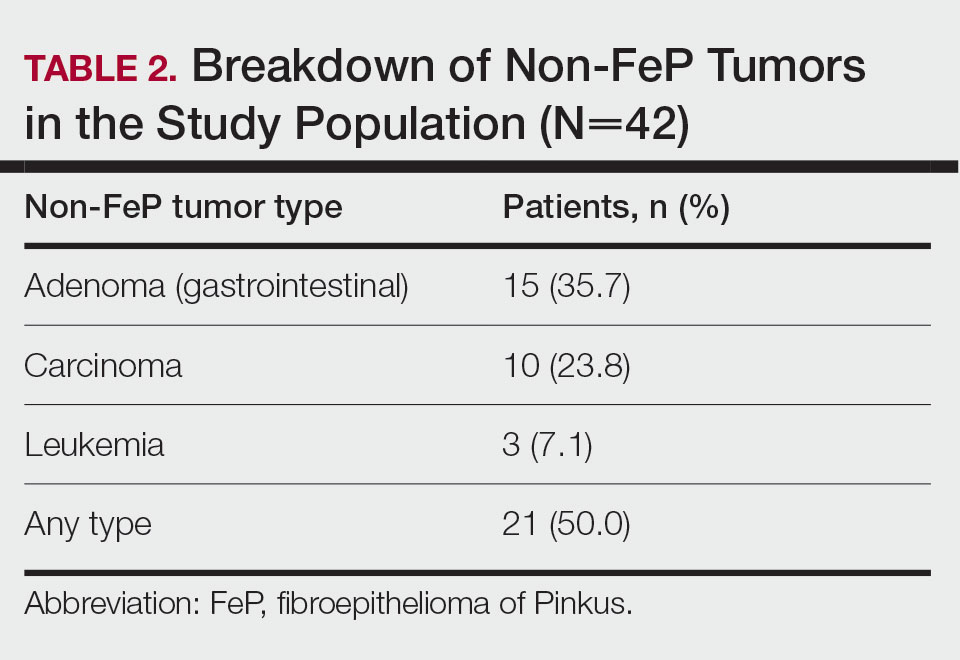
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
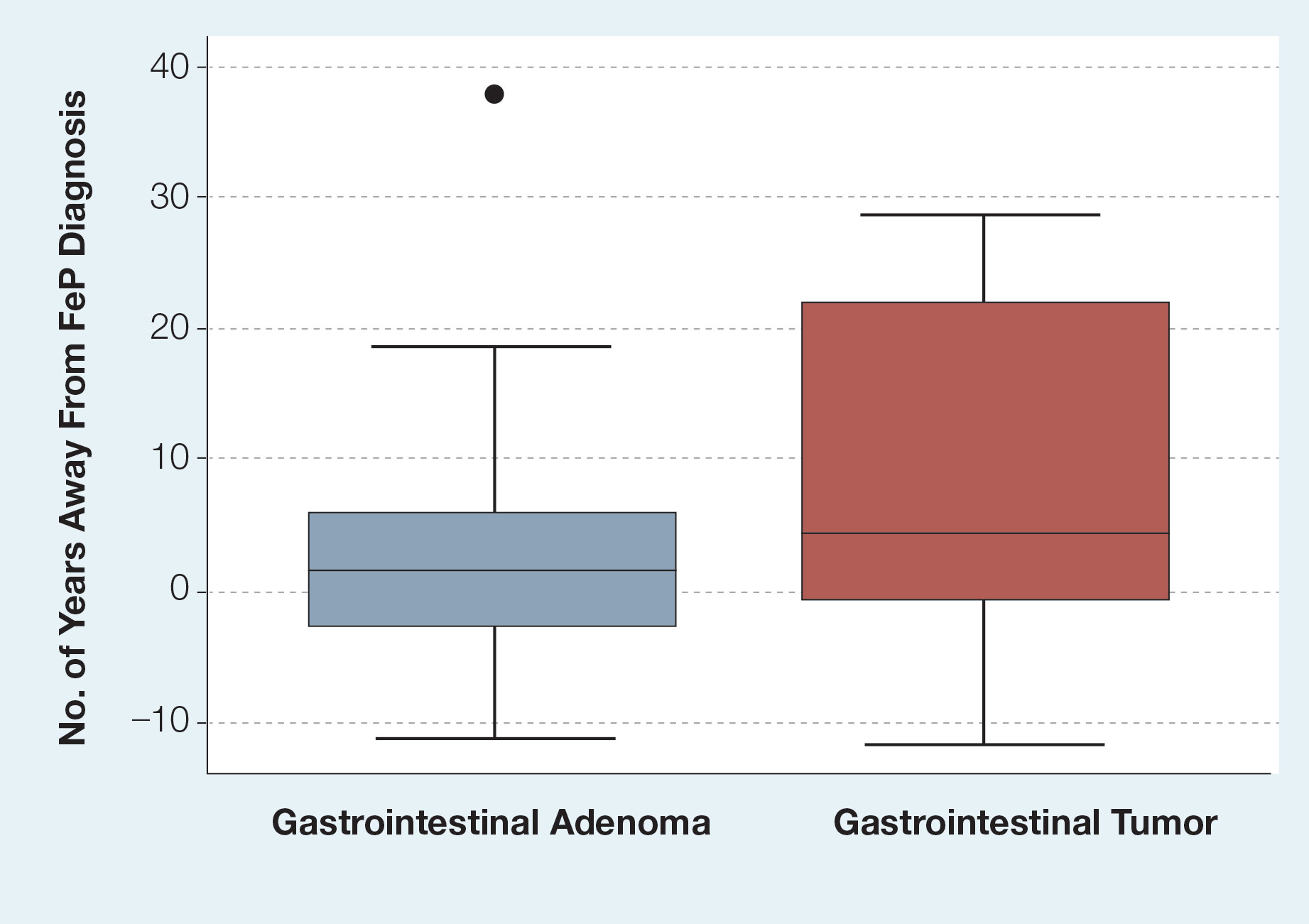
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Chronic Ulcerative Lesion
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.
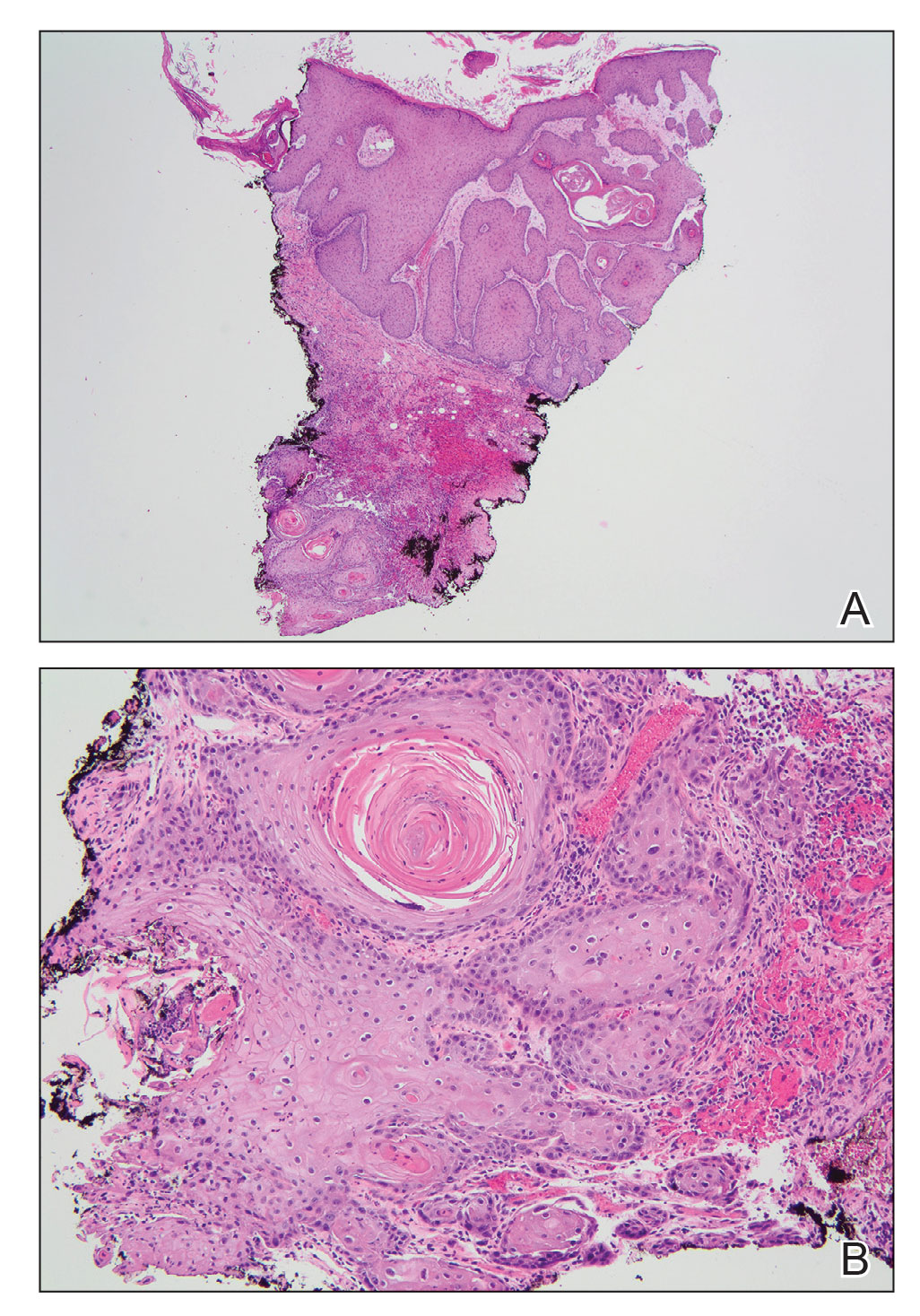
Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
A 46-year-old man with a history of a left leg burn during childhood that was unsuccessfully treated with multiple skin grafts presented as a hospital follow-up for outpatient management of an ulcer. The patient had an ulcer that gradually increased in size over 7 years. Over the course of 2 weeks prior to the hospital presentation, he noted increased pain and severe difficulty with ambulation but remained afebrile without other systemic symptoms. Prior to the outpatient follow-up, he had been admitted to the hospital where he underwent imaging, laboratory studies, and skin biopsy, as well as treatment with empiric vancomycin. Physical examination revealed a large undermined ulcer with an elevated peripheral margin and crusting on the left lower leg with surrounding chronic scarring.
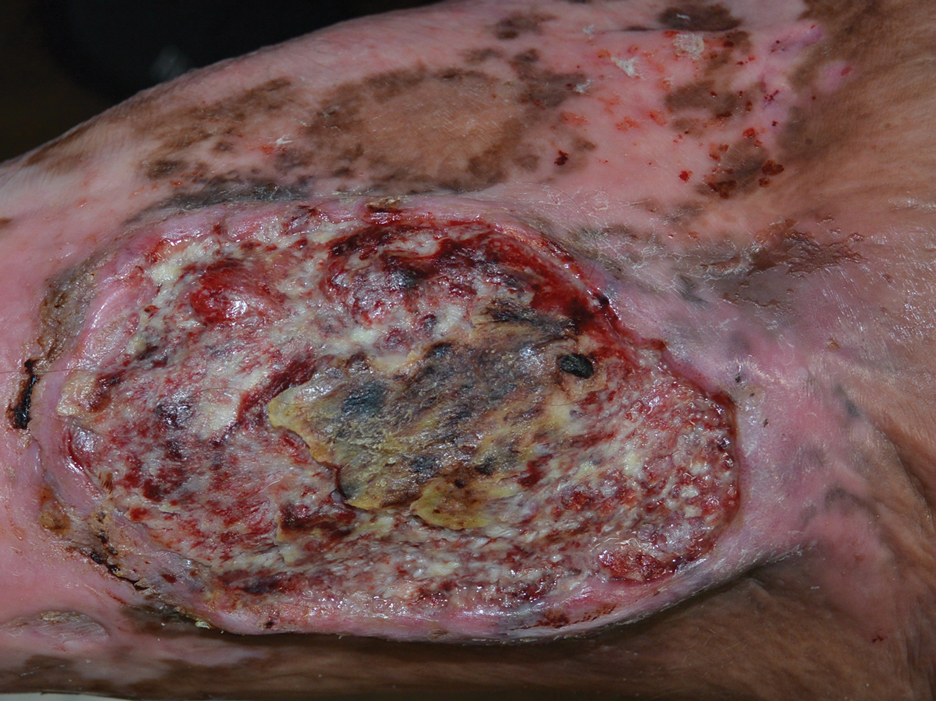
Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
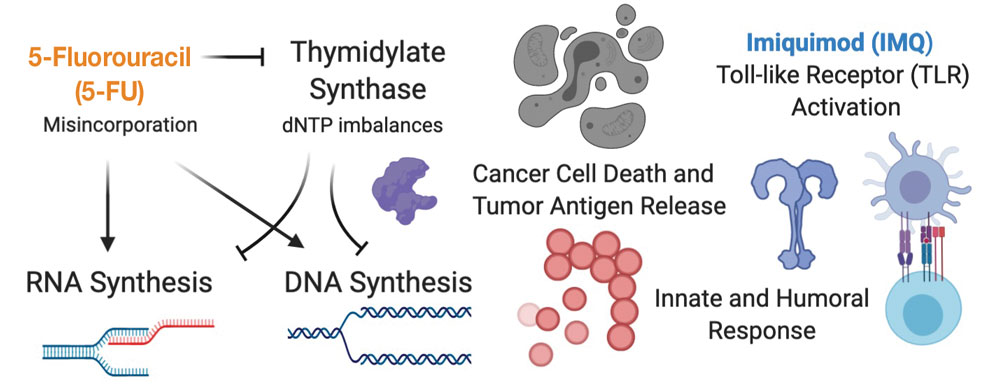
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea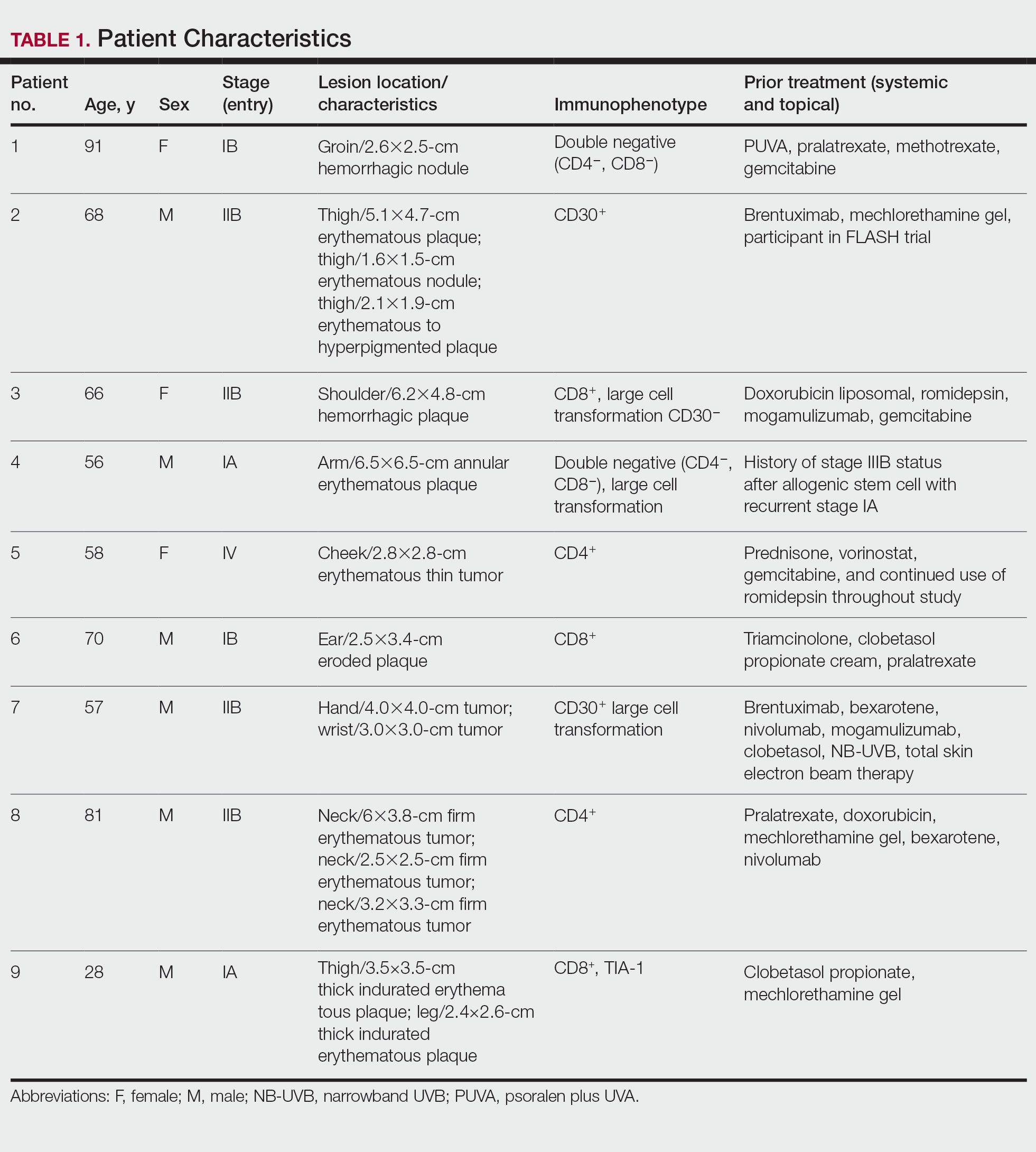
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
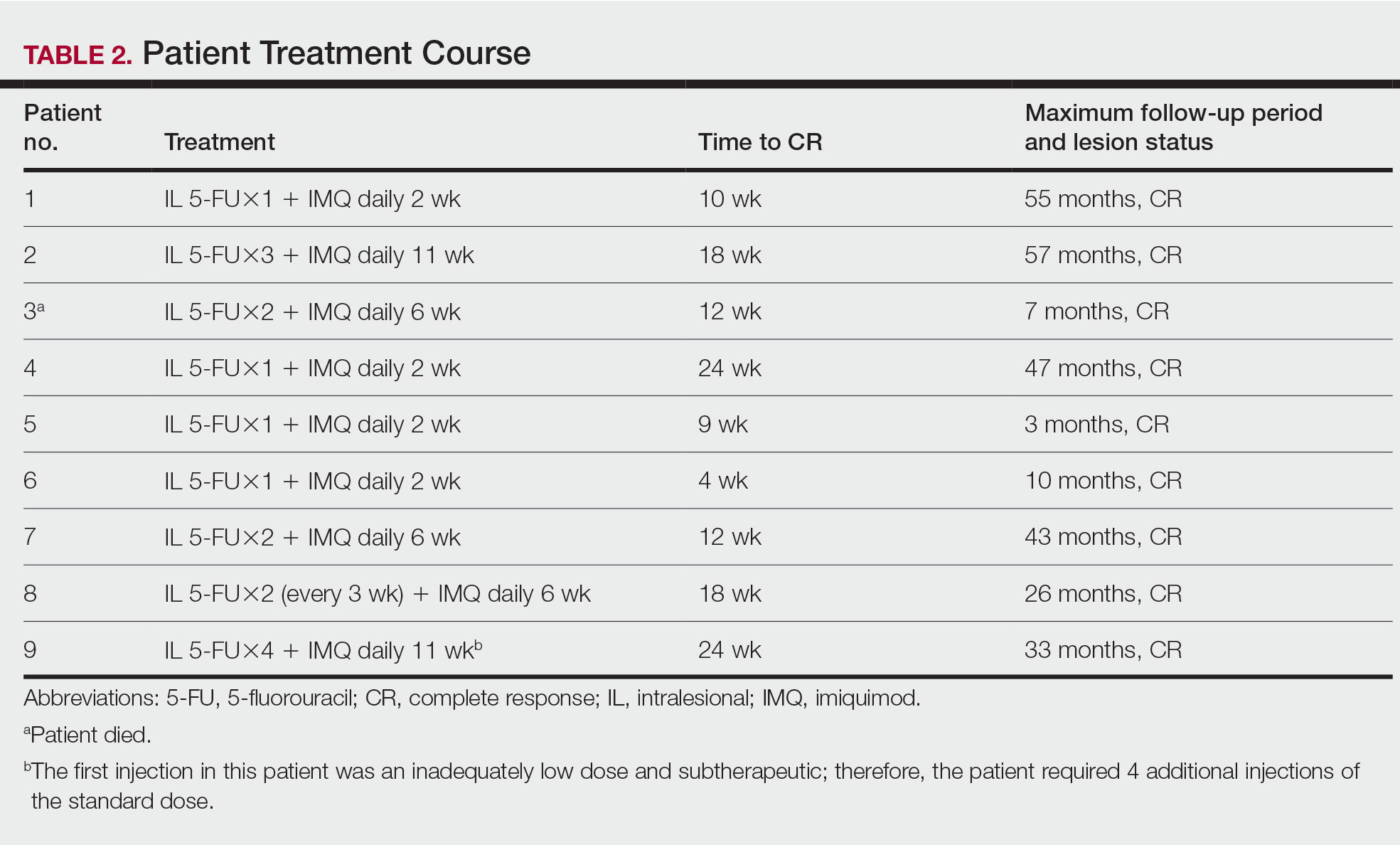
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
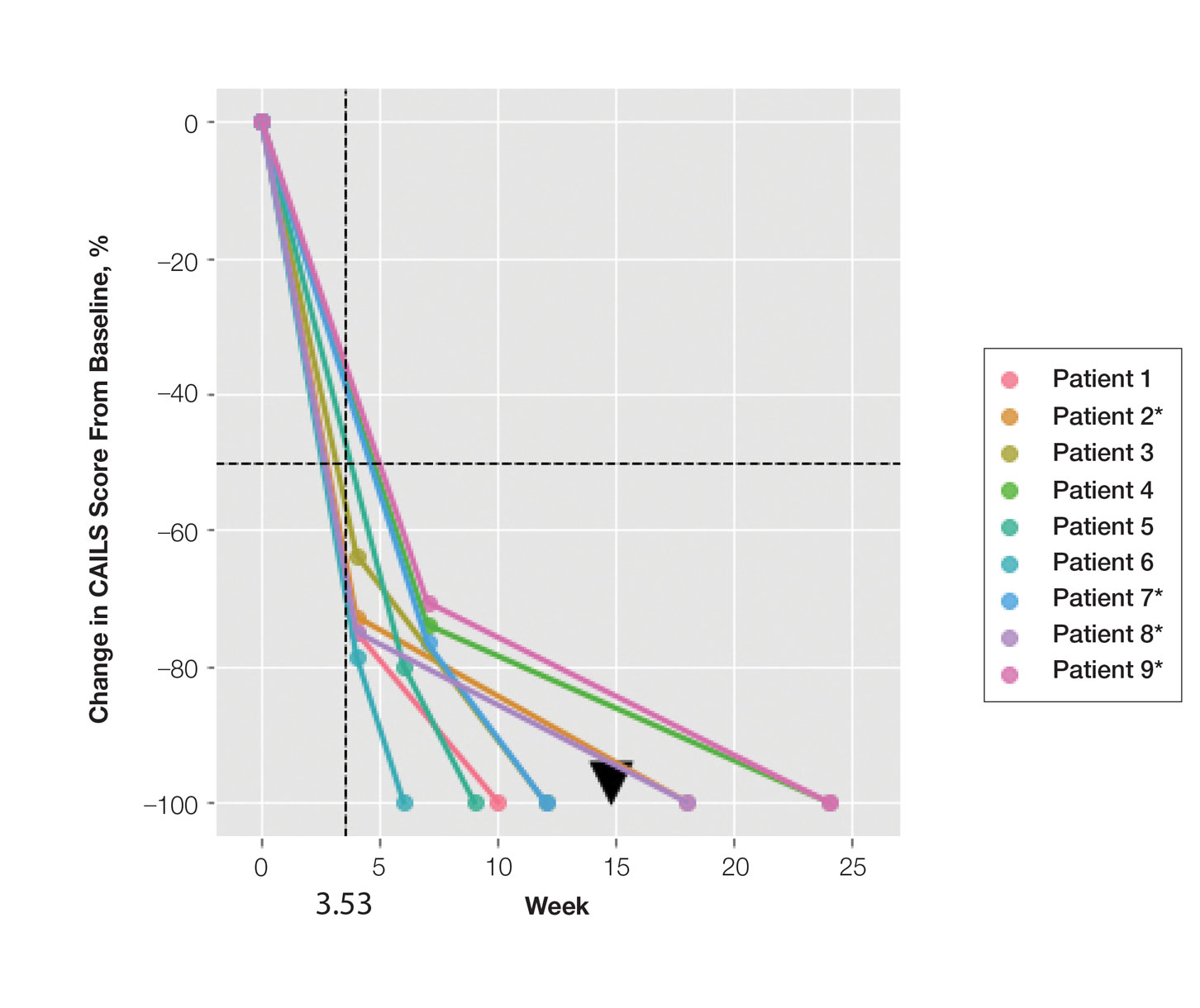
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
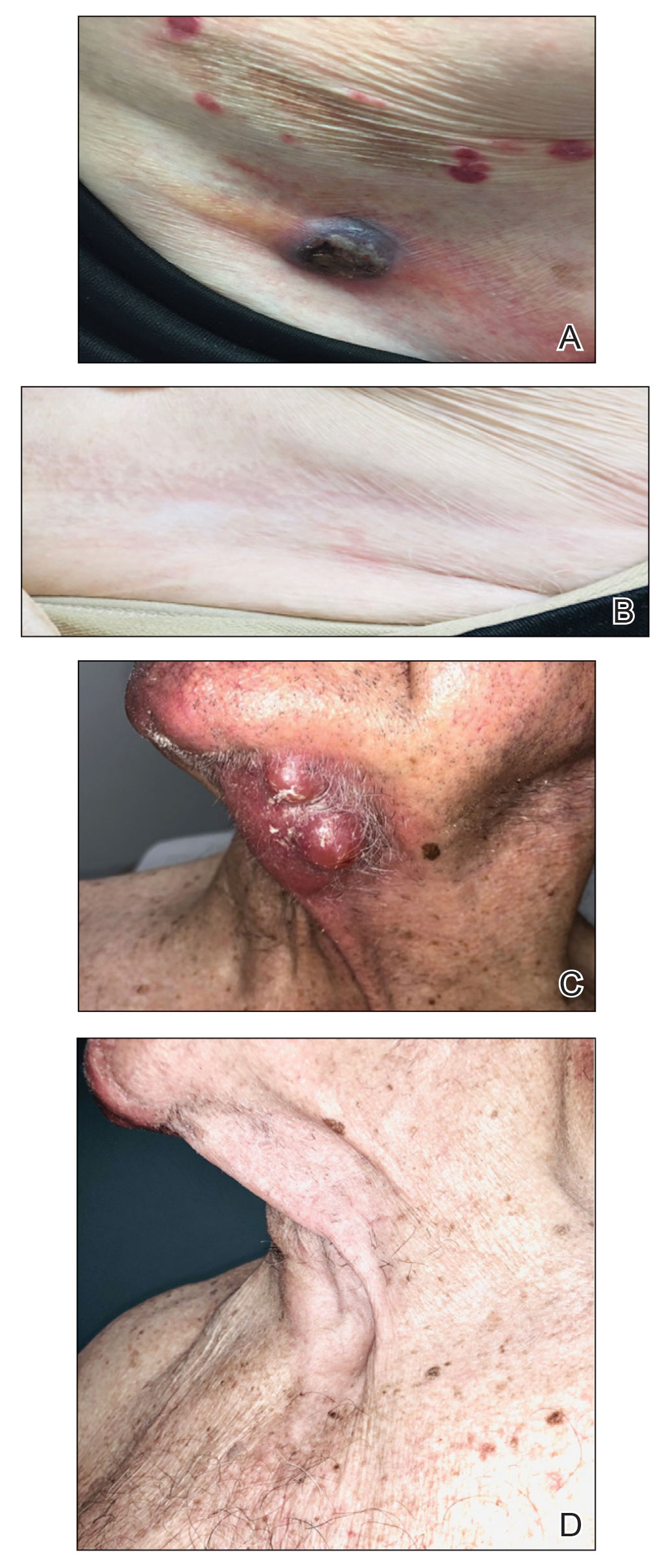
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.
Long-pulsed 1,064 nm Nd:YAG for nonaggressive BCC ‘effective and easy’
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
AT MOAS 2022
Manicure gone wrong leads to cancer diagnosis
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”