User login
Guidelines for assessing cancer risk may need updating
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
The authors of the clinical trial suggest that these guidelines may need to be revised.
Individuals with hereditary breast and ovarian cancer (HBOC) have an 80% lifetime risk of breast cancer and are at greater risk of ovarian cancer, pancreatic cancer, prostate cancer, and melanoma. Those with Lynch syndrome (LS) have an 80% lifetime risk of colorectal cancer, a 60% lifetime risk of endometrial cancer, and heightened risk of upper gastrointestinal, urinary tract, skin, and other tumors, said study coauthor N. Jewel Samadder, MD in a statement.
The National Cancer Control Network has guidelines for determining family risk for colorectal cancer and breast, ovarian, and pancreatic cancer to identify individuals who should be screened for LS and HBOC, but these rely on personal and family health histories.
“These criteria were created at a time when genetic testing was cost prohibitive and thus aimed to identify those at the greatest chance of being a mutation carrier in the absence of population-wide whole-exome sequencing. However, [LS and HBOC] are poorly identified in current practice, and many patients are not aware of their cancer risk,” said Dr. Samadder, professor of medicine and coleader of the precision oncology program at the Mayo Clinic Comprehensive Cancer Center, Phoenix, in the statement.
Whole-exome sequencing covers only protein-coding regions of the genome, which is less than 2% of the total genome but includes more than 85% of known disease-related genetic variants, according to Emily Gay, who presented the trial results (Abstract 5768) on April 18 at the annual meeting of the American Association for Cancer Research.
“In recent years, the cost of whole-exome sequencing has been rapidly decreasing, allowing us to complete this test on saliva samples from thousands, if not tens of thousands of patients covering large populations and large health systems,” said Ms. Gay, a genetic counseling graduate student at the University of Arizona, during her presentation.
She described results from the TAPESTRY clinical trial, with 44,306 participants from Mayo Clinic centers in Arizona, Florida, and Minnesota, who were identified as definitely or likely to be harboring pathogenic mutations and consented to whole-exome sequencing from saliva samples. They used electronic health records to determine whether patients would satisfy the testing criteria from NCCN guidelines.
The researchers identified 1.24% of participants to be carriers of HBOC or LS. Of the HBOC carriers, 62.8% were female, and of the LS carriers, 62.6% were female. The percentages of HBOC and LS carriers who were White were 88.6 and 94.5, respectively. The median age of both groups was 57 years. Of HBOC carriers, 47.3% had personal histories of cancers; for LS carries, the percentage was 44.2.
Of HBOC carriers, 49.1% had been previously unaware of their genetic condition, while an even higher percentage of patients with LS – 59.3% – fell into that category. Thirty-two percent of those with HBOC and 56.2% of those with LS would not have qualified for screening using the relevant NCCN guidelines.
“Most strikingly,” 63.8% of individuals with mutations in the MSH6 gene and 83.7% of those mutations in the PMS2 gene would not have met NCCN criteria, Ms. Gay said.
Having a cancer type not known to be related to a genetic syndrome was a reason for 58.6% of individuals failing to meet NCCN guidelines, while 60.5% did not meet the guidelines because of an insufficient number of relatives known to have a history of cancer, and 63.3% did not because they had no personal history of cancer. Among individuals with a pathogenic mutation who met NCCN criteria, 34% were not aware of their condition.
“This suggests that the NCCN guidelines are underutilized in clinical practice, potentially due to the busy schedule of clinicians or because the complexity of using these criteria,” said Ms. Gay.
The numbers were even more striking among minorities: “There is additional data analysis and research needed in this area, but based on our preliminary findings, we saw that nearly 50% of the individuals who are [part of an underrepresented minority group] did not meet criteria, compared with 32% of the white cohort,” said Ms. Gay.
Asked what new NCCN guidelines should be, Ms. Gay replied: “I think maybe limiting the number of relatives that you have to have with a certain type of cancer, especially as we see families get smaller and smaller, especially in the United States – that family data isn’t necessarily available or as useful. And then also, I think, incorporating in the size of a family into the calculation, so more of maybe a point-based system like we see with other genetic conditions rather than a ‘yes you meet or no, you don’t.’ More of a range to say ‘you fall on the low-risk, medium-risk, or high-risk stage,’” said Ms. Gay.
During the Q&A period, session cochair Andrew Godwin, PhD, who is a professor of molecular oncology and pathology at University of Kansas Medical Center, Kansas City, said he wondered if whole-exome sequencing was capable of picking up cancer risk mutations that standard targeted tests don’t look for.
Dr. Samadder, who was in the audience, answered the question, saying that targeted tests are actually better at picking up some types of mutations like intronic mutations, single-nucleotide polymorphisms, and deletions.
“There are some limitations to whole-exome sequencing. Our estimate here of 1.2% [of participants carrying HBOC or LS mutations] is probably an underestimate. There are additional variants that exome sequencing probably doesn’t pick up easily or as well. That’s why we qualify that exome sequencing is a screening test, not a diagnostic,” he continued.
Ms. Gay and Dr. Samadder have no relevant financial disclosures. Dr. Godwin has financial relationships with Clara Biotech, VITRAC Therapeutics, and Sinochips Diagnostics.
FROM AACR 2023
Acute Onset of Vitiligolike Depigmentation After Nivolumab Therapy for Systemic Melanoma
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
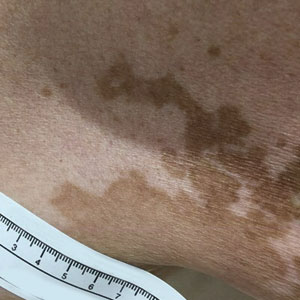
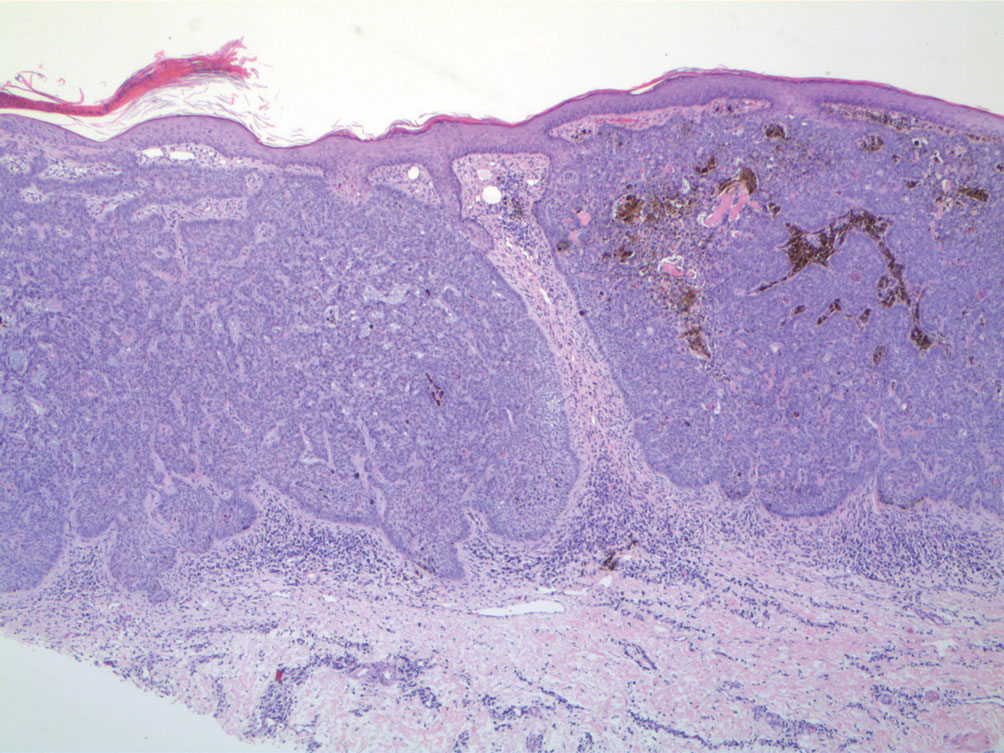
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.
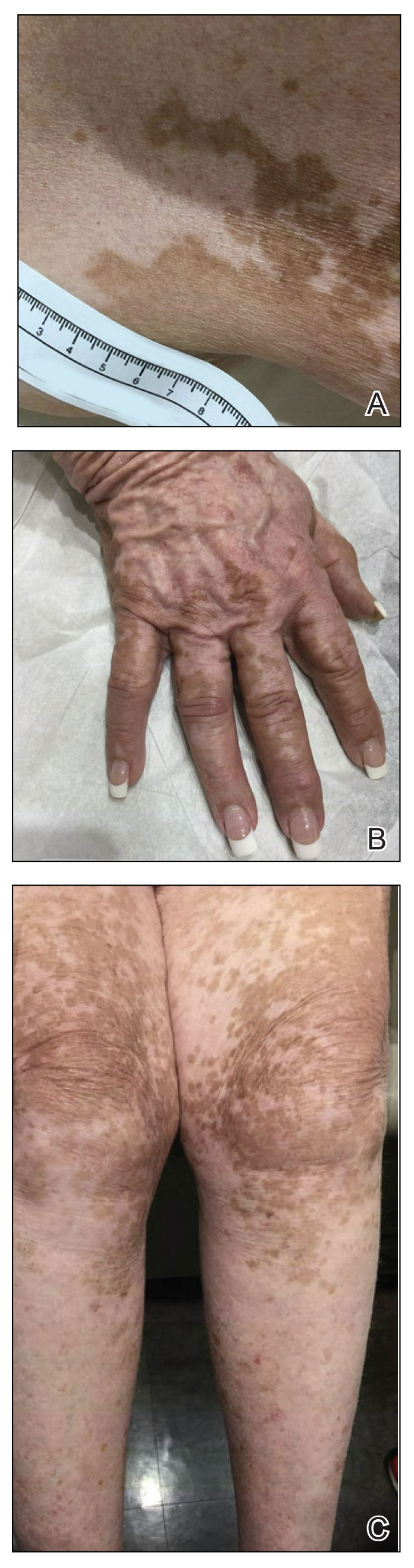
At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
To the Editor:
Vitiligolike depigmentation has been known to develop around the sites of origin of melanoma or more rarely in patients treated with antimelanoma therapy.1 Vitiligo is characterized by white patchy depigmentation of the skin caused by the loss of functional melanocytes from the epidermis. The exact mechanisms of disease are unknown and multifactorial; however, autoimmunity plays a central role. Interferon gamma (IFN-γ), C-X-C chemokine ligand 10, and IL-22 have been identified as key mediators in an inflammatory cascade leading to the stimulation of the innate immune response against melanocyte antigens.2,3 Research suggests melanoma-associated vitiligolike leukoderma also results from an immune reaction directed against antigenic determinants shared by both normal and malignant melanocytes.3 Vitiligolike lesions have been associated with the use of immunomodulatory agents such as nivolumab, a fully humanized monoclonal IgG4 antibody, which blocks the programmed cell death protein 1 (PD-1) receptor that normally is expressed on T cells during the effector phase of T-cell activation.4,5 In the tumor microenvironment, the PD-1 receptor is stimulated, leading to downregulation of the T-cell effector function and destruction of T cells.5 Due to T-cell apoptosis and consequent suppression of the immune response, tumorigenesis continues. By inhibiting the PD-1 receptor, nivolumab increases the number of active T cells and antitumor response. However, the distressing side effect of vitiligolike depigmentation has been reported in 15% to 25% of treated patients.6
In a meta-analysis by Teulings et al,7 patients with new-onset vitiligo and malignant melanoma demonstrated a 2-fold decrease in cancer progression and a 4-fold decreased risk for death vs patients without vitiligo development. Thus, in patients with melanoma, vitiligolike depigmentation should be considered a good prognostic indicator as well as a visible sign of spontaneous or therapy-induced antihumoral immune response against melanocyte differentiation antigens, as it is associated with a notable survival benefit in patients receiving immunotherapy for metastatic melanoma.3 We describe a case of diffuse vitiligolike depigmentation that developed suddenly during nivolumab treatment, causing much distress to the patient.
A 75-year-old woman presented to the clinic with a chief concern of sudden diffuse skin discoloration primarily affecting the face, hands, and extremities of 3 weeks’ duration. She had a medical history of metastatic melanoma—the site of the primary melanoma was never identified—and she was undergoing immune-modulating therapy with nivolumab. She was on her fifth month of treatment and was experiencing a robust therapeutic response with a reported 100% clearance of the metastatic melanoma as observed on a positron emission tomography scan. The patchy depigmentation of skin was causing her much distress. Physical examination revealed diffuse patches of hypopigmentation on the trunk, face, and extremities (Figure). Shave biopsies of the right lateral arm demonstrated changes consistent with vitiligo, with an adjacent biopsy illustrating normal skin characteristics. Triamcinolone ointment 0.1% was initiated, with instruction to apply it to affected areas twice daily for 2 weeks. However, there was no improvement, and she discontinued use.

At 3-month follow-up, the depigmentation persisted, prompting a trial of hydroquinone cream 4% to be used sparingly in cosmetically sensitive areas such as the face and dorsal aspects of the hands. Additionally, diligent photoprotection was advised. Upon re-evaluation 9 months later, the patient remained in cancer remission, continued nivolumab therapy, and reported improvement in the hypopigmentation with a more even skin color with topical hydroquinone use. She no longer noticed starkly contrasting hypopigmented patches.
Vitiligo is a benign skin condition characterized by white depigmented macules and patches. The key feature of the disorder is loss of functional melanocytes from the cutaneous epidermis and sometimes from the hair follicles, with various theories on the cause. It has been suggested that the disease is multifactorial, involving both genetics and environmental factors.2 Regardless of the exact mechanism, the result is always the same: loss of melanin pigment in cells due to loss of melanocytes.
Autoimmunity plays a central role in the causation of vitiligo and was first suspected as a possible cause due to the association of vitiligo with several other autoimmune disorders, such as thyroiditis.8 An epidemiological survey from the United Kingdom and North America (N=2624) found that 19.4% of vitiligo patients aged 20 years or older also reported a clinical history of autoimmune thyroid disease compared with 2.4% of the overall White population of the same age.9 Interferon gamma, C-X-C chemokine ligand 10, and IL-22 receptors stimulate the innate immune response, resulting in an overactive danger signaling cascade, which leads to proinflammatory signals against melanocyte antigens.2,3 The adaptive immune system also participates in the progression of vitiligo by activating dermal dendritic cells to attack melanocytes along with melanocyte-specific cytotoxic T cells.
Immunomodulatory agents utilized in the treatment of metastatic melanoma have been linked to vitiligolike depigmentation. In those receiving PD-1 immunotherapy for metastatic melanoma, vitiligolike lesions have been reported in 15% to 25% of patients.6 Typically, the PD-1 molecule has a regulatory function on effector T cells. Interaction of the PD-1 receptor with its ligands occurs primarily in peripheral tissue causing apoptosis and downregulation of effector T cells with the goal of decreasing collateral damage to surrounding tissues by active T cells.5 In the tumor microenvironment, however, suppression of the host’s immune response is enhanced by aberrant stimulation of the PD-1 receptor, causing downregulation of the T-cell effector function, T-cell destruction, and apoptosis, which results in continued tumor growth. Nivolumab, a fully humanized monoclonal IgG4 antibody, selectively inhibits the PD-1 receptor, disrupting the regulator pathway that would typically end in T-cell destruction.5 Accordingly, the population of active T cells is increased along with the antitumor response.4,10 Nivolumab exhibits success as an immunotherapeutic agent, with an overall survival rate in patients with metastatic melanoma undergoing nivolumab therapy of 41% to 42% at 3 years and 35% at 5 years.11 However, therapeutic manipulation of the host’s immune response does not come without a cost. Vitiligolike lesions have been reported in up to a quarter of patients receiving PD-1 immunotherapy for metastatic melanoma.6
The relationship between vitiligolike depigmentation and melanoma can be explained by the immune activation against antigens associated with melanoma that also are expressed by normal melanocytes. In clinical observations of patients with melanoma and patients with vitiligo, antibodies to human melanocyte antigens were present in 80% (24/30) of patients vs 7% (2/28) in the control group.12 The autoimmune response results from a cross-reaction of melanoma cells that share the same antigens as normal melanocytes, such as melanoma antigen recognized by T cells 1 (MART-1), gp100, and tyrosinase.13,14
Development of vitiligolike depigmentation in patients with metastatic melanoma treated with nivolumab has been reported to occur between 2 and 15 months after the start of PD-1 therapy. This side effect of treatment correlates with favorable clinical outcomes.15,16 Enhancing immune recognition of melanocytes in patients with melanoma confers a survival advantage, as studies by Koh et al17 and Norlund et al18 involving patients who developed vitiligolike hypopigmentation associated with malignant melanoma indicated a better prognosis than for those without hypopigmentation. The 5-year survival rate of patients with both malignant melanoma and vitiligo was reported as 60% to 67% when it was estimated that only 30% to 50% of patients should have survived that duration of time.17,18 Similarly, a systematic review of patients with melanoma stages III and IV reported that those with associated hypopigmentation had a 2- to 4-fold decreased risk of disease progression and death compared to patients without depigmentation.7
Use of traditional treatment therapies for vitiligo is based on the ability of the therapy to suppress the immune system. However, in patients with metastatic melanoma undergoing immune-modulating cancer therapies, traditional treatment options may counter the antitumor effects of the targeted immunotherapies and should be used with caution. Our patient displayed improvement in the appearance of her starkly contrasting hypopigmented patches with the use of hydroquinone cream 4%, which induced necrotic death of melanocytes by inhibiting the conversion of L-3,4-dihydroxyphenylalanine to melanin by tyrosinase.19 The effect achieved by using topical hydroquinone 4% was a lighter skin appearance in areas of application.
There is no cure for vitiligo, and although it is a benign condition, it can negatively impact a patient's quality of life. In some countries, vitiligo is confused with leprosy, resulting in a social stigma attached to the diagnosis. Many patients are frightened or embarrassed by the diagnosis of vitiligo and its effects, and they often experience discrimination.2 Patients with vitiligo also experience more psychological difficulties such as depression.20 The unpredictability of vitiligo is associated with negative emotions including fear of spreading the lesions, shame, insecurity, and sadness.21 Supportive care measures, including psychological support and counseling, are recommended. Additionally, upon initiation of anti–PD-1 therapies, expectations should be discussed with patients concerning the possibilities of depigmentation and associated treatment results. Although the occurrence of vitiligo may cause the patient concern, it should be communicated that its presence is a positive indicator of a vigorous antimelanoma immunity and an increased survival rate.7
Vitiligolike depigmentation is a known rare adverse effect of nivolumab treatment. Although aesthetically unfavorable for the patient, the development of vitiligolike lesions while undergoing immunotherapy for melanoma may be a sign of a promising clinical outcome due to an effective immune response to melanoma antigens. Our patient remains in remission without any evidence of melanoma after 9 months of therapy, which offers support for a promising outcome for melanoma patients who experience vitiligolike depigmentation.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
- de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Ortonne, JP, Passeron, T. Vitiligo and other disorders of hypopigmentation. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1087-1114.
- Opdivo. Package insert. Bristol-Myers Squibb Company; 2023.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300-5309.
- Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.e1.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168:756-761.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Hodi FS, Kluger H, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Cancer Res. 2016;76(14 suppl):CT001.
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314-318.
- Lynch SA, Bouchard BN, Vijayasaradhi S, et al. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10:141-150.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;15:1206-1212.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
- Koh HK, Sober AJ, Nakagawa H, et al. Malignant melanoma and vitiligo-like leukoderma: an electron microscope study. J Am Acad Dermatol. 1983;9:696-708.
- Nordlund JJ, Kirkwood JM, Forget BM, et al. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689-696.
- Palumbo A, d’Ischia M, Misuraca G, et al. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85-90.
- Lai YC, Yew YW, Kennedy C, et al. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177:708-718.
- Nogueira LSC, Zancanaro PCQ, Azambuja RD. Vitiligo and emotions. An Bras Dermatol. 2009;84:41-45.
Practice Points
- New-onset vitiligo coinciding with malignant melanoma should be considered a good prognostic indicator.
- Daily use of hydroquinone cream 4% in conjunction with diligent photoprotection was shown to even overall skin tone in a patient experiencing leukoderma from nivolumab therapy.
Collision Course of a Basal Cell Carcinoma and Apocrine Hidrocystoma on the Scalp
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.
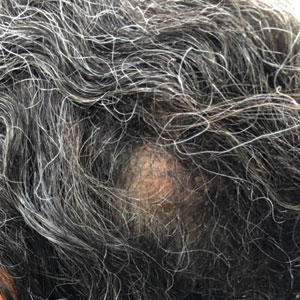
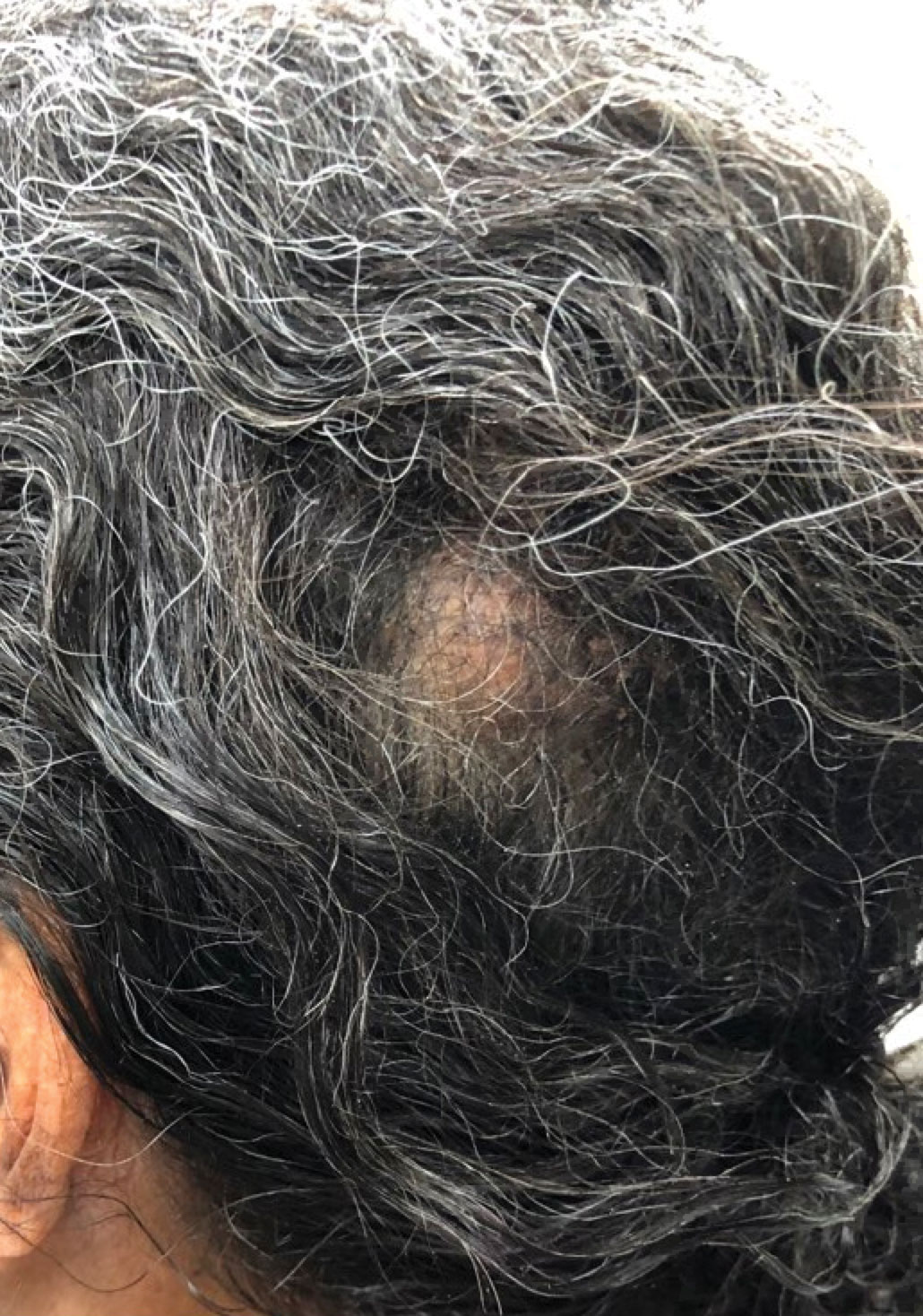
A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.
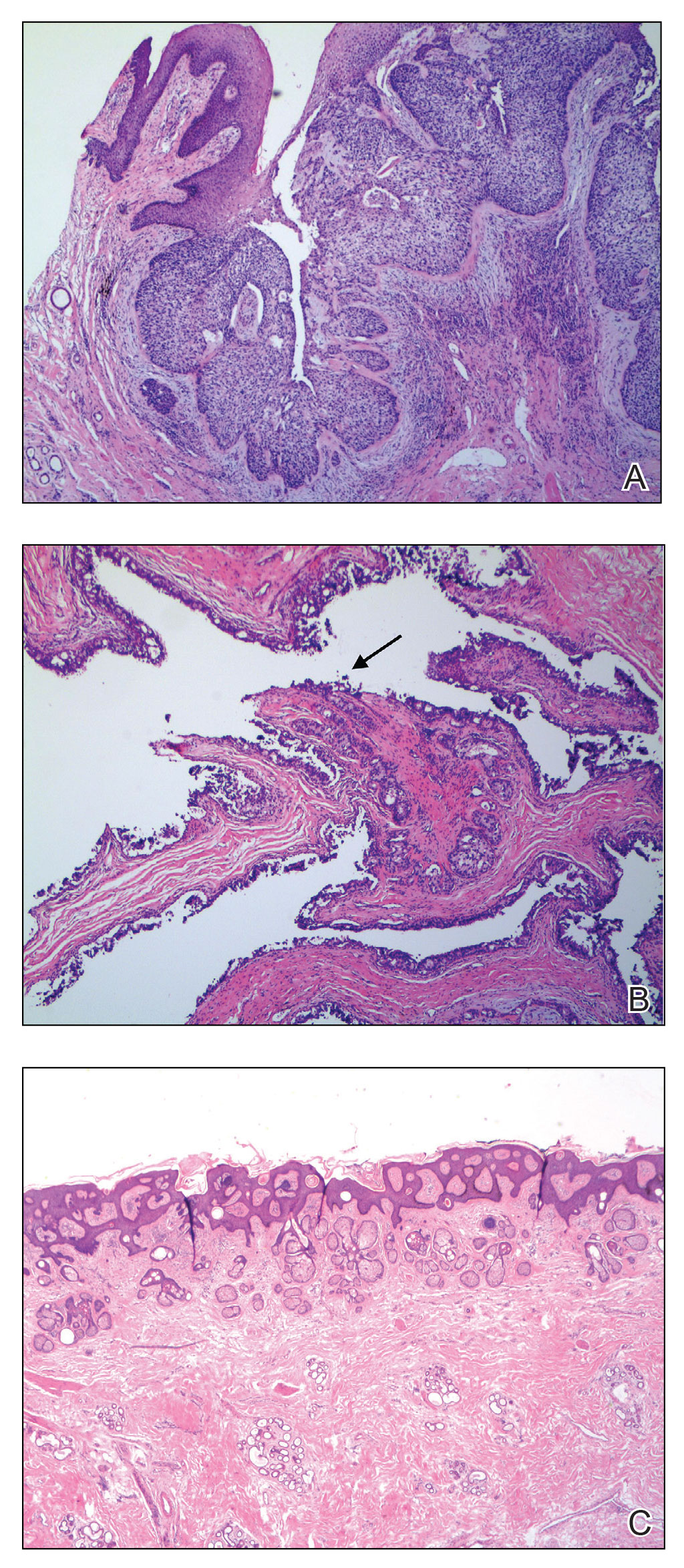
The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).
The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
PRACTICE POINTS
- When collision tumors are encountered during Mohs micrographic surgery, review of the initial diagnostic material is recommended.
- Permanent processing of Mohs excisions may be helpful in determining the diagnosis of the occult second tumor diagnosis.
USPSTF releases updated recommendations on skin cancer screening
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
.
This final recommendation applies to the general public and is not meant for those at higher risk, such as people with a family history of skin cancer or who have any signs or symptoms, such as irregular moles.
“The new recommendations are consistent with those from 2016, and we are unable to balance benefits and harms,” said Task Force member Katrina Donahue, MD, MPH, professor and vice chair of research in the department of family medicine at the University of North Carolina, Chapel Hill. “Unfortunately, there is not enough evidence to recommend for or against screening, and health care professionals should use their judgment when deciding whether or not to screen.”
Dr. Donahue told this news organization that this is a call for more research: “Our recommendations are for patients who present to primary care without symptoms, and after a careful assessment of benefit and harms, we didn’t have evidence to push us towards screening as a benefit. We did look at data from two large screening programs, but they were from Europe and not representative of the U.S. population. They also did not show a benefit for reducing melanoma-related mortality.”
The USPSTF final recommendation statement and corresponding evidence summary have been published online in JAMA, as well as on the USPSTF website.
Skin cancer is the most commonly diagnosed cancer in the United States, but there are different types that vary in their incidence and severity. Basal and squamous cell carcinomas are the most common types of skin cancer, but they infrequently lead to death or substantial morbidity, notes the USPTSF. Melanomas represent about 1% of skin cancer and cause the most skin cancer deaths. An estimated 8,000 individuals in the United States will die of melanoma in 2023.
There are racial differences in melanoma incidence; it is about 30 times more common in White versus Black persons, but disease in persons with darker skin color tends to be diagnosed at a later stage. These disparities may be due to differences in risk factors, access to care, and clinical presentation.
In an accompanying editorial, Maryam M. Asgari, MD, MPH, of the department of dermatology, Massachusetts General Hospital, Boston, and Lori A. Crane, PhD, MPH, of the Colorado School of Public Health, University of Colorado, Aurora, point out that people with darker skin phenotypes also tend to be affected by skin cancers that are not associated with UV radiation, such as acral melanoma, which arises on the palms and soles, and skin cancers that arise in areas of chronic inflammation, such as wounds.
Thus, differences in anatomical distribution of skin cancers in in the various subpopulations needs to be considered when performing skin screening, they write. “Furthermore, while skin cancer risk is lower among people with darker skin pigmentation, survival is often worse for cancers like melanoma, highlighting the potential need for screening.”
“More data are needed, particularly regarding genetic and environmental risk factors for skin cancer in people with darker pigmentation, to help inform guidelines that can be broadly applied to the U.S. population,” add Dr. Asgari and Dr. Crane. “The diversity of the U.S. population extends also to geography, culture, and socioeconomic status, all of which affect skin cancer risk.”
Review of evidence
The USPSTF commissioned a systematic review to evaluate the benefits and harms of screening for skin cancer in asymptomatic adolescents and adults, including evidence for both keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma) and cutaneous melanoma.
Foundational evidence showed that the sensitivity of visual skin examination by a clinician to detect melanoma ranged from 40% to 70% and specificity ranged from 86% to 98%. Evidence that evaluated the diagnostic accuracy of visual skin examination to detect keratinocyte carcinoma was limited and inconsistent. There were no new studies reporting on diagnostic accuracy for an asymptomatic screening population.
The USPSTF also reviewed 20 studies in 29 articles (n = 6,053,411). This included three nonrandomized studies evaluating two skin cancer screening programs in Germany, but results were inconsistent. In addition, the ecological and nonrandomized design of the studies limited the conclusions that could be drawn and the applicability to a U.S. population was difficult to assess because of differences in population diversity and health care delivery in the United States.
Other nonrandomized studies that looked at various outcomes, such as harms and stage at diagnosis and melanoma or all-cause mortality, also did not provide sufficient evidence to support screening.
Research is needed
In a second accompanying editorial published in JAMA Dermatology, Adewole S. Adamson, MD, MPP, of the division of dermatology and dermatologic surgery at the University of Texas, Austin, pointed out that unlike other cancer screening programs, such as those for breast, colon, and prostate cancer, skin cancer screening programs are somewhat less organized.
The other programs focus on defined groups of the population, generally with easily identifiable characteristics such as age, sex, and family history, and importantly, there are always defined ages for initiation and halting of screening and intervals for screening frequency. None of these basic screening parameters have been widely adopted among dermatologists in the United States, he wrote. “One important reason why skin cancer screening has remained inconsistent is that it is not covered by Medicare or by many commercial insurance companies,” Dr. Adamson told this news organization. “The test, in this case the skin exam, is often performed as part of a routine dermatology visit.”
Dermatologists should take the lead on this, he said. “Dermatologists should push for a high quality prospective clinical trial of skin cancer screening, preferably in a high-risk population.”
Dr. Donahue agrees that research is needed, as noted in the recommendation. For example, studies are needed demonstrating consistent data of the effects of screening on morbidity and mortality or early detection of skin cancer, and clearer descriptions of skin color and inclusion of a full spectrum of skin colors in study participants. Clinical research is also needed on outcomes in participants that reflect the diversity of the U.S. population.
“I hope funding agencies will be interested in this area of study,” she said. “We put out the whole systematic review and point out the gaps. We need consistent evidence in detecting cancer early and reducing complications from skin cancer.”
The U.S. Congress mandates that the Agency for Healthcare Research and Quality support the operations of the USPSTF.
None of the USPSTF authors report any disclosures. Dr. Asgari reported receiving royalties from UpToDate. Dr. Crane did not make any disclosures. Dr. Adamson reported serving as an expert reviewer for the U.S. Preventive Services Task Force skin cancer screening report, as well as support from the Robert Wood Johnson Foundation, the Dermatology Foundation Public Health Career Development Award, the National Institutes of Health, the American Cancer Society, and Meredith’s Mission for Melanoma.
A version of this article originally appeared on Medscape.com.
Bergamot
Citrus bergamia (bergamot) is a fruit tree thought to originate in the Mediterranean area; its fruit has been a part of the diet in that region since the early 18th century.1 Bergamot is known to confer antioxidant as well as anti-inflammatory activity, and yields proapoptotic effects in the sebaceous gland.2,3 The plant contains the natural furocoumarin bergapten, which is also known as 5-methoxypsoralen.4
5 In this capacity, bergamot oil has been used for photodynamic therapy of cutaneous conditions such as vitiligo.6 In fact, for several years 5-methoxypsoralen and 8-methoxypsoralen have been used to achieve acceptable clearance rates of psoriasis and vitiligo.7 This column focuses on bergapten, as well as the cutaneous conditions for which bergamot has been shown to have some benefits warranting application or further investigation.
Bergapten
In a 2021 literature review, Liang et al. cited the anti-inflammatory, antimicrobial, anticancer, and other salutary effects associated with bergapten. Based on numerous citations, they also cautioned about the phototoxicity of the compound combined with ultraviolet (UV) light while noting the photoactivation of bergapten for anticancer uses.4
The following year, Quetglas-Llabrés et al. acknowledged, in another literature review, the numerous preclinical and in vitro studies demonstrating the therapeutic activity of bergapten and highlighted clinical trials revealing notable lesion clearance rates of psoriasis or vitiligo imparted by oral or topical bergapten along with UV irradiation. Bergapten was also found to be effective as hypolipemic therapy.5
Anti-inflammatory topical uses
In a 2017 study by Han et al. of 10 essential oils, bergamot was among the investigated oils, all of which exhibited significant anti-proliferative activity in a preinflamed human dermal fibroblast system simulating chronic inflammation. Bergamot was among three essential oils that also suppressed protein molecules involved with inflammation, immune responses, and tissue remodeling, indicating anti-inflammatory and wound healing characteristics.8
More recently, Cristiano et al. reported that ultradeformable nanocarriers containing bergamot essential oil and ammonium glycyrrhizinate were demonstrated in healthy human volunteers to be characterized by the appropriate mean size, size distribution, surface charge, and long-term stability for topical administration. Topical administration on human volunteers also revealed greater activity of the combined agents as compared with a nanosystem loaded only with ammonium glycyrrhizinate. The researchers concluded that this combination of ingredients in ultradeformable vesicles shows potential as topical anti-inflammatory treatment.3
Acne
In a 2020 study using golden hamsters, Sun et al. assessed the effects of the juice and essential oils of bergamot and sweet orange on acne vulgaris engendered by excessive androgen secretion. Among 80 male hamsters randomly divided into 10 groups ranging from low to high doses, all results demonstrated improvement with treatment as seen by decreased growth rates of sebaceous glands, suppressed triglyceride accumulation, lowered inflammatory cytokine release, and apoptosis promotion in sebaceous glands. The authors noted that the essential oils yielded better dose-dependent effects than the juices.2
Psoriasis
In 2019, Perna et al. conducted a literature review on the effects of bergamot essential oil, extract, juice, and polyphenolic fraction on various health metrics. Thirty-one studies (20 involving humans with 1,709 subjects and 11 in rats and mice) were identified. Animal models indicated that bergamot essential oil (10 mg/kg or 20 mg/kg daily for 20 weeks) reduced psoriatic plaques, increased skin collagen content, and fostered hair growth and that bergamot juice (20 mg/kg) diminished proinflammatory cytokines. Human studies showed that bergamot extract and essential oil may reduce blood pressure and improve mental conditions.9
Vitiligo
In 2019, Shaaban et al. prepared elastic nanocarriers (spanlastics) to deliver psoralen-containing bergamot oil along with PUVB with the intention of harnessing melanogenic activity to treat vitiligo. Histopathologic assessment on rat skin was conducted before clinical treatment in patients with vitiligo. The spanlastics were deemed to be of suitable nanosize and deformable, yielding consistent bergamot oil release. The bergamot oil included in the nanocarrier was found to enhance photostability and photodynamic activity, with the researchers concluding that bergamot oil nanospanlastics with psoralen-UVB therapy shows potential as a vitiligo therapy.10
Two years later, Shaaban evaluated bergamot oil formulated in nanostructured lipid carriers as a photosensitizer for photodynamic treatment of vitiligo. The botanical oil was effectively used in the nanostructured lipid carriers with a gel consistency that delivered sustained release of the oil for 24 hours. Preclinical and clinical results in patients were encouraging for the topical photodynamic treatment of vitiligo, with the nanostructured lipid carriers improving the photostability and photodynamic activity of bergamot oil.6
Photoaging, photoprotection, and safety concerns
Three decades ago, an international cooperative study of the photophysical, photomutagenic, and photocarcinogenic characteristics of bergamot oil and the effect of UVA and UVB sunscreens found that UVB and UVA sunscreens at low concentration (0.5%-1%) in perfumes could not inhibit the phototoxicity of bergamot oil on human skin.11
In a 2015 study assessing the impact of 38% bergamot polyphenolic fraction (a highly concentrated Citrus bergamia fruit extract) on UVB-generated photoaging, Nisticò et al. found that the bergamot compound dose-dependently protected HaCaT cells against UVB-caused oxidative stress and photoaging markers. Suggesting that the high-antioxidant bergamot polyphenolic fraction has potential for use in skin care formulations, the researchers added that the extract seems to induce antiproliferative, immune-modulating, and antiaging activity.12In 2022, Alexa et al. performed in vitro tests and found that natural preparations containing bergamot, orange, and clove essential oils do not significantly alter physiological skin parameters and were deemed safe for topical use. An emulsion with bergamot essential oil was also found to reduce the viability of oral squamous cell carcinoma cells.13
Conclusion
As a photosensitizing agent, bergamot has an established role in skin care. Beyond its niche role in treatments for vitiligo and psoriasis, this botanical product appears to show potential as an anti-inflammatory agent as well as an ingredient to combat photoaging and skin cancer. Much more research is needed to elucidate the possible wider benefits of this Mediterranean staple.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Juber M. Health benefits of bergamot. WebMD. November 29, 2022. Accessed March 21, 2023.
2. Sun P et al. Mediators Inflamm. 2020 Oct 6;2020:8868107.
3. Cristiano MC et al. Biomedicines. 2022 Apr 30;10(5):1039.
4. Liang Y et al. Phytother Res. 2021 Nov;35(11):6131-47.
5. Quetglas-Llabrés MM et al. Oxid Med Cell Longev. 2022 Apr 25;2022:8615242.
6. Shaaban M et al. Expert Opin Drug Deliv. 2021 Jan;18(1):139-50.
7. McNeely W, Goa KL. Drugs. 1998 Oct;56(4):667-90.
8. Han X, Beaumont C, Stevens N. Biochim Open. 2017 Apr 26;5:1-7.
9. Perna S et al. Food Sci Nutr. 2019 Jan 25;7(2):369-84.
10. Shaaban M et al. Drug Deliv Transl Res. 2019 Dec;9(6):1106-16.
11. Dubertret L et al. J Photochem Photobiol B. 1990 Nov;7(2-4):251-9.
12. Nisticò S et al. J Biol Regul Homeost Agents. 2015 Jul-Sep;29(3):723-8.
13. Alexa VT et al. Molecules. 2022 Feb 1;27(3):990.
Citrus bergamia (bergamot) is a fruit tree thought to originate in the Mediterranean area; its fruit has been a part of the diet in that region since the early 18th century.1 Bergamot is known to confer antioxidant as well as anti-inflammatory activity, and yields proapoptotic effects in the sebaceous gland.2,3 The plant contains the natural furocoumarin bergapten, which is also known as 5-methoxypsoralen.4
5 In this capacity, bergamot oil has been used for photodynamic therapy of cutaneous conditions such as vitiligo.6 In fact, for several years 5-methoxypsoralen and 8-methoxypsoralen have been used to achieve acceptable clearance rates of psoriasis and vitiligo.7 This column focuses on bergapten, as well as the cutaneous conditions for which bergamot has been shown to have some benefits warranting application or further investigation.
Bergapten
In a 2021 literature review, Liang et al. cited the anti-inflammatory, antimicrobial, anticancer, and other salutary effects associated with bergapten. Based on numerous citations, they also cautioned about the phototoxicity of the compound combined with ultraviolet (UV) light while noting the photoactivation of bergapten for anticancer uses.4
The following year, Quetglas-Llabrés et al. acknowledged, in another literature review, the numerous preclinical and in vitro studies demonstrating the therapeutic activity of bergapten and highlighted clinical trials revealing notable lesion clearance rates of psoriasis or vitiligo imparted by oral or topical bergapten along with UV irradiation. Bergapten was also found to be effective as hypolipemic therapy.5
Anti-inflammatory topical uses
In a 2017 study by Han et al. of 10 essential oils, bergamot was among the investigated oils, all of which exhibited significant anti-proliferative activity in a preinflamed human dermal fibroblast system simulating chronic inflammation. Bergamot was among three essential oils that also suppressed protein molecules involved with inflammation, immune responses, and tissue remodeling, indicating anti-inflammatory and wound healing characteristics.8
More recently, Cristiano et al. reported that ultradeformable nanocarriers containing bergamot essential oil and ammonium glycyrrhizinate were demonstrated in healthy human volunteers to be characterized by the appropriate mean size, size distribution, surface charge, and long-term stability for topical administration. Topical administration on human volunteers also revealed greater activity of the combined agents as compared with a nanosystem loaded only with ammonium glycyrrhizinate. The researchers concluded that this combination of ingredients in ultradeformable vesicles shows potential as topical anti-inflammatory treatment.3
Acne
In a 2020 study using golden hamsters, Sun et al. assessed the effects of the juice and essential oils of bergamot and sweet orange on acne vulgaris engendered by excessive androgen secretion. Among 80 male hamsters randomly divided into 10 groups ranging from low to high doses, all results demonstrated improvement with treatment as seen by decreased growth rates of sebaceous glands, suppressed triglyceride accumulation, lowered inflammatory cytokine release, and apoptosis promotion in sebaceous glands. The authors noted that the essential oils yielded better dose-dependent effects than the juices.2
Psoriasis
In 2019, Perna et al. conducted a literature review on the effects of bergamot essential oil, extract, juice, and polyphenolic fraction on various health metrics. Thirty-one studies (20 involving humans with 1,709 subjects and 11 in rats and mice) were identified. Animal models indicated that bergamot essential oil (10 mg/kg or 20 mg/kg daily for 20 weeks) reduced psoriatic plaques, increased skin collagen content, and fostered hair growth and that bergamot juice (20 mg/kg) diminished proinflammatory cytokines. Human studies showed that bergamot extract and essential oil may reduce blood pressure and improve mental conditions.9
Vitiligo
In 2019, Shaaban et al. prepared elastic nanocarriers (spanlastics) to deliver psoralen-containing bergamot oil along with PUVB with the intention of harnessing melanogenic activity to treat vitiligo. Histopathologic assessment on rat skin was conducted before clinical treatment in patients with vitiligo. The spanlastics were deemed to be of suitable nanosize and deformable, yielding consistent bergamot oil release. The bergamot oil included in the nanocarrier was found to enhance photostability and photodynamic activity, with the researchers concluding that bergamot oil nanospanlastics with psoralen-UVB therapy shows potential as a vitiligo therapy.10
Two years later, Shaaban evaluated bergamot oil formulated in nanostructured lipid carriers as a photosensitizer for photodynamic treatment of vitiligo. The botanical oil was effectively used in the nanostructured lipid carriers with a gel consistency that delivered sustained release of the oil for 24 hours. Preclinical and clinical results in patients were encouraging for the topical photodynamic treatment of vitiligo, with the nanostructured lipid carriers improving the photostability and photodynamic activity of bergamot oil.6
Photoaging, photoprotection, and safety concerns
Three decades ago, an international cooperative study of the photophysical, photomutagenic, and photocarcinogenic characteristics of bergamot oil and the effect of UVA and UVB sunscreens found that UVB and UVA sunscreens at low concentration (0.5%-1%) in perfumes could not inhibit the phototoxicity of bergamot oil on human skin.11
In a 2015 study assessing the impact of 38% bergamot polyphenolic fraction (a highly concentrated Citrus bergamia fruit extract) on UVB-generated photoaging, Nisticò et al. found that the bergamot compound dose-dependently protected HaCaT cells against UVB-caused oxidative stress and photoaging markers. Suggesting that the high-antioxidant bergamot polyphenolic fraction has potential for use in skin care formulations, the researchers added that the extract seems to induce antiproliferative, immune-modulating, and antiaging activity.12In 2022, Alexa et al. performed in vitro tests and found that natural preparations containing bergamot, orange, and clove essential oils do not significantly alter physiological skin parameters and were deemed safe for topical use. An emulsion with bergamot essential oil was also found to reduce the viability of oral squamous cell carcinoma cells.13
Conclusion
As a photosensitizing agent, bergamot has an established role in skin care. Beyond its niche role in treatments for vitiligo and psoriasis, this botanical product appears to show potential as an anti-inflammatory agent as well as an ingredient to combat photoaging and skin cancer. Much more research is needed to elucidate the possible wider benefits of this Mediterranean staple.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Juber M. Health benefits of bergamot. WebMD. November 29, 2022. Accessed March 21, 2023.
2. Sun P et al. Mediators Inflamm. 2020 Oct 6;2020:8868107.
3. Cristiano MC et al. Biomedicines. 2022 Apr 30;10(5):1039.
4. Liang Y et al. Phytother Res. 2021 Nov;35(11):6131-47.
5. Quetglas-Llabrés MM et al. Oxid Med Cell Longev. 2022 Apr 25;2022:8615242.
6. Shaaban M et al. Expert Opin Drug Deliv. 2021 Jan;18(1):139-50.
7. McNeely W, Goa KL. Drugs. 1998 Oct;56(4):667-90.
8. Han X, Beaumont C, Stevens N. Biochim Open. 2017 Apr 26;5:1-7.
9. Perna S et al. Food Sci Nutr. 2019 Jan 25;7(2):369-84.
10. Shaaban M et al. Drug Deliv Transl Res. 2019 Dec;9(6):1106-16.
11. Dubertret L et al. J Photochem Photobiol B. 1990 Nov;7(2-4):251-9.
12. Nisticò S et al. J Biol Regul Homeost Agents. 2015 Jul-Sep;29(3):723-8.
13. Alexa VT et al. Molecules. 2022 Feb 1;27(3):990.
Citrus bergamia (bergamot) is a fruit tree thought to originate in the Mediterranean area; its fruit has been a part of the diet in that region since the early 18th century.1 Bergamot is known to confer antioxidant as well as anti-inflammatory activity, and yields proapoptotic effects in the sebaceous gland.2,3 The plant contains the natural furocoumarin bergapten, which is also known as 5-methoxypsoralen.4
5 In this capacity, bergamot oil has been used for photodynamic therapy of cutaneous conditions such as vitiligo.6 In fact, for several years 5-methoxypsoralen and 8-methoxypsoralen have been used to achieve acceptable clearance rates of psoriasis and vitiligo.7 This column focuses on bergapten, as well as the cutaneous conditions for which bergamot has been shown to have some benefits warranting application or further investigation.
Bergapten
In a 2021 literature review, Liang et al. cited the anti-inflammatory, antimicrobial, anticancer, and other salutary effects associated with bergapten. Based on numerous citations, they also cautioned about the phototoxicity of the compound combined with ultraviolet (UV) light while noting the photoactivation of bergapten for anticancer uses.4
The following year, Quetglas-Llabrés et al. acknowledged, in another literature review, the numerous preclinical and in vitro studies demonstrating the therapeutic activity of bergapten and highlighted clinical trials revealing notable lesion clearance rates of psoriasis or vitiligo imparted by oral or topical bergapten along with UV irradiation. Bergapten was also found to be effective as hypolipemic therapy.5
Anti-inflammatory topical uses
In a 2017 study by Han et al. of 10 essential oils, bergamot was among the investigated oils, all of which exhibited significant anti-proliferative activity in a preinflamed human dermal fibroblast system simulating chronic inflammation. Bergamot was among three essential oils that also suppressed protein molecules involved with inflammation, immune responses, and tissue remodeling, indicating anti-inflammatory and wound healing characteristics.8
More recently, Cristiano et al. reported that ultradeformable nanocarriers containing bergamot essential oil and ammonium glycyrrhizinate were demonstrated in healthy human volunteers to be characterized by the appropriate mean size, size distribution, surface charge, and long-term stability for topical administration. Topical administration on human volunteers also revealed greater activity of the combined agents as compared with a nanosystem loaded only with ammonium glycyrrhizinate. The researchers concluded that this combination of ingredients in ultradeformable vesicles shows potential as topical anti-inflammatory treatment.3
Acne
In a 2020 study using golden hamsters, Sun et al. assessed the effects of the juice and essential oils of bergamot and sweet orange on acne vulgaris engendered by excessive androgen secretion. Among 80 male hamsters randomly divided into 10 groups ranging from low to high doses, all results demonstrated improvement with treatment as seen by decreased growth rates of sebaceous glands, suppressed triglyceride accumulation, lowered inflammatory cytokine release, and apoptosis promotion in sebaceous glands. The authors noted that the essential oils yielded better dose-dependent effects than the juices.2
Psoriasis
In 2019, Perna et al. conducted a literature review on the effects of bergamot essential oil, extract, juice, and polyphenolic fraction on various health metrics. Thirty-one studies (20 involving humans with 1,709 subjects and 11 in rats and mice) were identified. Animal models indicated that bergamot essential oil (10 mg/kg or 20 mg/kg daily for 20 weeks) reduced psoriatic plaques, increased skin collagen content, and fostered hair growth and that bergamot juice (20 mg/kg) diminished proinflammatory cytokines. Human studies showed that bergamot extract and essential oil may reduce blood pressure and improve mental conditions.9
Vitiligo
In 2019, Shaaban et al. prepared elastic nanocarriers (spanlastics) to deliver psoralen-containing bergamot oil along with PUVB with the intention of harnessing melanogenic activity to treat vitiligo. Histopathologic assessment on rat skin was conducted before clinical treatment in patients with vitiligo. The spanlastics were deemed to be of suitable nanosize and deformable, yielding consistent bergamot oil release. The bergamot oil included in the nanocarrier was found to enhance photostability and photodynamic activity, with the researchers concluding that bergamot oil nanospanlastics with psoralen-UVB therapy shows potential as a vitiligo therapy.10
Two years later, Shaaban evaluated bergamot oil formulated in nanostructured lipid carriers as a photosensitizer for photodynamic treatment of vitiligo. The botanical oil was effectively used in the nanostructured lipid carriers with a gel consistency that delivered sustained release of the oil for 24 hours. Preclinical and clinical results in patients were encouraging for the topical photodynamic treatment of vitiligo, with the nanostructured lipid carriers improving the photostability and photodynamic activity of bergamot oil.6
Photoaging, photoprotection, and safety concerns
Three decades ago, an international cooperative study of the photophysical, photomutagenic, and photocarcinogenic characteristics of bergamot oil and the effect of UVA and UVB sunscreens found that UVB and UVA sunscreens at low concentration (0.5%-1%) in perfumes could not inhibit the phototoxicity of bergamot oil on human skin.11
In a 2015 study assessing the impact of 38% bergamot polyphenolic fraction (a highly concentrated Citrus bergamia fruit extract) on UVB-generated photoaging, Nisticò et al. found that the bergamot compound dose-dependently protected HaCaT cells against UVB-caused oxidative stress and photoaging markers. Suggesting that the high-antioxidant bergamot polyphenolic fraction has potential for use in skin care formulations, the researchers added that the extract seems to induce antiproliferative, immune-modulating, and antiaging activity.12In 2022, Alexa et al. performed in vitro tests and found that natural preparations containing bergamot, orange, and clove essential oils do not significantly alter physiological skin parameters and were deemed safe for topical use. An emulsion with bergamot essential oil was also found to reduce the viability of oral squamous cell carcinoma cells.13
Conclusion
As a photosensitizing agent, bergamot has an established role in skin care. Beyond its niche role in treatments for vitiligo and psoriasis, this botanical product appears to show potential as an anti-inflammatory agent as well as an ingredient to combat photoaging and skin cancer. Much more research is needed to elucidate the possible wider benefits of this Mediterranean staple.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Juber M. Health benefits of bergamot. WebMD. November 29, 2022. Accessed March 21, 2023.
2. Sun P et al. Mediators Inflamm. 2020 Oct 6;2020:8868107.
3. Cristiano MC et al. Biomedicines. 2022 Apr 30;10(5):1039.
4. Liang Y et al. Phytother Res. 2021 Nov;35(11):6131-47.
5. Quetglas-Llabrés MM et al. Oxid Med Cell Longev. 2022 Apr 25;2022:8615242.
6. Shaaban M et al. Expert Opin Drug Deliv. 2021 Jan;18(1):139-50.
7. McNeely W, Goa KL. Drugs. 1998 Oct;56(4):667-90.
8. Han X, Beaumont C, Stevens N. Biochim Open. 2017 Apr 26;5:1-7.
9. Perna S et al. Food Sci Nutr. 2019 Jan 25;7(2):369-84.
10. Shaaban M et al. Drug Deliv Transl Res. 2019 Dec;9(6):1106-16.
11. Dubertret L et al. J Photochem Photobiol B. 1990 Nov;7(2-4):251-9.
12. Nisticò S et al. J Biol Regul Homeost Agents. 2015 Jul-Sep;29(3):723-8.
13. Alexa VT et al. Molecules. 2022 Feb 1;27(3):990.
What happens to melanocytic nevi during laser hair removal?
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
PHOENIX – , while common histologic changes include mild atypia and thermal damage, according to results from a systematic review of literature on the topic. To date, no severe cases of severe dysplasia or melanoma have been reported.
“That’s reassuring,” study author Ahuva Cices, MD, said in an interview at the annual conference of the American Society for Laser Medicine and Surgery, where she presented the results during an abstract session. “But, with that in mind, we want to avoid treating nevi with laser hair removal to avoid changes that could be concerning. We also recommend baseline skin exams so we know what we’re looking at before we start treating with lasers, and any changes can be recognized from that baseline status. It’s important to keep an eye out for changes and always be evaluating.”
In December of 2022, Dr. Cices, chief dermatology resident at Mount Sinai Health System, New York, searched PubMed for articles that evaluated changes in melanocytic nevi after laser hair removal procedures. She used the search terms “nevi laser hair removal,” “nevi diode,” “nevi long pulse alexandrite,” “nevi long pulse neodymium doped yttrium aluminum garnet,” and “melanoma laser hair removal,” and limited the analysis to English language patient-based reports that discussed incidental treatment of melanocytic nevi while undergoing hair removal with a laser.
Reports excluded from the analysis were those that focused on changes following hair removal with nonlaser devices such as intense pulsed light (IPL), those evaluating nonmelanocytic nevi such as Becker’s nevus or nevus of Ota, and those evaluating the intentional ablation or removal of melanocytic lesions.
The search yielded 10 relevant studies for systematic review: seven case reports or series and three observational trials, two of which were prospective and one retrospective.
The results of the review, according to Dr. Cices, revealed that clinical and dermoscopic changes were noted to present as early as 15 days after treatment and persist to the maximum follow up time, at 3 years. Commonly reported changes included regression, decreased size, laser-induced asymmetry, bleaching, darkening, and altered pattern on dermoscopy. Histologic changes included mild atypia, thermal damage, scar formation, and regression.
“Although some of the clinical and dermoscopic alterations may be concerning for malignancy, to our knowledge, there are no documented cases of malignant transformation of nevi following treatment with laser hair removal,” she wrote in the abstract.
Dr. Cices acknowledged certain limitations of the systematic review, including the low number of relevant reports and their generally small sample size, many of which were limited to single cases.
Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was asked to comment on the review, characterized the findings as important because laser hair removal is such a commonly performed procedure.
While the study is limited by the small number of studies on the subject matter, “it brings up an important discussion,” Dr. Ibrahimi said in an interview. “Generally speaking, we know that most hair removal lasers do indeed target melanin pigment and can be absorbed by melanocytes. While the wavelengths used for LHR [laser hair removal] will not result in DNA damage or cause mutations that can lead to melanoma, they can sometimes alter the appearance of pigmented lesions and that may change the dermatologist’s ability to monitor them for atypia,” he noted.
“For that reason, I would recommend all patients see a dermatologist for evaluation of their nevi prior to any treatments and they consider very carefully where they get their laser treatments. If they have any atypical pigmented lesions, then that information should be disclosed with the person performing the laser hair removal procedure particularly if there are lesions that are being specifically monitored.”
Dr. Cices reported having no disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Study highlights potential skin cancer risk of UV nail polish dryers
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
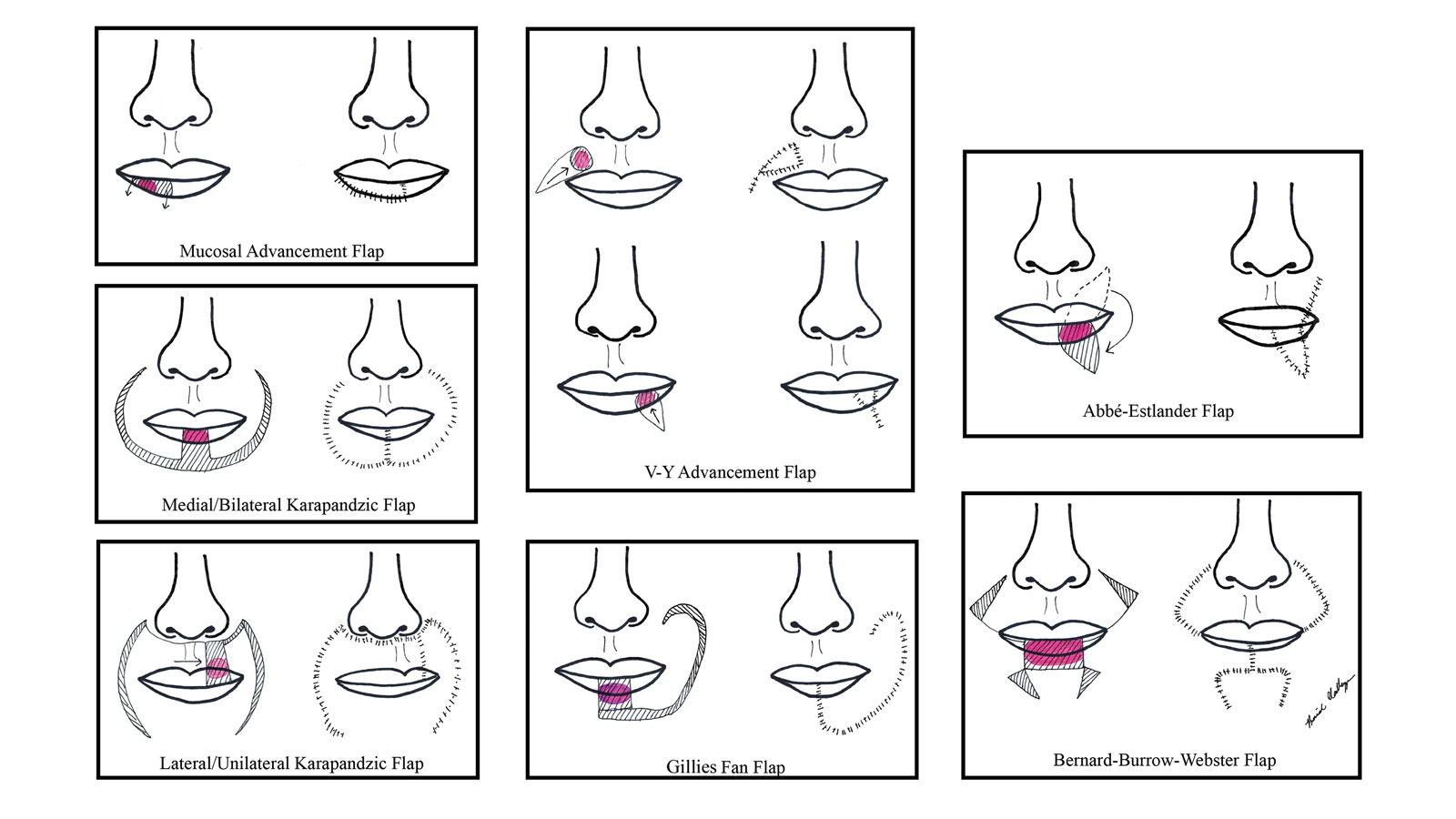
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
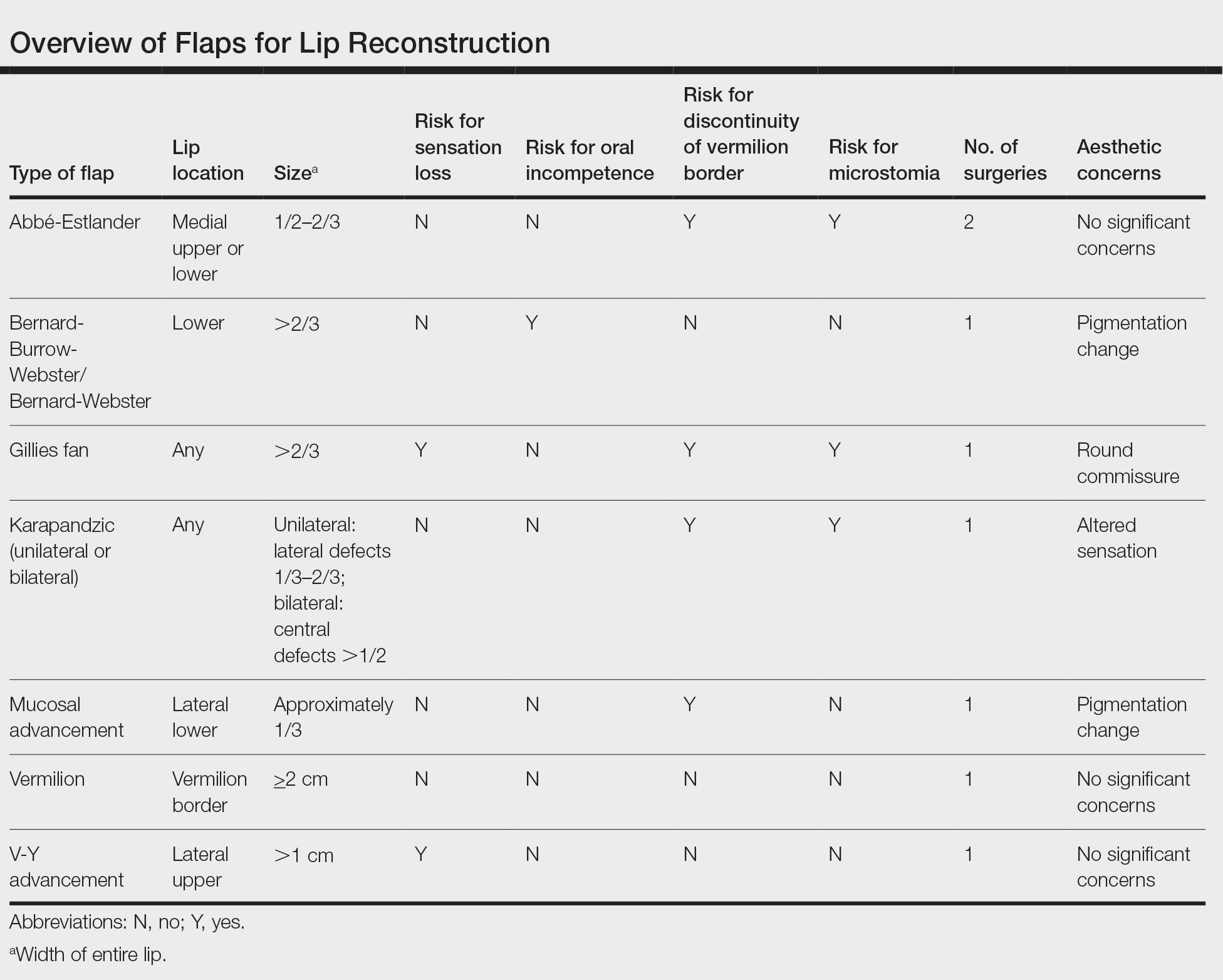
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Subcutaneous Panniculitic T-cell Lymphoma Presenting With Anasarca in a Patient With Known Chronic Lymphocytic Leukemia
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.
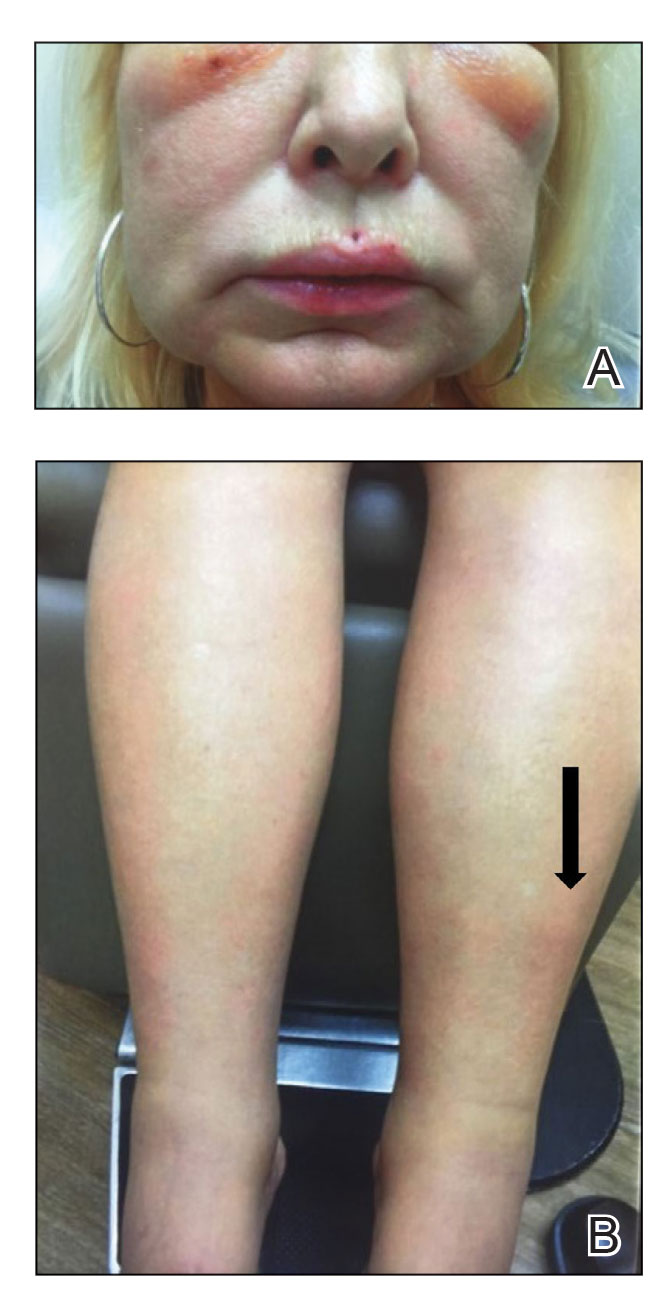
During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.
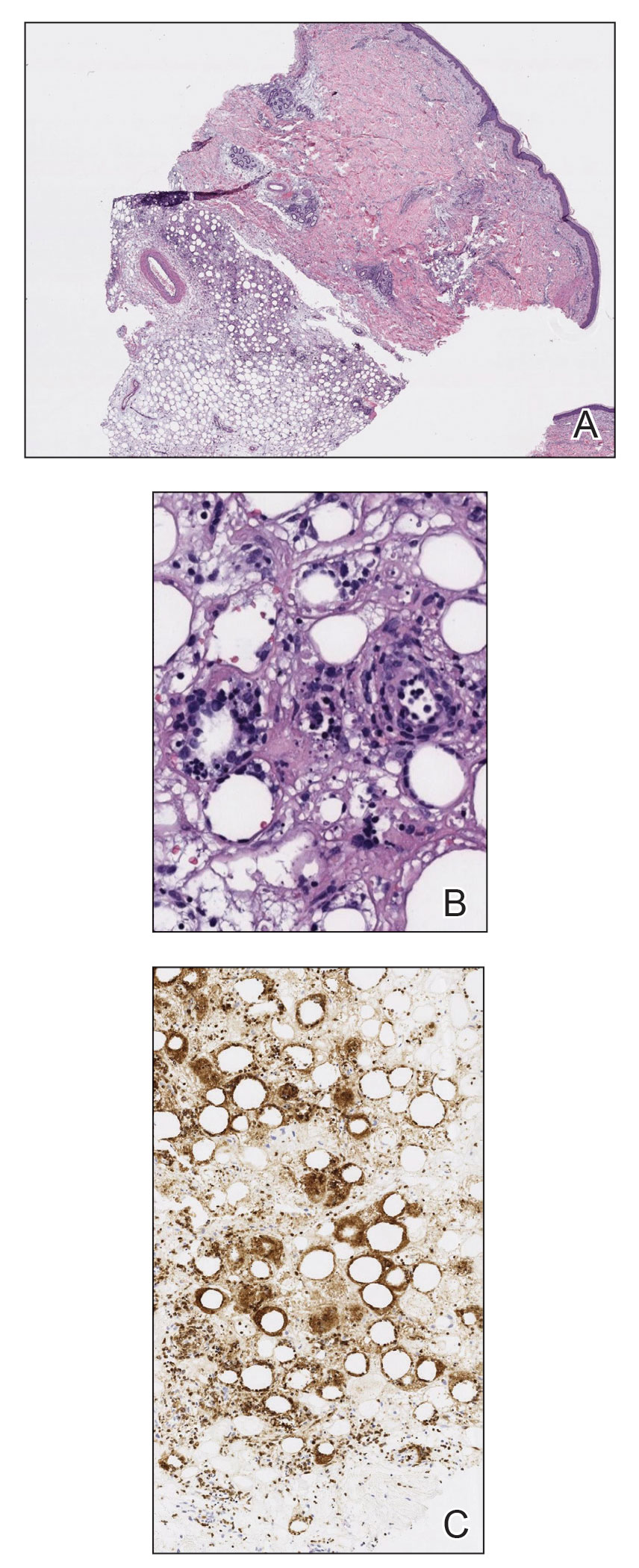
Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.

During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.

Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.

During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.

Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
Practice Points
- Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare type of cutaneous T-cell lymphoma that may be complicated by fatal hemophagocytic syndrome.
- Patients typically present with deep-seated plaques or nodules that may be masked by localized edema.
- A biopsy is necessary to diagnose SPTCL, as well as to assess the degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and angioinvasion to distinguish it from other panniculitides.
- In patients with a known hematologic malignancy, a secondary malignancy must be considered in the differential diagnosis of paraneoplastic edema.
FDA approves new Merkel cell carcinoma treatment
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.