User login
Does Exercise Reduce Cancer Risk? It’s Just Not That Simple
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.
“Exercise is medicine” has become something of a mantra, with good reason. There’s no doubt that regular physical activity has a broad range of health benefits. Exercise can improve circulation, help control weight, reduce stress, and boost mood — take your pick.
Lower cancer risk is also on the list — with exercise promoted as a risk-cutting strategy in government guidelines and in recommendations from professional groups such as the American Cancer Society.
The bulk of the data hangs on less rigorous, observational studies that have linked physical activity to lower risks for certain cancers, but plenty of questions remain.
What are the cancer types where exercise makes a difference? How significant is that impact? And what, exactly, defines a physical activity pattern powerful enough to move the needle on cancer risk?
Here’s an overview of the state of the evidence.
Exercise and Cancer Types: A Mixed Bag
When it comes to cancer prevention strategies, guidelines uniformly endorse less couch time and more movement. But a deeper look at the science reveals a complex and often poorly understood connection between exercise and cancer risk.
For certain cancer types, the benefits of exercise on cancer risk seem fairly well established.
The latest edition of the Physical Activity Guidelines for Americans, published in 2018, cites “strong evidence” that regular exercise might curb the risks for breast and colon cancers as well as bladder, endometrial, esophageal, kidney, and gastric cancers. These guidelines also point to “moderate”-strength evidence of a protective association with lung cancer.
The evidence of a protective effect, however, is strongest for breast and colon cancers, said Jennifer Ligibel, MD, senior physician in the Breast Oncology Center at Dana-Farber Cancer Institute, Boston, . “But,” she pointed out, “that may be because they’re some of the most common cancers, and it’s been easier to detect an association.”
Guidelines from the American Cancer Society, published in 2020, align with the 2018 recommendations.
“We believe there’s strong evidence to suggest at least eight different types of cancer are associated with physical activity,” said Erika Rees-Punia, PhD, MPH, senior principal scientist, epidemiology and behavioral research at the American Cancer Society.
That view is not universal, however. Current recommendations from the World Cancer Research Fund and American Institute for Cancer Research, for example, are more circumspect, citing only three cancers with good evidence of a protective effect from exercise: Breast (postmenopausal), colon, and endometrial.
“We definitely can’t say exercise reduces the risk of all cancers,” said Lee Jones, PhD, head of the Exercise Oncology Program at Memorial Sloan Kettering Cancer Center in New York City. “The data suggest it’s just not that simple.”
And it’s challenging to put all the evidence together, Dr. Jones added.
The physical activity guidelines are based on published systematic reviews, meta-analyses, and pooled analyses of data from observational studies that examined the relationship between physical activity — aerobic exercise, specifically — and cancer incidence. That means the evidence comes with all the limitations observational studies entail, such as how they collect information on participants’ exercise habits — which, Dr. Jones noted, is typically done via “monster questionnaires” that gauge physical activity in broad strokes.
Pooling all those findings into a meta-analysis is tricky, Dr. Jones added, because individual studies vary in important ways — from follow-up periods to how they quantify exercise and track cancer incidence.
In a study published in February in Cancer Cell, Dr. Jones and his colleagues attempted to address some of those issues by leveraging data from the PLCO screening trial.
The PLCO was a prospective study of over 60,000 US adults that compared the effects of annual screening vs usual care on cancer mortality. At enrollment, participants completed questionnaires that included an assessment of “vigorous” exercise. Based on that, Dr. Jones and his colleagues classified 55% as “exercisers” — meaning they reported 2 or more hours of vigorous exercise per week. The remaining 45%, who were in the 0 to 1 hour per week range, were deemed non-exercisers.
Over a median of 18 years, nearly 16,000 first-time invasive cancers were diagnosed, and some interesting differences between exercisers and non-exercisers emerged. The active group had lower risks for three cancers: Head and neck, with a 26% lower risk (hazard ratio [HR], 0.74), lung (a 20% lower risk), and breast (an 11% lower risk).
What was striking, however, was the lack of connection between exercise and many cancers cited in the guidelines, including colon, gastric, bladder, endometrial, and renal cancers.
Perhaps even more surprising — exercisers had higher risks for prostate cancer (12%) and melanoma (20%). This finding, Dr. Jones said, is in line with a previous pooled analysis of data from 12 US and European prospective cohorts. In this study, the most physically active participants (90th percentile) had higher risks for melanoma and prostate cancer, compared with the least active group (10th percentile).
The melanoma findings do make sense, Dr. Jones said, given that highly active people may spend a lot of time in the sun. “My advice,” Dr. Jones said, “is, if you’re exercising outside, wear sunscreen.” The prostate cancer findings, however, are more puzzling and warrant further research, he noted.
But the bottom line is that the relationship between exercise and cancer types is mixed and far from nailed down.
How Big Is the Effect?
Even if exercise reduces the risk for only certain cancers, that’s still important, particularly when those links appear strongest for common cancer types, such as breast and colon.
But how much of a difference can exercise make?
Based on the evidence, it may only be a modest one. A 2019 systematic review by the Physical Activity Guidelines Advisory Committee provided a rough estimate: Across hundreds of epidemiological studies, people with the highest physical activity levels had a 10%-20% lower risk for the cancers cited in the 2018 exercise guidelines compared with people who were least active.
These figures, however, are probably an underestimate, said Anne McTiernan, MD, PhD, a member of the advisory committee and professor of epidemiology, at Fred Hutchinson Cancer Center, Seattle.
“This is what we usually see when a factor is not measured very well,” said Dr. McTiernan, explaining that the individual studies differed in their categories of “highest” and “lowest” physical activity, such that one study’s “highest” could be another’s mid-range.
“In other words, the effects of physical activity are likely larger” than the review found, Dr. McTiernan said.
The next logical question is whether a bigger exercise “dose” — more time or higher intensity — would have a greater impact on cancer risk. A 2019 study published in the Journal of Clinical Oncology tried to clarify that by pooling data on over 750,000 participants from nine prospective cohorts.
Overall, people meeting government recommendations for exercise — equivalent to about 2.5-5 hours of weekly moderate activity, such as a brisk walk, or about 1.25-2.5 hours of more vigorous activities, like running — had lower risks for seven of 15 cancer types studied compared with less active people.
For cancers with positive findings, being on the higher end of the recommended 2.5- to 5-hour weekly range was better. Risk reductions for breast cancer, for instance, were 6% at 2.5 hours of physical activity per week and 10% at 5 hours per week. Similar trends emerged for other cancer types, including colon (8%-14%), endometrial (10%-18%), liver cancer (18%-27%), and non-Hodgkin lymphoma in women (11%-18%).
But there may be an exercise sweet spot that maximizes the cancer risk benefit.
Among people who surpassed the recommendations — exercising for more time or more intensely — the risk reduction benefit did not necessarily improve in a linear fashion. For certain cancer types, such as colon and endometrial, the benefits of more vigorous exercise “eroded at higher levels of activity,” the authors said.
The issue here is that most studies have not dug deeply into aerobic exercise habits. Often, studies present participants with a list of activities — walking, biking, and running — and ask them to estimate how often and for what duration they do each.
Plus, “we’ve usually lumped moderate and vigorous activities together,” Dr. Rees-Punia said, which means there’s a lack of “granular data” to say whether certain intensities or frequencies of exercise are optimal and for whom.
Why Exercise May Lower Cancer Risk
Exercise habits do not, of course, exist in a vacuum. Highly active people, Dr. Ligibel said, tend to be of higher socioeconomic status, leaner, and have generally healthier lifestyles than sedentary people.
Body weight is a big confounder as well. However, Dr. Rees-Punia noted, it’s also probably a reason that exercise is linked to lower cancer risks, particularly by preventing weight gain. Still, studies have found that the association between exercise and many cancers remains significant after adjusting for body mass index.
The why remains unclear, though some studies offer clues.
“There’s been some really interesting mechanistic research, suggesting that exercise may help inhibit tumor growth or upregulate the immune system,” Dr. Ligibel said.
That includes not only lab research but small intervention studies. While these studies have largely involved people who already have cancer, some have also focused on healthy individuals.
A 2019 study from Dr. Ligibel and her colleagues, which randomly assigned 49 women newly diagnosed with breast cancer to start either an exercise program or mind-body practices ahead of surgery, found exercisers, who had been active for about a month at the time of surgery, showed signs of immune system upregulation in their tumors, while the control group did not.
Among healthy postmenopausal women, a meta-analysis of six clinical trials from Dr. McTiernan and her colleagues found that exercise plus calorie reduction can reduce levels of breast cancer-related endogenous hormones, more so than calorie-cutting alone. And a 2023 study found that high-intensity exercise boosted the ranks of certain immune cells and reduced inflammation in the colon among people at high risk for colon and endometrial cancers due to Lynch syndrome.
Defining an Exercise ‘Prescription’
Despite the gaps and uncertainties in the research, government guidelines as well as those from the American Cancer Society and other medical groups are in lockstep in their exercise recommendations: Adults should strive for 150-300 minutes of moderate-intensity aerobic exercise (like brisk walking), 75-150 minutes of vigorous activity (like running), or some combination each week.
The guidelines also encourage strength training twice a week — advice that’s based on research tying those activity levels to lower risks for heart disease, diabetes, and other chronic conditions.
But there’s no “best” exercise prescription for lowering cancer risk specifically. Most epidemiological studies have examined only aerobic activity, Dr. Rees-Punia said, and there’s very little known about whether strength conditioning or other moderate heart rate-elevating activities, such as daily household chores, may reduce the risk for cancer.
Given the lack of nuance in the literature, it’s hard to say what intensities, types, or amounts of exercise are best for each individual.
Going forward, device-based measurements of physical activity could “help us sort out the effects of different intensities of exercise and possibly types,” Dr. Rees-Punia said.
But overall, Dr. McTiernan said, the data do show that the risks for several cancers are lower at the widely recommended activity levels.
“The bottom-line advice is still to exercise at least 150 minutes per week at a moderate-intensity level or greater,” Dr. McTiernan said.
Or put another way, moving beats being sedentary. It’s probably wise for everyone to sit less, noted Dr. Rees-Punia, for overall health and based on evidence tying sedentary time to the risks for certain cancers, including colon, endometrial, and lung.
There’s a practical element to consider in all of this: What physical activities will people actually do on the regular? In the big epidemiological studies, Dr. McTiernan noted, middle-aged and older adults most often report walking, suggesting that’s the preferred, or most accessible activity, for many.
“You can only benefit from the physical activity you’ll actually do,” Dr. Rees-Punia said.
Dr. Ligibel echoed that sentiment, saying she encourages patients to think about physical activity as a process: “You need to find things you like to do and work them into your daily life, in a sustainable way.
“People often talk about exercise being medicine,” Dr. Ligibel said. “But I think you could take that too far. If we get too prescriptive about it, that could take the joy away.”
A version of this article appeared on Medscape.com.
How to Optimize Epidermal Approximation During Wound Suturing Using a Smartphone Camera
Practice Gap
Precise wound approximation during cutaneous suturing is of vital importance for optimal closure and long-term scar outcomes. Although buried dermal sutures achieve wound-edge approximation and eversion, meticulous placement of epidermal sutures allows for fine-tuning of the wound edges through epidermal approximation, eversion, and the correction of minor height discrepancies (step-offs).
Several percutaneous suture techniques and materials are available to dermatologic surgeons. However, precise, gap- and tension-free approximation of the wound edges is desired for prompt re-epithelialization and a barely visible scar.1,2
Epidermal sutures should be placed under minimal tension to align the papillary dermis and epidermis precisely. The dermatologic surgeon can evaluate the effectiveness of their suturing technique by carefully examining the closure for visibility of the bilateral wound edges, which should show equally if approximation is precise; small gaps between the wound edges (undesired); or dermal bleeding, which is a manifestation of inaccurate approximation.
Advances in smartphone camera technology have led to high-quality photography in a variety of settings. Although smartphone photography often is used for documentation purposes in health care, we recommend incorporating it as a quality-control checkpoint for objective evaluation, allowing the dermatologic surgeon to scrutinize the wound edges and refine their surgical technique to improve scar outcomes.
The Technique
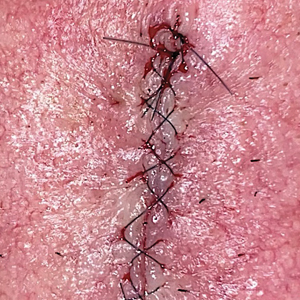
After suturing the wound closed, we routinely use a 12-megapixel smartphone camera (up to 2× optical zoom) to photograph the closed wound at 1× or 2× magnification to capture more details and use the zoom function to further evaluate the wound edges close-up (Figure). In any area where inadequate epidermal approximation is noted on the photograph, an additional stitch can be placed. Photography can be repeated until ideal reapproximation occurs.
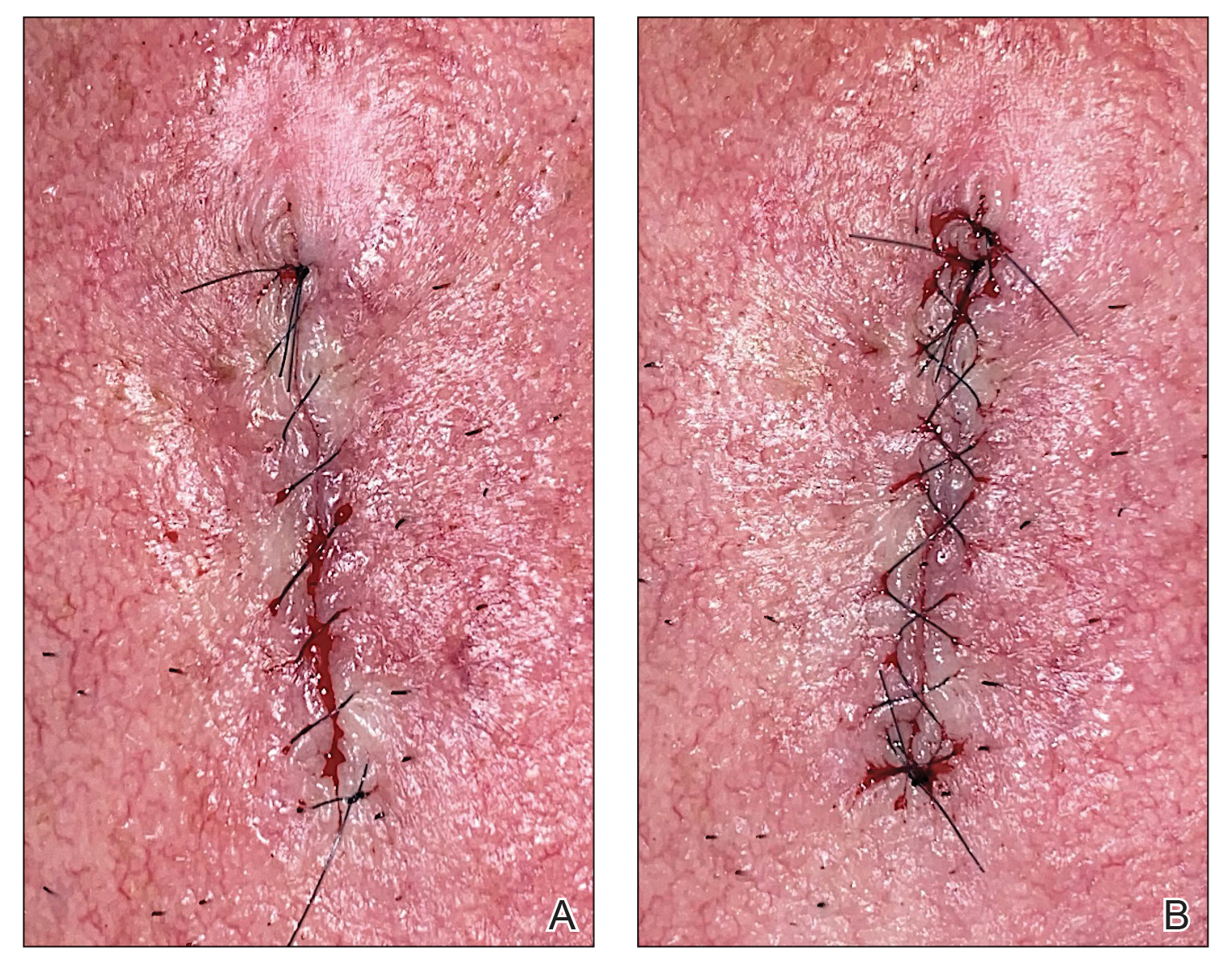
Practice Implications
Most smartphones released in recent years have a 12-megapixel camera, making them more easily accessible than surgical loupes. Additionally, surgical loupes are expensive, come with a learning curve, and can be intimidating to new or inexperienced surgeons or dermatology residents. Because virtually every dermatologic surgeon has access to a smartphone and snapping an image takes no more than a few seconds, we believe this technique is a valuable new self-assessment tool for dermatologic surgeons. It may be particularly valuable to dermatology residents and new/inexperienced surgeons looking to improve their techniques and scar outcomes.
- Perry AW, McShane RH. Fine-tuning of the skin edges in the closure of surgical wounds. Controlling inversion and eversion with the path of the needle—the right stitch at the right time. J Dermatol Surg Oncol. 1981;7:471-476. doi:10.1111/j.1524-4725.1981.tb00680.x
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
Practice Gap
Precise wound approximation during cutaneous suturing is of vital importance for optimal closure and long-term scar outcomes. Although buried dermal sutures achieve wound-edge approximation and eversion, meticulous placement of epidermal sutures allows for fine-tuning of the wound edges through epidermal approximation, eversion, and the correction of minor height discrepancies (step-offs).
Several percutaneous suture techniques and materials are available to dermatologic surgeons. However, precise, gap- and tension-free approximation of the wound edges is desired for prompt re-epithelialization and a barely visible scar.1,2
Epidermal sutures should be placed under minimal tension to align the papillary dermis and epidermis precisely. The dermatologic surgeon can evaluate the effectiveness of their suturing technique by carefully examining the closure for visibility of the bilateral wound edges, which should show equally if approximation is precise; small gaps between the wound edges (undesired); or dermal bleeding, which is a manifestation of inaccurate approximation.
Advances in smartphone camera technology have led to high-quality photography in a variety of settings. Although smartphone photography often is used for documentation purposes in health care, we recommend incorporating it as a quality-control checkpoint for objective evaluation, allowing the dermatologic surgeon to scrutinize the wound edges and refine their surgical technique to improve scar outcomes.
The Technique
After suturing the wound closed, we routinely use a 12-megapixel smartphone camera (up to 2× optical zoom) to photograph the closed wound at 1× or 2× magnification to capture more details and use the zoom function to further evaluate the wound edges close-up (Figure). In any area where inadequate epidermal approximation is noted on the photograph, an additional stitch can be placed. Photography can be repeated until ideal reapproximation occurs.

Practice Implications
Most smartphones released in recent years have a 12-megapixel camera, making them more easily accessible than surgical loupes. Additionally, surgical loupes are expensive, come with a learning curve, and can be intimidating to new or inexperienced surgeons or dermatology residents. Because virtually every dermatologic surgeon has access to a smartphone and snapping an image takes no more than a few seconds, we believe this technique is a valuable new self-assessment tool for dermatologic surgeons. It may be particularly valuable to dermatology residents and new/inexperienced surgeons looking to improve their techniques and scar outcomes.
Practice Gap
Precise wound approximation during cutaneous suturing is of vital importance for optimal closure and long-term scar outcomes. Although buried dermal sutures achieve wound-edge approximation and eversion, meticulous placement of epidermal sutures allows for fine-tuning of the wound edges through epidermal approximation, eversion, and the correction of minor height discrepancies (step-offs).
Several percutaneous suture techniques and materials are available to dermatologic surgeons. However, precise, gap- and tension-free approximation of the wound edges is desired for prompt re-epithelialization and a barely visible scar.1,2
Epidermal sutures should be placed under minimal tension to align the papillary dermis and epidermis precisely. The dermatologic surgeon can evaluate the effectiveness of their suturing technique by carefully examining the closure for visibility of the bilateral wound edges, which should show equally if approximation is precise; small gaps between the wound edges (undesired); or dermal bleeding, which is a manifestation of inaccurate approximation.
Advances in smartphone camera technology have led to high-quality photography in a variety of settings. Although smartphone photography often is used for documentation purposes in health care, we recommend incorporating it as a quality-control checkpoint for objective evaluation, allowing the dermatologic surgeon to scrutinize the wound edges and refine their surgical technique to improve scar outcomes.
The Technique
After suturing the wound closed, we routinely use a 12-megapixel smartphone camera (up to 2× optical zoom) to photograph the closed wound at 1× or 2× magnification to capture more details and use the zoom function to further evaluate the wound edges close-up (Figure). In any area where inadequate epidermal approximation is noted on the photograph, an additional stitch can be placed. Photography can be repeated until ideal reapproximation occurs.

Practice Implications
Most smartphones released in recent years have a 12-megapixel camera, making them more easily accessible than surgical loupes. Additionally, surgical loupes are expensive, come with a learning curve, and can be intimidating to new or inexperienced surgeons or dermatology residents. Because virtually every dermatologic surgeon has access to a smartphone and snapping an image takes no more than a few seconds, we believe this technique is a valuable new self-assessment tool for dermatologic surgeons. It may be particularly valuable to dermatology residents and new/inexperienced surgeons looking to improve their techniques and scar outcomes.
- Perry AW, McShane RH. Fine-tuning of the skin edges in the closure of surgical wounds. Controlling inversion and eversion with the path of the needle—the right stitch at the right time. J Dermatol Surg Oncol. 1981;7:471-476. doi:10.1111/j.1524-4725.1981.tb00680.x
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Perry AW, McShane RH. Fine-tuning of the skin edges in the closure of surgical wounds. Controlling inversion and eversion with the path of the needle—the right stitch at the right time. J Dermatol Surg Oncol. 1981;7:471-476. doi:10.1111/j.1524-4725.1981.tb00680.x
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
FDA Removes Harmful Chemicals From Food Packaging
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
What Happens to Surgery Candidates with BHDs and Cancer?
based on data from a new study of nearly 700,000 individuals.
The reason for this association remains unclear, and highlights the need to address existing behavioral health disorders (BHDs), which can be exacerbated after a patient is diagnosed with cancer, wrote Timothy M. Pawlik, MD, of The Ohio State University, Columbus, and colleagues. A cancer diagnosis can cause not only physical stress, but mental, emotional, social, and economic stress that can prompt a new BHD, cause relapse of a previous BHD, or exacerbate a current BHD, the researchers noted.
What is Known About BHDs and Cancer?
Although previous studies have shown a possible association between BHDs and increased cancer risk, as well as reduced compliance with care, the effect of BHDs on outcomes in cancer patients undergoing surgical resection has not been examined, wrote Dr. Pawlik and colleagues.
Previous research has focused on the impact of having a preexisting serious mental illness (SMI) such as schizophrenia and bipolar disorder on cancer care.
A 2023 literature review of 27 studies published in the Journal of Medical Imaging and Radiation Sciences showed that patients with preexisting severe mental illness (such as schizophrenia or bipolar disorder) had greater cancer-related mortality. In that study, the researchers also found that patients with severe mental illness were more likely to have metastatic disease at diagnosis, but less likely to receive optimal treatments, than individuals without SMIs.
Many studies also have focused on patients developing mental health problems (including BHDs) after a cancer diagnosis, but the current study is the first known to examine outcomes in those with BHDs before cancer.
Why Was It Important to Conduct This Study?
“BHDs are a diverse set of mental illnesses that affect an individual’s psychosocial wellbeing, potentially resulting in maladaptive behaviors,” Dr. Pawlik said in an interview. BHDs, which include substance abuse, eating disorders, and sleep disorders, are less common than anxiety/depression, but have an estimated prevalence of 1.3%-3.1% among adults in the United States, he said.
What Does the New Study Add?
In the new review by Dr. Pawlik and colleagues, published in the Journal of the American College of Surgeons (Katayama ES. J Am Coll Surg. 2024 Feb 29. doi: 2024. 10.1097/XCS.0000000000000954), BHDs were defined as substance abuse, eating disorders, or sleep disorders, which had not been the focus of previous studies. The researchers reviewed data from 694,836 adult patients with lung, esophageal, gastric, liver, pancreatic, or colorectal cancer between 2018-2021 using the Medicare Standard Analytic files. A total of 46,719 patients (6.7%) had at least one BHD.
Overall, patients with a BHD were significantly less likely than those without a BHD to undergo surgical resection (20.3% vs. 23.4%). Patients with a BHD also had significantly worse long-term postoperative survival than those without BHDs (median 37.1 months vs. 46.6 months) and significantly higher in-hospital costs ($17,432 vs. 16,159, P less than .001 for all).
Among patients who underwent cancer surgery, the odds of any complication were significantly higher for those with a BHD compared to those with no BHD (odds ratio 1.32), as were the odds of a prolonged length of stay (OR 1.67) and 90-day readmission (OR 1.57).
Dr. Pawlik said he was surprised by several of the findings, including that 1 in 15 Medicare beneficiaries had a BHD diagnosis, “with male sex and minority racial status, as well as higher social vulnerability, being associated with a higher prevalence of BHD.”
Also, the independent association of having a BHD with 30%-50% higher odds of a complication, prolonged length of stay, and 90-day readmission was higher than Dr. Pawlik had anticipated.
Why Do Patients With BHDs Have Fewer Surgeries and Worse Outcomes?
The reasons for this association were likely multifactorial and may reflect the greater burden of medical comorbidity and chronic illness in many patients with BHDs because of maladaptive lifestyles or poor nutrition status, Dr. Pawlik said.
“Patients with BHDs also likely face barriers to accessing care, which was noted particularly among patients with BHDs who lived in socially vulnerable areas,” he said. BHD patients also were more likely to be treated at low-volume rather than high-volume hospitals, “which undoubtedly contributed in part to worse outcomes in this cohort of patients,” he added.
What Can Oncologists Do to Help?
The take-home message for clinicians is that BHDs are linked to worse surgical outcomes and higher health care costs in cancer patients, Dr. Pawlik said in an interview.
“Enhanced accessibility to behavioral healthcare, as well as comprehensive policy reform related to mental health services are needed to improve care of patients with BHDs,” he said. “For example, implementing psychiatry compensation programs may encourage practice in vulnerable areas,” he said.
Other strategies include a following a collaborative care model involving mental health professionals working in tandem with primary care and mid-level practitioners and increasing use and establishment of telehealth systems to improve patient access to BHD services, he said.
What Are the Limitations?
The study by Dr. Pawlik and colleagues was limited by several factors, including the lack of data on younger patients and the full range of BHDs, as well as underreporting of BHDs and the high copays for mental health care, the researchers noted. However, the results suggest that concomitant BHDs are associated with worse cancer outcomes and higher in-hospital costs, and illustrate the need to screen for and target these conditions in cancer patients, the researchers concluded.
What Are the Next Steps for Research?
The current study involved Medicare beneficiaries aged 65 years or older, and more research is needed to investigate the impact of BHDs among younger cancer patients in whom the prevalence may be higher and the impact of BHDs may be different, Dr. Pawlik said in an interview. In addition, the analysis of BHDs as a composite of substance abuse, eating disorders, and sleep disorders (because the numbers were too small to break out data for each disorder, separately) prevented investigation of potential differences and unique challenges faced by distinct subpopulations of BHD patients, he said.
“Future studies should examine the individual impact of substance abuse, eating disorders, and sleep disorders on access to surgery, as well as the potential different impact that each one of these different BHDs may have on postoperative outcomes,” Dr. Pawlik suggested.
The study was supported by The Ohio State University College of Medicine Roessler Summer Research Scholarship. The researchers had no financial conflicts to disclose.
based on data from a new study of nearly 700,000 individuals.
The reason for this association remains unclear, and highlights the need to address existing behavioral health disorders (BHDs), which can be exacerbated after a patient is diagnosed with cancer, wrote Timothy M. Pawlik, MD, of The Ohio State University, Columbus, and colleagues. A cancer diagnosis can cause not only physical stress, but mental, emotional, social, and economic stress that can prompt a new BHD, cause relapse of a previous BHD, or exacerbate a current BHD, the researchers noted.
What is Known About BHDs and Cancer?
Although previous studies have shown a possible association between BHDs and increased cancer risk, as well as reduced compliance with care, the effect of BHDs on outcomes in cancer patients undergoing surgical resection has not been examined, wrote Dr. Pawlik and colleagues.
Previous research has focused on the impact of having a preexisting serious mental illness (SMI) such as schizophrenia and bipolar disorder on cancer care.
A 2023 literature review of 27 studies published in the Journal of Medical Imaging and Radiation Sciences showed that patients with preexisting severe mental illness (such as schizophrenia or bipolar disorder) had greater cancer-related mortality. In that study, the researchers also found that patients with severe mental illness were more likely to have metastatic disease at diagnosis, but less likely to receive optimal treatments, than individuals without SMIs.
Many studies also have focused on patients developing mental health problems (including BHDs) after a cancer diagnosis, but the current study is the first known to examine outcomes in those with BHDs before cancer.
Why Was It Important to Conduct This Study?
“BHDs are a diverse set of mental illnesses that affect an individual’s psychosocial wellbeing, potentially resulting in maladaptive behaviors,” Dr. Pawlik said in an interview. BHDs, which include substance abuse, eating disorders, and sleep disorders, are less common than anxiety/depression, but have an estimated prevalence of 1.3%-3.1% among adults in the United States, he said.
What Does the New Study Add?
In the new review by Dr. Pawlik and colleagues, published in the Journal of the American College of Surgeons (Katayama ES. J Am Coll Surg. 2024 Feb 29. doi: 2024. 10.1097/XCS.0000000000000954), BHDs were defined as substance abuse, eating disorders, or sleep disorders, which had not been the focus of previous studies. The researchers reviewed data from 694,836 adult patients with lung, esophageal, gastric, liver, pancreatic, or colorectal cancer between 2018-2021 using the Medicare Standard Analytic files. A total of 46,719 patients (6.7%) had at least one BHD.
Overall, patients with a BHD were significantly less likely than those without a BHD to undergo surgical resection (20.3% vs. 23.4%). Patients with a BHD also had significantly worse long-term postoperative survival than those without BHDs (median 37.1 months vs. 46.6 months) and significantly higher in-hospital costs ($17,432 vs. 16,159, P less than .001 for all).
Among patients who underwent cancer surgery, the odds of any complication were significantly higher for those with a BHD compared to those with no BHD (odds ratio 1.32), as were the odds of a prolonged length of stay (OR 1.67) and 90-day readmission (OR 1.57).
Dr. Pawlik said he was surprised by several of the findings, including that 1 in 15 Medicare beneficiaries had a BHD diagnosis, “with male sex and minority racial status, as well as higher social vulnerability, being associated with a higher prevalence of BHD.”
Also, the independent association of having a BHD with 30%-50% higher odds of a complication, prolonged length of stay, and 90-day readmission was higher than Dr. Pawlik had anticipated.
Why Do Patients With BHDs Have Fewer Surgeries and Worse Outcomes?
The reasons for this association were likely multifactorial and may reflect the greater burden of medical comorbidity and chronic illness in many patients with BHDs because of maladaptive lifestyles or poor nutrition status, Dr. Pawlik said.
“Patients with BHDs also likely face barriers to accessing care, which was noted particularly among patients with BHDs who lived in socially vulnerable areas,” he said. BHD patients also were more likely to be treated at low-volume rather than high-volume hospitals, “which undoubtedly contributed in part to worse outcomes in this cohort of patients,” he added.
What Can Oncologists Do to Help?
The take-home message for clinicians is that BHDs are linked to worse surgical outcomes and higher health care costs in cancer patients, Dr. Pawlik said in an interview.
“Enhanced accessibility to behavioral healthcare, as well as comprehensive policy reform related to mental health services are needed to improve care of patients with BHDs,” he said. “For example, implementing psychiatry compensation programs may encourage practice in vulnerable areas,” he said.
Other strategies include a following a collaborative care model involving mental health professionals working in tandem with primary care and mid-level practitioners and increasing use and establishment of telehealth systems to improve patient access to BHD services, he said.
What Are the Limitations?
The study by Dr. Pawlik and colleagues was limited by several factors, including the lack of data on younger patients and the full range of BHDs, as well as underreporting of BHDs and the high copays for mental health care, the researchers noted. However, the results suggest that concomitant BHDs are associated with worse cancer outcomes and higher in-hospital costs, and illustrate the need to screen for and target these conditions in cancer patients, the researchers concluded.
What Are the Next Steps for Research?
The current study involved Medicare beneficiaries aged 65 years or older, and more research is needed to investigate the impact of BHDs among younger cancer patients in whom the prevalence may be higher and the impact of BHDs may be different, Dr. Pawlik said in an interview. In addition, the analysis of BHDs as a composite of substance abuse, eating disorders, and sleep disorders (because the numbers were too small to break out data for each disorder, separately) prevented investigation of potential differences and unique challenges faced by distinct subpopulations of BHD patients, he said.
“Future studies should examine the individual impact of substance abuse, eating disorders, and sleep disorders on access to surgery, as well as the potential different impact that each one of these different BHDs may have on postoperative outcomes,” Dr. Pawlik suggested.
The study was supported by The Ohio State University College of Medicine Roessler Summer Research Scholarship. The researchers had no financial conflicts to disclose.
based on data from a new study of nearly 700,000 individuals.
The reason for this association remains unclear, and highlights the need to address existing behavioral health disorders (BHDs), which can be exacerbated after a patient is diagnosed with cancer, wrote Timothy M. Pawlik, MD, of The Ohio State University, Columbus, and colleagues. A cancer diagnosis can cause not only physical stress, but mental, emotional, social, and economic stress that can prompt a new BHD, cause relapse of a previous BHD, or exacerbate a current BHD, the researchers noted.
What is Known About BHDs and Cancer?
Although previous studies have shown a possible association between BHDs and increased cancer risk, as well as reduced compliance with care, the effect of BHDs on outcomes in cancer patients undergoing surgical resection has not been examined, wrote Dr. Pawlik and colleagues.
Previous research has focused on the impact of having a preexisting serious mental illness (SMI) such as schizophrenia and bipolar disorder on cancer care.
A 2023 literature review of 27 studies published in the Journal of Medical Imaging and Radiation Sciences showed that patients with preexisting severe mental illness (such as schizophrenia or bipolar disorder) had greater cancer-related mortality. In that study, the researchers also found that patients with severe mental illness were more likely to have metastatic disease at diagnosis, but less likely to receive optimal treatments, than individuals without SMIs.
Many studies also have focused on patients developing mental health problems (including BHDs) after a cancer diagnosis, but the current study is the first known to examine outcomes in those with BHDs before cancer.
Why Was It Important to Conduct This Study?
“BHDs are a diverse set of mental illnesses that affect an individual’s psychosocial wellbeing, potentially resulting in maladaptive behaviors,” Dr. Pawlik said in an interview. BHDs, which include substance abuse, eating disorders, and sleep disorders, are less common than anxiety/depression, but have an estimated prevalence of 1.3%-3.1% among adults in the United States, he said.
What Does the New Study Add?
In the new review by Dr. Pawlik and colleagues, published in the Journal of the American College of Surgeons (Katayama ES. J Am Coll Surg. 2024 Feb 29. doi: 2024. 10.1097/XCS.0000000000000954), BHDs were defined as substance abuse, eating disorders, or sleep disorders, which had not been the focus of previous studies. The researchers reviewed data from 694,836 adult patients with lung, esophageal, gastric, liver, pancreatic, or colorectal cancer between 2018-2021 using the Medicare Standard Analytic files. A total of 46,719 patients (6.7%) had at least one BHD.
Overall, patients with a BHD were significantly less likely than those without a BHD to undergo surgical resection (20.3% vs. 23.4%). Patients with a BHD also had significantly worse long-term postoperative survival than those without BHDs (median 37.1 months vs. 46.6 months) and significantly higher in-hospital costs ($17,432 vs. 16,159, P less than .001 for all).
Among patients who underwent cancer surgery, the odds of any complication were significantly higher for those with a BHD compared to those with no BHD (odds ratio 1.32), as were the odds of a prolonged length of stay (OR 1.67) and 90-day readmission (OR 1.57).
Dr. Pawlik said he was surprised by several of the findings, including that 1 in 15 Medicare beneficiaries had a BHD diagnosis, “with male sex and minority racial status, as well as higher social vulnerability, being associated with a higher prevalence of BHD.”
Also, the independent association of having a BHD with 30%-50% higher odds of a complication, prolonged length of stay, and 90-day readmission was higher than Dr. Pawlik had anticipated.
Why Do Patients With BHDs Have Fewer Surgeries and Worse Outcomes?
The reasons for this association were likely multifactorial and may reflect the greater burden of medical comorbidity and chronic illness in many patients with BHDs because of maladaptive lifestyles or poor nutrition status, Dr. Pawlik said.
“Patients with BHDs also likely face barriers to accessing care, which was noted particularly among patients with BHDs who lived in socially vulnerable areas,” he said. BHD patients also were more likely to be treated at low-volume rather than high-volume hospitals, “which undoubtedly contributed in part to worse outcomes in this cohort of patients,” he added.
What Can Oncologists Do to Help?
The take-home message for clinicians is that BHDs are linked to worse surgical outcomes and higher health care costs in cancer patients, Dr. Pawlik said in an interview.
“Enhanced accessibility to behavioral healthcare, as well as comprehensive policy reform related to mental health services are needed to improve care of patients with BHDs,” he said. “For example, implementing psychiatry compensation programs may encourage practice in vulnerable areas,” he said.
Other strategies include a following a collaborative care model involving mental health professionals working in tandem with primary care and mid-level practitioners and increasing use and establishment of telehealth systems to improve patient access to BHD services, he said.
What Are the Limitations?
The study by Dr. Pawlik and colleagues was limited by several factors, including the lack of data on younger patients and the full range of BHDs, as well as underreporting of BHDs and the high copays for mental health care, the researchers noted. However, the results suggest that concomitant BHDs are associated with worse cancer outcomes and higher in-hospital costs, and illustrate the need to screen for and target these conditions in cancer patients, the researchers concluded.
What Are the Next Steps for Research?
The current study involved Medicare beneficiaries aged 65 years or older, and more research is needed to investigate the impact of BHDs among younger cancer patients in whom the prevalence may be higher and the impact of BHDs may be different, Dr. Pawlik said in an interview. In addition, the analysis of BHDs as a composite of substance abuse, eating disorders, and sleep disorders (because the numbers were too small to break out data for each disorder, separately) prevented investigation of potential differences and unique challenges faced by distinct subpopulations of BHD patients, he said.
“Future studies should examine the individual impact of substance abuse, eating disorders, and sleep disorders on access to surgery, as well as the potential different impact that each one of these different BHDs may have on postoperative outcomes,” Dr. Pawlik suggested.
The study was supported by The Ohio State University College of Medicine Roessler Summer Research Scholarship. The researchers had no financial conflicts to disclose.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Are Food Emulsifiers Associated With Increased Cancer Risk?
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Democratic Lawmakers Press Pfizer on Chemotherapy Drug Shortages
In a statement about their February 21 action, the legislators, led by Rep. Jamie Raskin (D-Md.), the committee’s ranking minority member, described their work as a follow up to an earlier investigation into price hikes of generic drugs. While the committee members queried Pfizer over the three oncology medications only, they also sent letters to drugmakers Teva and Sandoz with respect to shortages in other drug classes.
A representative for Pfizer confirmed to MDedge Oncology that the company had received the representatives’ letter but said “we have no further details to provide at this time.”
What is the basis for concern?
All three generic chemotherapy drugs are mainstay treatments used across a broad array of cancers. Though shortages have been reported for several years, they became especially acute after December 2022, when an inspection by the US Food and Drug Administration (FDA) led to regulatory action against an Indian manufacturer, Intas, that produced up to half of the platinum-based therapies supplied globally. The National Comprehensive Cancer Care Network reported in October 2023 that more than 90% of its member centers were struggling to maintain adequate supplies of carboplatin, and 70% had trouble obtaining cisplatin, while the American Society of Clinical Oncology published clinical guidance on alternative treatment strategies.
What has the government done in response to the recent shortages?
The White House and the FDA announced in September that they were working with several manufacturers to help increase supplies of the platinum-based chemotherapies and of methotrexate, and taking measures that included relaxing rules on imports. Recent guidance under a pandemic-era federal law, the 2020 CARES Act, strengthened manufacturer reporting requirements related to drug shortages, and other measures have been proposed. While federal regulators have many tools with which to address drug shortages, they cannot legally oblige a manufacturer to increase production of a drug.
What can the lawmakers expect to achieve with their letter?
By pressuring Pfizer publicly, the lawmakers may be able to nudge the company to take measures to assure more consistent supplies of the three drugs. The lawmakers also said they hoped to glean from Pfizer more insight into the root causes of the shortages and potential remedies. They noted that, in a May 2023 letter by Pfizer to customers, the company had warned of depleted and limited supplies of the three drugs and said it was “working diligently” to increase output. However, the lawmakers wrote, “the root cause is not yet resolved and carboplatin, cisplatin, and methotrexate continue to experience residual delays.”
Why did the committee target Pfizer specifically?
Pfizer and its subsidiaries are among the major manufacturers of the three generic chemotherapy agents mentioned in the letter. The legislators noted that “pharmaceutical companies may not be motivated to produce generic drugs like carboplatin, cisplatin, and methotrexate, because they are not as lucrative as producing patented brand name drugs,” and that “as a principal supplier of carboplatin, cisplatin, and methotrexate, it is critical that Pfizer continues to increase production of these life-sustaining cancer medications, even amidst potential lower profitability.”
The committee members also made reference to news reports of price-gouging with these medications, as smaller hospitals or oncology centers are forced to turn to unscrupulous third-party suppliers.
What is being demanded of Pfizer?
Pfizer was given until March 6 to respond, in writing and in a briefing with committee staff, to a six questions. These queries concern what specific steps the company has taken to increase supplies of the three generic oncology drugs, what Pfizer is doing to help avert price-gouging, whether further oncology drug shortages are anticipated, and how the company is working with the FDA on the matter.
In a statement about their February 21 action, the legislators, led by Rep. Jamie Raskin (D-Md.), the committee’s ranking minority member, described their work as a follow up to an earlier investigation into price hikes of generic drugs. While the committee members queried Pfizer over the three oncology medications only, they also sent letters to drugmakers Teva and Sandoz with respect to shortages in other drug classes.
A representative for Pfizer confirmed to MDedge Oncology that the company had received the representatives’ letter but said “we have no further details to provide at this time.”
What is the basis for concern?
All three generic chemotherapy drugs are mainstay treatments used across a broad array of cancers. Though shortages have been reported for several years, they became especially acute after December 2022, when an inspection by the US Food and Drug Administration (FDA) led to regulatory action against an Indian manufacturer, Intas, that produced up to half of the platinum-based therapies supplied globally. The National Comprehensive Cancer Care Network reported in October 2023 that more than 90% of its member centers were struggling to maintain adequate supplies of carboplatin, and 70% had trouble obtaining cisplatin, while the American Society of Clinical Oncology published clinical guidance on alternative treatment strategies.
What has the government done in response to the recent shortages?
The White House and the FDA announced in September that they were working with several manufacturers to help increase supplies of the platinum-based chemotherapies and of methotrexate, and taking measures that included relaxing rules on imports. Recent guidance under a pandemic-era federal law, the 2020 CARES Act, strengthened manufacturer reporting requirements related to drug shortages, and other measures have been proposed. While federal regulators have many tools with which to address drug shortages, they cannot legally oblige a manufacturer to increase production of a drug.
What can the lawmakers expect to achieve with their letter?
By pressuring Pfizer publicly, the lawmakers may be able to nudge the company to take measures to assure more consistent supplies of the three drugs. The lawmakers also said they hoped to glean from Pfizer more insight into the root causes of the shortages and potential remedies. They noted that, in a May 2023 letter by Pfizer to customers, the company had warned of depleted and limited supplies of the three drugs and said it was “working diligently” to increase output. However, the lawmakers wrote, “the root cause is not yet resolved and carboplatin, cisplatin, and methotrexate continue to experience residual delays.”
Why did the committee target Pfizer specifically?
Pfizer and its subsidiaries are among the major manufacturers of the three generic chemotherapy agents mentioned in the letter. The legislators noted that “pharmaceutical companies may not be motivated to produce generic drugs like carboplatin, cisplatin, and methotrexate, because they are not as lucrative as producing patented brand name drugs,” and that “as a principal supplier of carboplatin, cisplatin, and methotrexate, it is critical that Pfizer continues to increase production of these life-sustaining cancer medications, even amidst potential lower profitability.”
The committee members also made reference to news reports of price-gouging with these medications, as smaller hospitals or oncology centers are forced to turn to unscrupulous third-party suppliers.
What is being demanded of Pfizer?
Pfizer was given until March 6 to respond, in writing and in a briefing with committee staff, to a six questions. These queries concern what specific steps the company has taken to increase supplies of the three generic oncology drugs, what Pfizer is doing to help avert price-gouging, whether further oncology drug shortages are anticipated, and how the company is working with the FDA on the matter.
In a statement about their February 21 action, the legislators, led by Rep. Jamie Raskin (D-Md.), the committee’s ranking minority member, described their work as a follow up to an earlier investigation into price hikes of generic drugs. While the committee members queried Pfizer over the three oncology medications only, they also sent letters to drugmakers Teva and Sandoz with respect to shortages in other drug classes.
A representative for Pfizer confirmed to MDedge Oncology that the company had received the representatives’ letter but said “we have no further details to provide at this time.”
What is the basis for concern?
All three generic chemotherapy drugs are mainstay treatments used across a broad array of cancers. Though shortages have been reported for several years, they became especially acute after December 2022, when an inspection by the US Food and Drug Administration (FDA) led to regulatory action against an Indian manufacturer, Intas, that produced up to half of the platinum-based therapies supplied globally. The National Comprehensive Cancer Care Network reported in October 2023 that more than 90% of its member centers were struggling to maintain adequate supplies of carboplatin, and 70% had trouble obtaining cisplatin, while the American Society of Clinical Oncology published clinical guidance on alternative treatment strategies.
What has the government done in response to the recent shortages?
The White House and the FDA announced in September that they were working with several manufacturers to help increase supplies of the platinum-based chemotherapies and of methotrexate, and taking measures that included relaxing rules on imports. Recent guidance under a pandemic-era federal law, the 2020 CARES Act, strengthened manufacturer reporting requirements related to drug shortages, and other measures have been proposed. While federal regulators have many tools with which to address drug shortages, they cannot legally oblige a manufacturer to increase production of a drug.
What can the lawmakers expect to achieve with their letter?
By pressuring Pfizer publicly, the lawmakers may be able to nudge the company to take measures to assure more consistent supplies of the three drugs. The lawmakers also said they hoped to glean from Pfizer more insight into the root causes of the shortages and potential remedies. They noted that, in a May 2023 letter by Pfizer to customers, the company had warned of depleted and limited supplies of the three drugs and said it was “working diligently” to increase output. However, the lawmakers wrote, “the root cause is not yet resolved and carboplatin, cisplatin, and methotrexate continue to experience residual delays.”
Why did the committee target Pfizer specifically?
Pfizer and its subsidiaries are among the major manufacturers of the three generic chemotherapy agents mentioned in the letter. The legislators noted that “pharmaceutical companies may not be motivated to produce generic drugs like carboplatin, cisplatin, and methotrexate, because they are not as lucrative as producing patented brand name drugs,” and that “as a principal supplier of carboplatin, cisplatin, and methotrexate, it is critical that Pfizer continues to increase production of these life-sustaining cancer medications, even amidst potential lower profitability.”
The committee members also made reference to news reports of price-gouging with these medications, as smaller hospitals or oncology centers are forced to turn to unscrupulous third-party suppliers.
What is being demanded of Pfizer?
Pfizer was given until March 6 to respond, in writing and in a briefing with committee staff, to a six questions. These queries concern what specific steps the company has taken to increase supplies of the three generic oncology drugs, what Pfizer is doing to help avert price-gouging, whether further oncology drug shortages are anticipated, and how the company is working with the FDA on the matter.
Unleashing Our Immune Response to Quash Cancer
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
Oral Cancer: New System May Improve Prognostic Accuracy
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
FROM CANCER
Despite An AI Assist, Imaging Study Shows Disparities in Diagnosing Different Skin Tones
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
FROM NATURE MEDICINE