User login
For MD-IQ use only
3D Printing for the Development of Palatal Defect Prosthetics
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
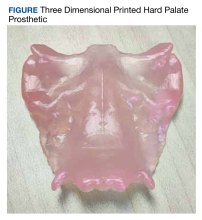
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Are Direct-to-Consumer Microbiome Tests Clinically Useful?
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Time to Lung Disease in Patients With Dermatomyositis Subtype Estimated
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
The time interval between onset of interstitial lung disease (ILD) and diagnosis of anti–melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) “has not been well described,” the authors say.
METHODOLOGY:
- , with the former having a particularly high mortality rate.
- In this retrospective cohort study using electronic medical records, researchers evaluated 774 patients with DM between 2008 and 2023 to learn more about the time interval between ILD and the time of an MDA5 antibody-positive DM diagnosis, which has not been well described.
- The primary outcome was ILD diagnosis and time in days between documented ILD and MDA5 antibody-positive DM diagnoses.
TAKEAWAY:
- Overall, 14 patients with DM (1.8%) were diagnosed with MDA5 antibody-positive DM in dermatology, rheumatology, or pulmonology departments (nine women and five men; age, 24-77 years; 79% were White and 7% were Black).
- ILD was diagnosed in 9 of the 14 patients (64%); 6 of the 14 (43%) met the criteria for RPILD. Two cases were diagnosed concurrently and two prior to MDA5 antibody-positive DM diagnosis.
- The median time between ILD and MDA5 antibody-positive DM diagnoses was 163 days.
- Gottron papules/sign and midfacial erythema were the most common dermatologic findings, and no association was seen between cutaneous signs and type of ILD.
IN PRACTICE:
“Establishing an accurate timeline between MDA5 antibody-positive DM and ILD can promote urgency among dermatologists to evaluate extracutaneous manifestations in their management of patients with DM for more accurate risk stratification and appropriate treatment,” the authors wrote.
SOURCE:
This study, led by Rachel R. Lin, from the University of Miami, Miami, Florida, was published online as a research letter in JAMA Dermatology.
LIMITATIONS:
Study limitations were the study’s retrospective design and small sample size.
DISCLOSURES:
No information on study funding was provided. One author reported personal fees from argenX outside this submitted work. Other authors did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Keratoacanthoma, SCC Relatively Rare With PD-1/PD-L1 Inhibitors, Study Suggests
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(AEs) reported to the US Food and Drug Administration (FDA).
METHODOLOGY:
- The risk for dermatologic immune-related side effects may be increased with immunologic-modifying drugs.
- To determine if there are significant signals between keratoacanthomas and cSCCs and PD-1/PD-L1 inhibitors, researchers analyzed AEs associated with these agents reported to the FDA’s Adverse Event Reporting System (FAERS) between January 2004 and May 2023.
- Pharmacovigilance signals were identified, and a significant signal was defined as the lower 95% CI of a reporting odds ratio (ROR) greater than one or the lower 95% CI of an information component (IC) greater than 0.
TAKEAWAY:
- Of the 158,000 reports of PD-1/PD-L1 inhibitor use, 43 were in patients who developed a keratoacanthoma (mean age, 77 years; 39% women) and 83 were in patients who developed cSCC (mean age, 71 years; 41% women). Patients aged 60-79 years were most likely to develop keratoacanthomas and cSCC on these treatments.
- A PD-1/PD-L1 inhibitor was listed as the suspect drug in all 43 keratoacanthoma reports and in 70 of 83 cSCC reports (the remaining 13 listed them as the concomitant drug).
- Significant signals were reported for both keratoacanthoma (ROR, 9.7; IC, 1.9) and cSCC (ROR, 3.0; IC, 0.9) with PD-1/PD-L1 inhibitor use.
- Of the reports where this information was available, all 10 cases of PD-1/PD-L1 inhibitor–linked keratoacanthoma and 10 of 17 cases (59%) of PD-1/PD-L1 inhibitor–linked cSCC, resolution was noted following discontinuation or dose reduction of the inhibitor.
IN PRACTICE:
“Given the large number of patients receiving immunotherapy, FAERS recording only 43 patients developing keratoacanthoma and 83 patients developing cSCC highlights that these conditions are relatively rare adverse events,” the authors wrote but added that more studies are needed to confirm these results.
SOURCE:
The study, led by Pushkar Aggarwal, MD, MBA, of the Department of Dermatology, University of Cincinnati, Cincinnati, Ohio, was published online in JAMA Dermatology.
LIMITATIONS:
The data obtained from FAERS did not contain information on all AEs from drugs. In addition, a causal association could not be determined.
DISCLOSURES:
The funding source was not reported. The authors did not report any conflicts of interest.
A version of this article appeared on Medscape.com.
PCOS: Laser, Light Therapy Helpful for Hirsutism
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Adding ACEI to Chemotherapy Does Not Prevent Cardiotoxicity
a new randomized trial showed.
The results suggested adding an ACE inhibitor doesn’t affect cardiac injury or cardiac function outcomes “and should not be used as a preventative strategy” in these patients, David Austin, MD, consultant cardiologist, Academic Cardiovascular Unit, The James Cook University Hospital, Middlesbrough, England, and chief investigator for the PROACT study, told this news organization.
But while these negative results are disappointing, he said, “we now have a definitive result in a robustly conducted trial that will take the field forward.”
The findings were presented on April 8, 2024, at the American College of Cardiology (ACC) Scientific Session 2024.
Anthracyclines, which are extracted from Streptomyces bacterium, are chemotherapy drugs widely used to treat several types of cancer. Doxorubicin is among the most clinically important anthracyclines.
While extremely effective, anthracyclines can cause irreversible damage to cardiac cells and ultimately impair cardiac function and even cause heart failure, which may only be evident years after exposure. “Cardiac injury is very common in patients treated with high dose anthracyclines,” noted Dr. Austin.
The open-label PROACT study included 111 adult patients, mean age 58 years and predominantly White and women, being treated for breast cancer (62%) or NHL (38%) at National Health Service hospitals in England with high-dose anthracycline-based chemotherapy.
Patients were randomized to standard care (six cycles of high-dose doxorubicin-equivalent anthracycline-based chemotherapy) plus the ACE inhibitor enalapril maleate or standard care alone. The mean chemotherapy dose was 328 mg/m2; any dose greater than 300 is considered high.
The starting dose of enalapril was 2.5 mg twice a day, which was titrated up to a maximum of 10 mg twice a day. The ACE inhibitor was started at least 2 days before chemotherapy began and finished 3 weeks after the last anthracycline dose.
During the study, enalapril was titrated to 20 mg in more than 75% of patients, with the mean dose being 17.7 mg.
Myocardial Injury Outcome
The primary outcome was myocardial injury measured by the presence (≥ 14 ng/L) of high sensitivity cardiac troponin T (cTnT) during anthracycline treatment and 1 month after the last dose of anthracycline.
cTnT is highly expressed in cardiomyocytes and has become a preferred biomarker for detecting acute myocardial infarction and other causes of myocardial injury.
Blood sampling for cTnT and cardiac troponin I (cTnI) was performed at baseline, within 72 hours prior to chemotherapy and at trial completion. All patients had negative troponin results at baseline, indicating no heart damage.
A majority of patients experienced elevations in troponin (78% in the enalapril group and 83% in the standard of care group), but there was no statistically significant difference between groups (adjusted odds ratio [OR], 0.65; 95% CI, 0.23-1.78; P = .405).
There was also no significant difference between groups in terms of cTnI, a secondary endpoint. However, the proportion of patients testing positive for cTnI (47% in the enalapril group and 45% in controls) was substantially lower than that for cTnT.
Large Discrepancy
The “large discrepancy in the rate of injury” with cTnT “has implications for the clinical interpretation of cardiac biomarkers in routine practice, and we should proceed with caution,” Dr. Austin told this news organization.
The finding has implications because guidelines don’t currently differentiate based on the type of troponin, Dr. Austin said in a press release. “I was surprised by the difference, and I think this raises the question of what troponin we should be using.”
Secondary outcomes focused on cardiac function, measured using echocardiography and included left ventricular global longitudinal strain (LVGLS) and left ventricular ejection fraction (LVEF). These were measured at baseline, 4 weeks after the last anthracycline dose and 1 year after the final chemotherapy.
There was no between-group difference in LVGLS cardiac function (21% for enalapril vs 22% for standard of care; adjusted OR, 0.95; 95% CI, 0.33-2.74; P = .921). This was also true for LVEF (4% for enalapril vs 0% for standard of care group; adjusted OR, 4.89; 95% CI, 0.40-674.62; P = .236).
Asked what the research team plans to do next, Dr. Austin said “the immediate first step” is to continue following PROACT patients. “We know heart failure events and cardiac dysfunction can occur later down the line.”
Due to the challenge of enrolling patients into trials like PROACT, “we should come together as a sort of a broader cardiovascular/oncology academic community to try to understand how we can better recruit patients into these studies,” said Dr. Austin.
“We need to solve that problem before we then go on to maybe examine other potential preventative therapies.”
He doesn’t think an alternative ACE inhibitor would prove beneficial. “We need to look elsewhere for effective therapies in this area.”
He noted these new findings are “broadly consistent” with other trials that investigated angiotensin receptor blockers.
Tough Population
Commenting on the study during a media briefing, Anita Deswal, chair, medicine, Department of Cardiology, Division of Internal Medicine, The University of Texas, commended the researchers for managing to enroll patients with cancer as this is “a tough” population to get to agree to being in a clinical trial.
“These patients are often overwhelmed financially, physically, and emotionally with the cancer diagnosis, as well as the cancer therapy and, therefore, to enroll them in something to prevent, maybe, some potential cardiac toxicity down the line, is really hard.”
Past trials investigating neuro-hormonal blockers to prevent cardiotoxicity have been criticized for enrolling patients at “too low risk,” said Dr. Deswal. “But investigators here went that step beyond and enrolled patients who were going to receive higher doses of anthracyclines, so kudos to that.”
And she noted investigators managed to get patients on almost the maximum dose of enalapril. “So, the drug was poised to have an effect — if it was there.”
The negative results may have something to do with endpoints. “Maybe we haven’t quite figured out what are the cutoffs for high sensitivity troponin I that identify patients truly at risk” of developing heart failure in the future.
Commenting on the study for this news organization, Anu Lala, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York City, said the results may come as a surprise to some.
“ACE inhibitors are considered cardioprotective and for this reason are often used prophylactically in patients receiving chemotherapy.”
Dr. Lala agrees troponin may not be the right endpoint. “Another question is whether clinical outcomes should be followed in addition to symptoms or onset of any heart failure symptoms, which may hold greater prognostic significance.”
The study was funded by the National Institute for Health and Care Research.
A version of this article appeared on Medscape.com.
a new randomized trial showed.
The results suggested adding an ACE inhibitor doesn’t affect cardiac injury or cardiac function outcomes “and should not be used as a preventative strategy” in these patients, David Austin, MD, consultant cardiologist, Academic Cardiovascular Unit, The James Cook University Hospital, Middlesbrough, England, and chief investigator for the PROACT study, told this news organization.
But while these negative results are disappointing, he said, “we now have a definitive result in a robustly conducted trial that will take the field forward.”
The findings were presented on April 8, 2024, at the American College of Cardiology (ACC) Scientific Session 2024.
Anthracyclines, which are extracted from Streptomyces bacterium, are chemotherapy drugs widely used to treat several types of cancer. Doxorubicin is among the most clinically important anthracyclines.
While extremely effective, anthracyclines can cause irreversible damage to cardiac cells and ultimately impair cardiac function and even cause heart failure, which may only be evident years after exposure. “Cardiac injury is very common in patients treated with high dose anthracyclines,” noted Dr. Austin.
The open-label PROACT study included 111 adult patients, mean age 58 years and predominantly White and women, being treated for breast cancer (62%) or NHL (38%) at National Health Service hospitals in England with high-dose anthracycline-based chemotherapy.
Patients were randomized to standard care (six cycles of high-dose doxorubicin-equivalent anthracycline-based chemotherapy) plus the ACE inhibitor enalapril maleate or standard care alone. The mean chemotherapy dose was 328 mg/m2; any dose greater than 300 is considered high.
The starting dose of enalapril was 2.5 mg twice a day, which was titrated up to a maximum of 10 mg twice a day. The ACE inhibitor was started at least 2 days before chemotherapy began and finished 3 weeks after the last anthracycline dose.
During the study, enalapril was titrated to 20 mg in more than 75% of patients, with the mean dose being 17.7 mg.
Myocardial Injury Outcome
The primary outcome was myocardial injury measured by the presence (≥ 14 ng/L) of high sensitivity cardiac troponin T (cTnT) during anthracycline treatment and 1 month after the last dose of anthracycline.
cTnT is highly expressed in cardiomyocytes and has become a preferred biomarker for detecting acute myocardial infarction and other causes of myocardial injury.
Blood sampling for cTnT and cardiac troponin I (cTnI) was performed at baseline, within 72 hours prior to chemotherapy and at trial completion. All patients had negative troponin results at baseline, indicating no heart damage.
A majority of patients experienced elevations in troponin (78% in the enalapril group and 83% in the standard of care group), but there was no statistically significant difference between groups (adjusted odds ratio [OR], 0.65; 95% CI, 0.23-1.78; P = .405).
There was also no significant difference between groups in terms of cTnI, a secondary endpoint. However, the proportion of patients testing positive for cTnI (47% in the enalapril group and 45% in controls) was substantially lower than that for cTnT.
Large Discrepancy
The “large discrepancy in the rate of injury” with cTnT “has implications for the clinical interpretation of cardiac biomarkers in routine practice, and we should proceed with caution,” Dr. Austin told this news organization.
The finding has implications because guidelines don’t currently differentiate based on the type of troponin, Dr. Austin said in a press release. “I was surprised by the difference, and I think this raises the question of what troponin we should be using.”
Secondary outcomes focused on cardiac function, measured using echocardiography and included left ventricular global longitudinal strain (LVGLS) and left ventricular ejection fraction (LVEF). These were measured at baseline, 4 weeks after the last anthracycline dose and 1 year after the final chemotherapy.
There was no between-group difference in LVGLS cardiac function (21% for enalapril vs 22% for standard of care; adjusted OR, 0.95; 95% CI, 0.33-2.74; P = .921). This was also true for LVEF (4% for enalapril vs 0% for standard of care group; adjusted OR, 4.89; 95% CI, 0.40-674.62; P = .236).
Asked what the research team plans to do next, Dr. Austin said “the immediate first step” is to continue following PROACT patients. “We know heart failure events and cardiac dysfunction can occur later down the line.”
Due to the challenge of enrolling patients into trials like PROACT, “we should come together as a sort of a broader cardiovascular/oncology academic community to try to understand how we can better recruit patients into these studies,” said Dr. Austin.
“We need to solve that problem before we then go on to maybe examine other potential preventative therapies.”
He doesn’t think an alternative ACE inhibitor would prove beneficial. “We need to look elsewhere for effective therapies in this area.”
He noted these new findings are “broadly consistent” with other trials that investigated angiotensin receptor blockers.
Tough Population
Commenting on the study during a media briefing, Anita Deswal, chair, medicine, Department of Cardiology, Division of Internal Medicine, The University of Texas, commended the researchers for managing to enroll patients with cancer as this is “a tough” population to get to agree to being in a clinical trial.
“These patients are often overwhelmed financially, physically, and emotionally with the cancer diagnosis, as well as the cancer therapy and, therefore, to enroll them in something to prevent, maybe, some potential cardiac toxicity down the line, is really hard.”
Past trials investigating neuro-hormonal blockers to prevent cardiotoxicity have been criticized for enrolling patients at “too low risk,” said Dr. Deswal. “But investigators here went that step beyond and enrolled patients who were going to receive higher doses of anthracyclines, so kudos to that.”
And she noted investigators managed to get patients on almost the maximum dose of enalapril. “So, the drug was poised to have an effect — if it was there.”
The negative results may have something to do with endpoints. “Maybe we haven’t quite figured out what are the cutoffs for high sensitivity troponin I that identify patients truly at risk” of developing heart failure in the future.
Commenting on the study for this news organization, Anu Lala, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York City, said the results may come as a surprise to some.
“ACE inhibitors are considered cardioprotective and for this reason are often used prophylactically in patients receiving chemotherapy.”
Dr. Lala agrees troponin may not be the right endpoint. “Another question is whether clinical outcomes should be followed in addition to symptoms or onset of any heart failure symptoms, which may hold greater prognostic significance.”
The study was funded by the National Institute for Health and Care Research.
A version of this article appeared on Medscape.com.
a new randomized trial showed.
The results suggested adding an ACE inhibitor doesn’t affect cardiac injury or cardiac function outcomes “and should not be used as a preventative strategy” in these patients, David Austin, MD, consultant cardiologist, Academic Cardiovascular Unit, The James Cook University Hospital, Middlesbrough, England, and chief investigator for the PROACT study, told this news organization.
But while these negative results are disappointing, he said, “we now have a definitive result in a robustly conducted trial that will take the field forward.”
The findings were presented on April 8, 2024, at the American College of Cardiology (ACC) Scientific Session 2024.
Anthracyclines, which are extracted from Streptomyces bacterium, are chemotherapy drugs widely used to treat several types of cancer. Doxorubicin is among the most clinically important anthracyclines.
While extremely effective, anthracyclines can cause irreversible damage to cardiac cells and ultimately impair cardiac function and even cause heart failure, which may only be evident years after exposure. “Cardiac injury is very common in patients treated with high dose anthracyclines,” noted Dr. Austin.
The open-label PROACT study included 111 adult patients, mean age 58 years and predominantly White and women, being treated for breast cancer (62%) or NHL (38%) at National Health Service hospitals in England with high-dose anthracycline-based chemotherapy.
Patients were randomized to standard care (six cycles of high-dose doxorubicin-equivalent anthracycline-based chemotherapy) plus the ACE inhibitor enalapril maleate or standard care alone. The mean chemotherapy dose was 328 mg/m2; any dose greater than 300 is considered high.
The starting dose of enalapril was 2.5 mg twice a day, which was titrated up to a maximum of 10 mg twice a day. The ACE inhibitor was started at least 2 days before chemotherapy began and finished 3 weeks after the last anthracycline dose.
During the study, enalapril was titrated to 20 mg in more than 75% of patients, with the mean dose being 17.7 mg.
Myocardial Injury Outcome
The primary outcome was myocardial injury measured by the presence (≥ 14 ng/L) of high sensitivity cardiac troponin T (cTnT) during anthracycline treatment and 1 month after the last dose of anthracycline.
cTnT is highly expressed in cardiomyocytes and has become a preferred biomarker for detecting acute myocardial infarction and other causes of myocardial injury.
Blood sampling for cTnT and cardiac troponin I (cTnI) was performed at baseline, within 72 hours prior to chemotherapy and at trial completion. All patients had negative troponin results at baseline, indicating no heart damage.
A majority of patients experienced elevations in troponin (78% in the enalapril group and 83% in the standard of care group), but there was no statistically significant difference between groups (adjusted odds ratio [OR], 0.65; 95% CI, 0.23-1.78; P = .405).
There was also no significant difference between groups in terms of cTnI, a secondary endpoint. However, the proportion of patients testing positive for cTnI (47% in the enalapril group and 45% in controls) was substantially lower than that for cTnT.
Large Discrepancy
The “large discrepancy in the rate of injury” with cTnT “has implications for the clinical interpretation of cardiac biomarkers in routine practice, and we should proceed with caution,” Dr. Austin told this news organization.
The finding has implications because guidelines don’t currently differentiate based on the type of troponin, Dr. Austin said in a press release. “I was surprised by the difference, and I think this raises the question of what troponin we should be using.”
Secondary outcomes focused on cardiac function, measured using echocardiography and included left ventricular global longitudinal strain (LVGLS) and left ventricular ejection fraction (LVEF). These were measured at baseline, 4 weeks after the last anthracycline dose and 1 year after the final chemotherapy.
There was no between-group difference in LVGLS cardiac function (21% for enalapril vs 22% for standard of care; adjusted OR, 0.95; 95% CI, 0.33-2.74; P = .921). This was also true for LVEF (4% for enalapril vs 0% for standard of care group; adjusted OR, 4.89; 95% CI, 0.40-674.62; P = .236).
Asked what the research team plans to do next, Dr. Austin said “the immediate first step” is to continue following PROACT patients. “We know heart failure events and cardiac dysfunction can occur later down the line.”
Due to the challenge of enrolling patients into trials like PROACT, “we should come together as a sort of a broader cardiovascular/oncology academic community to try to understand how we can better recruit patients into these studies,” said Dr. Austin.
“We need to solve that problem before we then go on to maybe examine other potential preventative therapies.”
He doesn’t think an alternative ACE inhibitor would prove beneficial. “We need to look elsewhere for effective therapies in this area.”
He noted these new findings are “broadly consistent” with other trials that investigated angiotensin receptor blockers.
Tough Population
Commenting on the study during a media briefing, Anita Deswal, chair, medicine, Department of Cardiology, Division of Internal Medicine, The University of Texas, commended the researchers for managing to enroll patients with cancer as this is “a tough” population to get to agree to being in a clinical trial.
“These patients are often overwhelmed financially, physically, and emotionally with the cancer diagnosis, as well as the cancer therapy and, therefore, to enroll them in something to prevent, maybe, some potential cardiac toxicity down the line, is really hard.”
Past trials investigating neuro-hormonal blockers to prevent cardiotoxicity have been criticized for enrolling patients at “too low risk,” said Dr. Deswal. “But investigators here went that step beyond and enrolled patients who were going to receive higher doses of anthracyclines, so kudos to that.”
And she noted investigators managed to get patients on almost the maximum dose of enalapril. “So, the drug was poised to have an effect — if it was there.”
The negative results may have something to do with endpoints. “Maybe we haven’t quite figured out what are the cutoffs for high sensitivity troponin I that identify patients truly at risk” of developing heart failure in the future.
Commenting on the study for this news organization, Anu Lala, MD, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai, New York City, said the results may come as a surprise to some.
“ACE inhibitors are considered cardioprotective and for this reason are often used prophylactically in patients receiving chemotherapy.”
Dr. Lala agrees troponin may not be the right endpoint. “Another question is whether clinical outcomes should be followed in addition to symptoms or onset of any heart failure symptoms, which may hold greater prognostic significance.”
The study was funded by the National Institute for Health and Care Research.
A version of this article appeared on Medscape.com.
FROM THE ACC 2024
New and Improved Option for Detecting Neurologic Pathogens?
DENVER — , results of a real-world analysis show.
Metagenomic next-generation sequencing (mNGS) of RNA and DNA from cerebrospinal fluid (CSF) simultaneously tests for a wide range of infectious agents and identifies individual pathogens, including viruses, bacteria, fungi, and parasites. About half of patients with a suspected central nervous system (CNS) infection may go undiagnosed due to a lack of tools that detect rare pathogens. Although mNGS is currently available only in specialized laboratories, expanding access to the diagnostic could address this problem, investigators noted.
“Our results justify incorporation of CSF mNGS testing as part of the routine diagnostic workup in hospitalized patients who present with potential central nervous system infections,” study investigator Charles Chiu, MD, PhD, professor in the Department of Laboratory Medicine as well as Medicine and Department of Medicine – Infectious Diseases and director of the Clinical Microbiology Laboratory, University of California San Fransisco (UCSF), said at a press conference.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology (AAN).
‘Real-World’ Performance
Accurate diagnosis of CNS infections on the basis of CSF, imaging, patient history, and presentation is challenging, the researchers noted. “Roughly 50% of patients who present with a presumed central nervous system infection actually end up without a diagnosis,” Dr. Chiu said.
This is due to the lack of diagnostic tests for rare pathogens and because noninfectious conditions like cancer, autoantibody syndrome, or vasculitis can mimic an infection, he added.
CSF is “very limiting,” Dr. Chiu noted. “We are unable, practically, from a volume perspective, as well as a cost and turnaround time perspective, to be able to send off every possible test for every possible organism.”
The inability to rapidly pinpoint the cause of an infectious disease like meningitis or encephalitis can cause delays in appropriate treatment.
To assess the “real-world” performance of mNGS, researchers collected 4828 samples from mainly hospitalized patients across the United States and elsewhere from 2016 to 2023.
Overall, the test detected at least one pathogen in 16.6% of cases. More than 70% were DNA or RNA viruses, followed by bacteria, fungi, and parasites.
High Sensitivity
The technology was also able to detect novel or emerging neurotropic pathogens, including a yellow fever virus responsible for a transfusion-transmitted encephalitis outbreak and Fusarium solani, which caused a fungal meningitis outbreak.
Investigators also conducted a chart review on a subset of 1052 patients at UCSF to compare the performance of CSF nMGS testing with commonly used in-hospital diagnostic tests.
“We showed that as a single test, spinal fluid mNGS has an overall sensitivity of 63%, specificity of 99%, and accuracy of 90%,” said Dr. Chiu.
The sensitivity of mNGS was significantly higher compared with direct-detection testing from CSF (46%); direct-detection testing performed on samples other than CSF, such as blood (15%); and indirect serologic testing looking for antibodies (29%) (P < .001 for all).
This suggests that mNGS could potentially “detect the hundreds of different pathogens that cause clinically indistinguishable infections,” Dr. Chui said.
mNGS testing is currently confined to large specialized or reference laboratories. For greater access to the test, routine clinical labs or hospital labs would have to implement it, said Dr. Chiu.
“If you can bring the technology to the point of care, directly to the hospital lab that’s running the test, we can produce results that would have a more rapid impact on patients,” he said.
Guiding Therapy
Ultimately, he added, the purpose of a diagnostic test is to “generate actionable information that could potentially guide therapy.”
Researchers are now evaluating medical charts of the same subcohort of patients to determine whether the test made a clinical difference.
“We want to know if it had a positive or negative or no clinical impact on the management and treatment of patients,” said Dr. Chiu. “Producing data like this will help us define the role of this test in the future as part of the diagnostic paradigm.”
The researchers are also working on a cost/benefit analysis, and Dr. Chiu said that US Food and Drug Administration approval of the test is needed “to establish a blueprint for reimbursement.”
Commenting on the findings, Jessica Robinson-Papp, MD, professor and vice chair of clinical research, Department of Neurology, Icahn School of Medicine, New York, said that the technology could be useful, especially in developing countries with higher rates of CNS infections.
“What’s really exciting about it is you can take a very small CSF sample, like 1 mL, and in an unbiased way just screen for all different kinds of pathogens including both DNA and RNA viruses, parasites, bacteria, and fungi, and sort of come up with whether there’s a pathogen there or whether there is no pathogen there,” she said.
However, there’s a chance that this sensitive technique will pick up contaminants, she added. “For example, if there’s a little environmental bacterium either on the skin or in the water used for processing, it can get reads on that.”
The study received support from Delve Bio and the Chan-Zuckerberg Biohub.
Dr. Chiu has received personal compensation for serving on a Scientific Advisory or Data Safety Monitoring Board for Biomeme and has stock in Delve Bio, Poppy Health, Mammoth Biosciences, and BiomeSense and has received intellectual property interests from a discovery or technology relating to healthcare. Dr. Robinson-Papp has no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , results of a real-world analysis show.
Metagenomic next-generation sequencing (mNGS) of RNA and DNA from cerebrospinal fluid (CSF) simultaneously tests for a wide range of infectious agents and identifies individual pathogens, including viruses, bacteria, fungi, and parasites. About half of patients with a suspected central nervous system (CNS) infection may go undiagnosed due to a lack of tools that detect rare pathogens. Although mNGS is currently available only in specialized laboratories, expanding access to the diagnostic could address this problem, investigators noted.
“Our results justify incorporation of CSF mNGS testing as part of the routine diagnostic workup in hospitalized patients who present with potential central nervous system infections,” study investigator Charles Chiu, MD, PhD, professor in the Department of Laboratory Medicine as well as Medicine and Department of Medicine – Infectious Diseases and director of the Clinical Microbiology Laboratory, University of California San Fransisco (UCSF), said at a press conference.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology (AAN).
‘Real-World’ Performance
Accurate diagnosis of CNS infections on the basis of CSF, imaging, patient history, and presentation is challenging, the researchers noted. “Roughly 50% of patients who present with a presumed central nervous system infection actually end up without a diagnosis,” Dr. Chiu said.
This is due to the lack of diagnostic tests for rare pathogens and because noninfectious conditions like cancer, autoantibody syndrome, or vasculitis can mimic an infection, he added.
CSF is “very limiting,” Dr. Chiu noted. “We are unable, practically, from a volume perspective, as well as a cost and turnaround time perspective, to be able to send off every possible test for every possible organism.”
The inability to rapidly pinpoint the cause of an infectious disease like meningitis or encephalitis can cause delays in appropriate treatment.
To assess the “real-world” performance of mNGS, researchers collected 4828 samples from mainly hospitalized patients across the United States and elsewhere from 2016 to 2023.
Overall, the test detected at least one pathogen in 16.6% of cases. More than 70% were DNA or RNA viruses, followed by bacteria, fungi, and parasites.
High Sensitivity
The technology was also able to detect novel or emerging neurotropic pathogens, including a yellow fever virus responsible for a transfusion-transmitted encephalitis outbreak and Fusarium solani, which caused a fungal meningitis outbreak.
Investigators also conducted a chart review on a subset of 1052 patients at UCSF to compare the performance of CSF nMGS testing with commonly used in-hospital diagnostic tests.
“We showed that as a single test, spinal fluid mNGS has an overall sensitivity of 63%, specificity of 99%, and accuracy of 90%,” said Dr. Chiu.
The sensitivity of mNGS was significantly higher compared with direct-detection testing from CSF (46%); direct-detection testing performed on samples other than CSF, such as blood (15%); and indirect serologic testing looking for antibodies (29%) (P < .001 for all).
This suggests that mNGS could potentially “detect the hundreds of different pathogens that cause clinically indistinguishable infections,” Dr. Chui said.
mNGS testing is currently confined to large specialized or reference laboratories. For greater access to the test, routine clinical labs or hospital labs would have to implement it, said Dr. Chiu.
“If you can bring the technology to the point of care, directly to the hospital lab that’s running the test, we can produce results that would have a more rapid impact on patients,” he said.
Guiding Therapy
Ultimately, he added, the purpose of a diagnostic test is to “generate actionable information that could potentially guide therapy.”
Researchers are now evaluating medical charts of the same subcohort of patients to determine whether the test made a clinical difference.
“We want to know if it had a positive or negative or no clinical impact on the management and treatment of patients,” said Dr. Chiu. “Producing data like this will help us define the role of this test in the future as part of the diagnostic paradigm.”
The researchers are also working on a cost/benefit analysis, and Dr. Chiu said that US Food and Drug Administration approval of the test is needed “to establish a blueprint for reimbursement.”
Commenting on the findings, Jessica Robinson-Papp, MD, professor and vice chair of clinical research, Department of Neurology, Icahn School of Medicine, New York, said that the technology could be useful, especially in developing countries with higher rates of CNS infections.
“What’s really exciting about it is you can take a very small CSF sample, like 1 mL, and in an unbiased way just screen for all different kinds of pathogens including both DNA and RNA viruses, parasites, bacteria, and fungi, and sort of come up with whether there’s a pathogen there or whether there is no pathogen there,” she said.
However, there’s a chance that this sensitive technique will pick up contaminants, she added. “For example, if there’s a little environmental bacterium either on the skin or in the water used for processing, it can get reads on that.”
The study received support from Delve Bio and the Chan-Zuckerberg Biohub.
Dr. Chiu has received personal compensation for serving on a Scientific Advisory or Data Safety Monitoring Board for Biomeme and has stock in Delve Bio, Poppy Health, Mammoth Biosciences, and BiomeSense and has received intellectual property interests from a discovery or technology relating to healthcare. Dr. Robinson-Papp has no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , results of a real-world analysis show.
Metagenomic next-generation sequencing (mNGS) of RNA and DNA from cerebrospinal fluid (CSF) simultaneously tests for a wide range of infectious agents and identifies individual pathogens, including viruses, bacteria, fungi, and parasites. About half of patients with a suspected central nervous system (CNS) infection may go undiagnosed due to a lack of tools that detect rare pathogens. Although mNGS is currently available only in specialized laboratories, expanding access to the diagnostic could address this problem, investigators noted.
“Our results justify incorporation of CSF mNGS testing as part of the routine diagnostic workup in hospitalized patients who present with potential central nervous system infections,” study investigator Charles Chiu, MD, PhD, professor in the Department of Laboratory Medicine as well as Medicine and Department of Medicine – Infectious Diseases and director of the Clinical Microbiology Laboratory, University of California San Fransisco (UCSF), said at a press conference.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology (AAN).
‘Real-World’ Performance
Accurate diagnosis of CNS infections on the basis of CSF, imaging, patient history, and presentation is challenging, the researchers noted. “Roughly 50% of patients who present with a presumed central nervous system infection actually end up without a diagnosis,” Dr. Chiu said.
This is due to the lack of diagnostic tests for rare pathogens and because noninfectious conditions like cancer, autoantibody syndrome, or vasculitis can mimic an infection, he added.
CSF is “very limiting,” Dr. Chiu noted. “We are unable, practically, from a volume perspective, as well as a cost and turnaround time perspective, to be able to send off every possible test for every possible organism.”
The inability to rapidly pinpoint the cause of an infectious disease like meningitis or encephalitis can cause delays in appropriate treatment.
To assess the “real-world” performance of mNGS, researchers collected 4828 samples from mainly hospitalized patients across the United States and elsewhere from 2016 to 2023.
Overall, the test detected at least one pathogen in 16.6% of cases. More than 70% were DNA or RNA viruses, followed by bacteria, fungi, and parasites.
High Sensitivity
The technology was also able to detect novel or emerging neurotropic pathogens, including a yellow fever virus responsible for a transfusion-transmitted encephalitis outbreak and Fusarium solani, which caused a fungal meningitis outbreak.
Investigators also conducted a chart review on a subset of 1052 patients at UCSF to compare the performance of CSF nMGS testing with commonly used in-hospital diagnostic tests.
“We showed that as a single test, spinal fluid mNGS has an overall sensitivity of 63%, specificity of 99%, and accuracy of 90%,” said Dr. Chiu.
The sensitivity of mNGS was significantly higher compared with direct-detection testing from CSF (46%); direct-detection testing performed on samples other than CSF, such as blood (15%); and indirect serologic testing looking for antibodies (29%) (P < .001 for all).
This suggests that mNGS could potentially “detect the hundreds of different pathogens that cause clinically indistinguishable infections,” Dr. Chui said.
mNGS testing is currently confined to large specialized or reference laboratories. For greater access to the test, routine clinical labs or hospital labs would have to implement it, said Dr. Chiu.
“If you can bring the technology to the point of care, directly to the hospital lab that’s running the test, we can produce results that would have a more rapid impact on patients,” he said.
Guiding Therapy
Ultimately, he added, the purpose of a diagnostic test is to “generate actionable information that could potentially guide therapy.”
Researchers are now evaluating medical charts of the same subcohort of patients to determine whether the test made a clinical difference.
“We want to know if it had a positive or negative or no clinical impact on the management and treatment of patients,” said Dr. Chiu. “Producing data like this will help us define the role of this test in the future as part of the diagnostic paradigm.”
The researchers are also working on a cost/benefit analysis, and Dr. Chiu said that US Food and Drug Administration approval of the test is needed “to establish a blueprint for reimbursement.”
Commenting on the findings, Jessica Robinson-Papp, MD, professor and vice chair of clinical research, Department of Neurology, Icahn School of Medicine, New York, said that the technology could be useful, especially in developing countries with higher rates of CNS infections.
“What’s really exciting about it is you can take a very small CSF sample, like 1 mL, and in an unbiased way just screen for all different kinds of pathogens including both DNA and RNA viruses, parasites, bacteria, and fungi, and sort of come up with whether there’s a pathogen there or whether there is no pathogen there,” she said.
However, there’s a chance that this sensitive technique will pick up contaminants, she added. “For example, if there’s a little environmental bacterium either on the skin or in the water used for processing, it can get reads on that.”
The study received support from Delve Bio and the Chan-Zuckerberg Biohub.
Dr. Chiu has received personal compensation for serving on a Scientific Advisory or Data Safety Monitoring Board for Biomeme and has stock in Delve Bio, Poppy Health, Mammoth Biosciences, and BiomeSense and has received intellectual property interests from a discovery or technology relating to healthcare. Dr. Robinson-Papp has no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Most Targeted Cancer Drugs Lack Substantial Clinical Benefit
TOPLINE:
METHODOLOGY:
- The strength and quality of evidence supporting genome-targeted cancer drug approvals vary. A big reason is the growing number of cancer drug approvals based on surrogate endpoints, such as disease-free and progression-free survival, instead of clinical endpoints, such as overall survival or quality of life. The US Food and Drug Administration (FDA) has also approved genome-targeted cancer drugs based on phase 1 or single-arm trials.
- Given these less rigorous considerations for approval, “the validity and value of the targets and surrogate measures underlying FDA genome-targeted cancer drug approvals are uncertain,” the researchers explained.
- In the current analysis, researchers assessed the validity of the molecular targets as well as the clinical benefits of genome-targeted cancer drugs approved in the United States from 2015 to 2022 based on results from pivotal trials.
- The researchers evaluated the strength of evidence supporting molecular targetability using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT) and the clinical benefit using the ESMO–Magnitude of Clinical Benefit Scale (ESMO-MCBS).
- The authors defined a substantial clinical benefit as an A or B grade for curative intent and a 4 or 5 for noncurative intent. High-benefit genomic-based cancer treatments were defined as those associated with a substantial clinical benefit (ESMO-MCBS) and that qualified as ESCAT category level I-A (a clinical benefit based on prospective randomized data) or I-B (prospective nonrandomized data).
TAKEAWAY:
- The analyses focused on 50 molecular-targeted cancer drugs covering 84 indications. Of which, 45 indications (54%) were approved based on phase 1 or 2 pivotal trials, 45 (54%) were supported by single-arm pivotal trials and the remaining 39 (46%) by randomized trial, and 48 (57%) were approved based on subgroup analyses.
- Among the 84 indications, more than half (55%) of the pivotal trials supporting approval used overall response rate as a primary endpoint, 31% used progression-free survival, and 6% used disease-free survival. Only seven indications (8%) were supported by pivotal trials demonstrating an improvement in overall survival.
- Among the 84 trials, 24 (29%) met the ESMO-MCBS threshold for substantial clinical benefit.
- Overall, when combining all ratings, only 24 of the 84 indications (29%) were considered high-benefit genomic-based cancer treatments.
IN PRACTICE:
“We applied the ESMO-MCBS and ESCAT value frameworks to identify therapies and molecular targets providing high clinical value that should be widely available to patients” and “found that drug indications supported by these characteristics represent a minority of cancer drug approvals in recent years,” the authors said. Using these value frameworks could help payers, governments, and individual patients “prioritize the availability of high-value molecular-targeted therapies.”
SOURCE:
The study, with first author Ariadna Tibau, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, was published online in JAMA Oncology.
LIMITATIONS:
The study evaluated only trials that supported regulatory approval and did not include outcomes of postapproval clinical studies, which could lead to changes in ESMO-MCBS grades and ESCAT levels of evidence over time.
DISCLOSURES:
The study was funded by the Kaiser Permanente Institute for Health Policy, Arnold Ventures, and the Commonwealth Fund. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The strength and quality of evidence supporting genome-targeted cancer drug approvals vary. A big reason is the growing number of cancer drug approvals based on surrogate endpoints, such as disease-free and progression-free survival, instead of clinical endpoints, such as overall survival or quality of life. The US Food and Drug Administration (FDA) has also approved genome-targeted cancer drugs based on phase 1 or single-arm trials.
- Given these less rigorous considerations for approval, “the validity and value of the targets and surrogate measures underlying FDA genome-targeted cancer drug approvals are uncertain,” the researchers explained.
- In the current analysis, researchers assessed the validity of the molecular targets as well as the clinical benefits of genome-targeted cancer drugs approved in the United States from 2015 to 2022 based on results from pivotal trials.
- The researchers evaluated the strength of evidence supporting molecular targetability using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT) and the clinical benefit using the ESMO–Magnitude of Clinical Benefit Scale (ESMO-MCBS).
- The authors defined a substantial clinical benefit as an A or B grade for curative intent and a 4 or 5 for noncurative intent. High-benefit genomic-based cancer treatments were defined as those associated with a substantial clinical benefit (ESMO-MCBS) and that qualified as ESCAT category level I-A (a clinical benefit based on prospective randomized data) or I-B (prospective nonrandomized data).
TAKEAWAY:
- The analyses focused on 50 molecular-targeted cancer drugs covering 84 indications. Of which, 45 indications (54%) were approved based on phase 1 or 2 pivotal trials, 45 (54%) were supported by single-arm pivotal trials and the remaining 39 (46%) by randomized trial, and 48 (57%) were approved based on subgroup analyses.
- Among the 84 indications, more than half (55%) of the pivotal trials supporting approval used overall response rate as a primary endpoint, 31% used progression-free survival, and 6% used disease-free survival. Only seven indications (8%) were supported by pivotal trials demonstrating an improvement in overall survival.
- Among the 84 trials, 24 (29%) met the ESMO-MCBS threshold for substantial clinical benefit.
- Overall, when combining all ratings, only 24 of the 84 indications (29%) were considered high-benefit genomic-based cancer treatments.
IN PRACTICE:
“We applied the ESMO-MCBS and ESCAT value frameworks to identify therapies and molecular targets providing high clinical value that should be widely available to patients” and “found that drug indications supported by these characteristics represent a minority of cancer drug approvals in recent years,” the authors said. Using these value frameworks could help payers, governments, and individual patients “prioritize the availability of high-value molecular-targeted therapies.”
SOURCE:
The study, with first author Ariadna Tibau, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, was published online in JAMA Oncology.
LIMITATIONS:
The study evaluated only trials that supported regulatory approval and did not include outcomes of postapproval clinical studies, which could lead to changes in ESMO-MCBS grades and ESCAT levels of evidence over time.
DISCLOSURES:
The study was funded by the Kaiser Permanente Institute for Health Policy, Arnold Ventures, and the Commonwealth Fund. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The strength and quality of evidence supporting genome-targeted cancer drug approvals vary. A big reason is the growing number of cancer drug approvals based on surrogate endpoints, such as disease-free and progression-free survival, instead of clinical endpoints, such as overall survival or quality of life. The US Food and Drug Administration (FDA) has also approved genome-targeted cancer drugs based on phase 1 or single-arm trials.
- Given these less rigorous considerations for approval, “the validity and value of the targets and surrogate measures underlying FDA genome-targeted cancer drug approvals are uncertain,” the researchers explained.
- In the current analysis, researchers assessed the validity of the molecular targets as well as the clinical benefits of genome-targeted cancer drugs approved in the United States from 2015 to 2022 based on results from pivotal trials.
- The researchers evaluated the strength of evidence supporting molecular targetability using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT) and the clinical benefit using the ESMO–Magnitude of Clinical Benefit Scale (ESMO-MCBS).
- The authors defined a substantial clinical benefit as an A or B grade for curative intent and a 4 or 5 for noncurative intent. High-benefit genomic-based cancer treatments were defined as those associated with a substantial clinical benefit (ESMO-MCBS) and that qualified as ESCAT category level I-A (a clinical benefit based on prospective randomized data) or I-B (prospective nonrandomized data).
TAKEAWAY:
- The analyses focused on 50 molecular-targeted cancer drugs covering 84 indications. Of which, 45 indications (54%) were approved based on phase 1 or 2 pivotal trials, 45 (54%) were supported by single-arm pivotal trials and the remaining 39 (46%) by randomized trial, and 48 (57%) were approved based on subgroup analyses.
- Among the 84 indications, more than half (55%) of the pivotal trials supporting approval used overall response rate as a primary endpoint, 31% used progression-free survival, and 6% used disease-free survival. Only seven indications (8%) were supported by pivotal trials demonstrating an improvement in overall survival.
- Among the 84 trials, 24 (29%) met the ESMO-MCBS threshold for substantial clinical benefit.
- Overall, when combining all ratings, only 24 of the 84 indications (29%) were considered high-benefit genomic-based cancer treatments.
IN PRACTICE:
“We applied the ESMO-MCBS and ESCAT value frameworks to identify therapies and molecular targets providing high clinical value that should be widely available to patients” and “found that drug indications supported by these characteristics represent a minority of cancer drug approvals in recent years,” the authors said. Using these value frameworks could help payers, governments, and individual patients “prioritize the availability of high-value molecular-targeted therapies.”
SOURCE:
The study, with first author Ariadna Tibau, MD, PhD, Brigham and Women’s Hospital and Harvard Medical School, Boston, was published online in JAMA Oncology.
LIMITATIONS:
The study evaluated only trials that supported regulatory approval and did not include outcomes of postapproval clinical studies, which could lead to changes in ESMO-MCBS grades and ESCAT levels of evidence over time.
DISCLOSURES:
The study was funded by the Kaiser Permanente Institute for Health Policy, Arnold Ventures, and the Commonwealth Fund. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
Survey Finds Mental Health Issues Increased After Cosmetic Procedure Complications
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
BALTIMORE — of patients with dermatology-related complications.
The study used an anonymous 40-question survey circulated to a Facebook cosmetic complication support group. Seventy-one of 100 individuals completed the questionnaire, reporting significantly higher rates of mental health issues after their complications than before. Results were presented at the annual conference of the American Society for Laser Medicine and Surgery (ASLMS). Almost all the survey respondents (99%) were female, with 61% aged 25-44 years and 34% aged 45-64 years.
“Cosmetic procedures have increased over the past decade, with procedures being increasingly performed by an evolving variety of providers,” the study’s lead author, Taryn Murray, MD, a dermatologist at Cleveland Clinic, Cleveland, Ohio, told this news organization. “Appropriate patient assessment and counseling and proper procedure technique are important for obtaining safe and effective results. Complications may not only impact patients physically but can also be harmful to their mental health.”
Rise in Mental Health Issues
The study found that before respondents had the treatment that led to their complications, 16% reported a history of generalized anxiety disorder, 15% a history of depression, and 1% a history of either BDD or PTSD. Following the complication, 50% reported a positive depression screening, 63% a positive BDD Questionnaire – Dermatology Version, and 63% a positive Primary Care PTSD screen, Dr. Murray said. “Almost half of respondents (46%) reported thinking about their complication for more than 3 hours a day,” she said in presenting the results.
Dr. Murray said the idea for the study grew out of her experience as a fellow working with Paul Friedman, MD, at the Dermatology and Laser Surgery Center at University of Texas Health in Houston.
“We were seeing a lot of complications,” Dr. Murray said in an interview. “Some of these were local. Some of these patients were flying in from out-of-state looking for help with the complication, and we could see what a mental and emotional burden this put on these patients. They were routinely in the office in tears saying it was interfering with their daily life, it was interfering with their job, saying they were going to lose their job, all because they were so distressed over what was happening to them.”
Yet, the research into psychological distress in patients with dermatologic complications is minimal, Dr. Murray added. “We think that body dysmorphic disorder is prevalent for patients seeking dermatology or plastic surgery services, but I don’t think either of the specialties do a great job in screening people for that when they come for treatment, so I think a lot of it goes undiagnosed. There’s been a trend looking at more at complications lately, but there’s been a gap in the literature.”
The treatments the patients in the survey had were microneedling with radiofrequency (29%), laser (24%), ultrasound for skin tightening (11%), radiofrequency for skin tightening (11%), microneedling (4%), chemical peel (3%), body contouring/sculpting (1%), and “other” (17%).
The study found that the largest share of procedures, 47%, were done by an esthetician/laser technician, followed by a nondermatologist physician (17%), a board-certified dermatologist (14%), an advanced practice provider (12%), and “other” (10%).
Self-reported complications included scarring (38%), hyperpigmentation (26%), erythema (24%), burn (23%), blisters (11%), and hypopigmentation (3%); 71% characterized their complications as “other,” and one respondent reported multiple complications.
“Respondents said they were satisfied with the previous cosmetic care they received,” Dr. Murray said during her presentation at the meeting. “And there was a consensus among the respondents that they did not feel adequately counseled on the risks of the procedure and that it did not meet their expectations and anticipated outcome.”
Take-Home Lesson
The lesson here is that practitioners who perform cosmetic procedures should be well-versed in the task and potential complications, Dr. Murray said in the interview. “If you’re going to be doing a procedure, make sure you know the proper techniques, the proper endpoints, and how to treat if you’re to have a complication,” she said. “If you don’t know how to treat a complication from the device, then you should think twice about using it.”
She also suggested screening patients for potentially undiagnosed mental health disorders. “It can play a role in the initial consultation and potentially any after-care they might need if there is a complication,” she said. “We may not have the adequate tools at this time to know how to best handle these patients and these scenarios, but hopefully my abstract will shed a little more light on it.”
She said she hopes her findings lead to more research in the future.
Asked to comment on the study, Jennifer Lin, MD, assistant professor of dermatology at Brigham and Women’s Hospital and Dana Farber Cancer Institute in Boston, Massachusetts, said one finding of the study stood out to her. “ I was very surprised from her dataset that patients think about it more than 3 hours a day,” she told this news organization. “That’s really significant. We talk about the side effects, but we don’t necessarily talk about the burden of how long the recovery will be or the psychological burden of potentially dealing with it.”
She noted that “there’s a bit of movement” toward developing guidelines for laser treatments, which would address the risk of complications. “That’s the goal: To have better guidelines to avoid these complications in the first place,” Dr. Lin said.
The study findings also point to a need for “premonitoring” individuals before procedures, she added. “We talked about patient selection and make sure someone doesn’t have body dysmorphic disorder, but we don’t formally screen for it,” she said. “We don’t our train our residents to screen for it. And I think doing more pre- and post-testing of how people are affected by laser treatment is going to become more important.”
Dr. Murray disclosed relationships with R2 Technologies. Dr. Lin had no relationships to disclose.
A version of this article appeared on Medscape.com.
FROM ASLMS 2024
Cannabis Constituent May Be Key to Easing THC-Induced Anxiety
, new data from a small study suggested.
Participants who inhaled vaporized D-limonene and THC reported significantly greater decreases in anxiogenic effects than did people who received either component alone or a placebo. Reductions were greater as the dose of the D-limonene was increased.
Investigators noted that the findings could have implications for the use of medicinal or recreational cannabis, which has increased in recent years due to state legalization efforts.
“People use cannabis to help reduce anxiety, depression, and posttraumatic stress disorder, but since THC levels vary widely, if a person overshoots their tolerance of THC, cannabis can induce anxiety rather than relieve it,” senior investigator Ryan Vandrey, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, said in a news release.
“Our study demonstrates that D-limonene can modulate the effects of THC in a meaningful way and make THC more tolerable to people using it for both therapeutic and non-therapeutic purposes,” he added.
The study was published online in Drug and Alcohol Dependence.
Entourage Theory
Cannabis legalization has opened the door to an increased range of medicinal and nonmedicinal uses, but its benefits can be limited by the anxiety and panic some people experience with its use, investigators noted.
Many cannabis plants have been bred to contain higher concentrations of THC, with some dispensaries selling cannabis with more than 20%-30% THC. The plants often include cannabidiol, “minor” cannabinoids, and terpenes, such as D-limonene.
Prior studies pointed to THC as the cause of acute behavioral and psychoactive effects some cannabis users experience. However, a new, untested theory, the “cannabis entourage effect theory,” suggested other components in cannabis, including D-limonene, may contribute to the anxiogenic symptoms.
“We were motivated by scientific publications that hypothesized D-limonene can attenuate the acute anxiogenic effects of cannabis, but for which empirical data did not exist,” Dr. Vandrey said.
Investigators designed a small double-blind, within-subjects crossover study of 20 healthy adults (median age, 26 years; 50% men). About half of participants were Caucasian/non-Hispanic, 30% African American/non-Hispanic, 10% Caucasian/Hispanic, and 10% Asian/non-Hispanic.
All participants completed nine outpatient drug administration sessions, during which they inhaled vaporized D-limonene alone (1 or 5 mg), THC alone (15 or 30 mg), the same doses of THC and D-limonene together, or placebo.
Primary outcomes included subjective drug effects, measured with the Drug Effect Questionnaire (DEQ) and the 20-item state subscale of the State-Trait Anxiety Inventory (STAI-S). Investigators also measured cognitive/psychomotor performance with the Digit Symbol Substitution Task (DSST) and the Paced Serial Addition Task.
Vital signs such as heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), and plasma D-limonene and THC concentrations were also tracked.
Participants’ responses were measured at baseline and then an additional nine times after initial exposure over the course of each 6-hour test session. Blood and urine samples were collected from participants before, during, and after each session.
First Evidence
There were no significant differences in outcomes between the D-limonene alone and placebo groups.
Receipt of 15- and 30-mg doses of THC alone was associated with subjective reports of acute cannabis exposure, including cognitive and physiological effects.
A treatment effect was observed for “anxious/nervous” (P < .01), “paranoid” (P < .01), and “heart racing” (P < .0001).
In planned comparisons, ratings of anxiety-like subjective effects qualitatively decreased as D-limonene dose increased, and concurrent administration of 30-mg THC plus 15-mg D-limonene significantly reduced ratings of “anxious/nervous” and “paranoid” on the DEQ compared to 30 mg of THC alone (P < .05).
Findings were similar on the composite score of the STAI-S, and although planned comparisons did not reach the threshold for statistical significance, reductions in anxiety approached significance in the THC plus D-limonene group compared with the THC alone condition (P = .08). The combination group also reported significantly lower subjective ratings of unpleasant drug effects than the THC alone group (P = .03).
In particular, a main effect of treatment was found for the anxious/nervous category on the DEQ (P < .01), as well as the “paranoid” (P < .01) and heart racing (P < .0001) categories.
On the other hand, ratings of anxious/nervous and paranoid categories were significantly lower in the 30-mg THC plus 15-mg D-limonene vs the 30-mg THC alone condition (P < .05, for all).
As for cognition, following drug administration, a significant main effect of treatment was observed for the DSST (P < .05), but no significant differences between THC and THC plus D-limonene combination conditions or between D-limonene alone and placebo were detected.
There were no differences within each THC dose and between D-limonene alone versus placebo conditions. Moreover, there were no main effects of treatment found for SBP or DBP.
The combination condition produced significantly greater concentrations of THC than the THC alone condition (P < .05).
“This study provides the first evidence that there are chemical constituents found naturally in the cannabis plant that can reduce some of the adverse effects of using delta-9-THC,” Dr. Vandrey said.
Although the exact mechanism by which vaporized D-limonene counters the anxiogenic effects of THC is unclear, “our best guess is that D-limonene is producing an anxiolytic effect on its own that is not mediated by cannabinoid receptors,” Dr. Vandrey said.
Significant Impact
Commenting on the research, Joshua Lile, PhD, professor, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, noted that the study seems to be the first of its kind to study the influence of terpene on THC response.
The research “makes a significant impact on our field,” and is “among the few controlled clinical studies that have demonstrated interactions between THC and other cannabis constituents, supporting the validity of the ‘entourage’ effect,” said Dr. Lile, who was not involved with the current research.
“This work is particularly important, given the unfounded claims sometimes made by the cannabis industry regarding the effects of different cannabis products,” he added.
Also commenting on the study, Ziva Cooper, PhD, professor and director of the UCLA Center for Cannabis and Cannabinoids, University of California Los Angeles, said the findings “have direct implications for improving the safety of cannabis, whether it’s being used for medical or nonmedical purposes, especially in people and patients who do not have experience with cannabis, a group that is at high risk for experiencing anxiety after using cannabis.”
In addition, “an important aspect to this study is that the effects of limonene in reducing anxiety attributed to delta-9-THC were observed at higher concentrations (or doses) than those usually present in the plant,” Dr. Copper said. “This calls for further investigation into new cannabis formulations specifically designed to leverage the potential protective effects of the terpene.”
This research was supported by the National Institute on Drug Abuse. Dr. Vandrey served as a consultant or received honoraria from Mira1a Therapeutics, Inc.; Jazz Pharmaceuticals; Charlotte’s Web; Syqe Medical Ltd.; and WebMD. The other authors’ disclosures are listed on the original paper. Dr. Lile declared no relevant financial relationships. Dr. Cooper reported receiving study drug from Canopy Growth Corp and True Terpenes, study-related materials from Storz & Bickel, and research support from the National Institute on Drug Abuse, National Center for Complementary and Integrative Health, California Department of Cannabis Control, Center for Medicinal Cannabis Research, and California Highway Patrol.
A version of this article appeared on Medscape.com.
, new data from a small study suggested.
Participants who inhaled vaporized D-limonene and THC reported significantly greater decreases in anxiogenic effects than did people who received either component alone or a placebo. Reductions were greater as the dose of the D-limonene was increased.
Investigators noted that the findings could have implications for the use of medicinal or recreational cannabis, which has increased in recent years due to state legalization efforts.
“People use cannabis to help reduce anxiety, depression, and posttraumatic stress disorder, but since THC levels vary widely, if a person overshoots their tolerance of THC, cannabis can induce anxiety rather than relieve it,” senior investigator Ryan Vandrey, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, said in a news release.
“Our study demonstrates that D-limonene can modulate the effects of THC in a meaningful way and make THC more tolerable to people using it for both therapeutic and non-therapeutic purposes,” he added.
The study was published online in Drug and Alcohol Dependence.
Entourage Theory
Cannabis legalization has opened the door to an increased range of medicinal and nonmedicinal uses, but its benefits can be limited by the anxiety and panic some people experience with its use, investigators noted.
Many cannabis plants have been bred to contain higher concentrations of THC, with some dispensaries selling cannabis with more than 20%-30% THC. The plants often include cannabidiol, “minor” cannabinoids, and terpenes, such as D-limonene.
Prior studies pointed to THC as the cause of acute behavioral and psychoactive effects some cannabis users experience. However, a new, untested theory, the “cannabis entourage effect theory,” suggested other components in cannabis, including D-limonene, may contribute to the anxiogenic symptoms.
“We were motivated by scientific publications that hypothesized D-limonene can attenuate the acute anxiogenic effects of cannabis, but for which empirical data did not exist,” Dr. Vandrey said.
Investigators designed a small double-blind, within-subjects crossover study of 20 healthy adults (median age, 26 years; 50% men). About half of participants were Caucasian/non-Hispanic, 30% African American/non-Hispanic, 10% Caucasian/Hispanic, and 10% Asian/non-Hispanic.
All participants completed nine outpatient drug administration sessions, during which they inhaled vaporized D-limonene alone (1 or 5 mg), THC alone (15 or 30 mg), the same doses of THC and D-limonene together, or placebo.
Primary outcomes included subjective drug effects, measured with the Drug Effect Questionnaire (DEQ) and the 20-item state subscale of the State-Trait Anxiety Inventory (STAI-S). Investigators also measured cognitive/psychomotor performance with the Digit Symbol Substitution Task (DSST) and the Paced Serial Addition Task.
Vital signs such as heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), and plasma D-limonene and THC concentrations were also tracked.
Participants’ responses were measured at baseline and then an additional nine times after initial exposure over the course of each 6-hour test session. Blood and urine samples were collected from participants before, during, and after each session.
First Evidence
There were no significant differences in outcomes between the D-limonene alone and placebo groups.
Receipt of 15- and 30-mg doses of THC alone was associated with subjective reports of acute cannabis exposure, including cognitive and physiological effects.
A treatment effect was observed for “anxious/nervous” (P < .01), “paranoid” (P < .01), and “heart racing” (P < .0001).
In planned comparisons, ratings of anxiety-like subjective effects qualitatively decreased as D-limonene dose increased, and concurrent administration of 30-mg THC plus 15-mg D-limonene significantly reduced ratings of “anxious/nervous” and “paranoid” on the DEQ compared to 30 mg of THC alone (P < .05).
Findings were similar on the composite score of the STAI-S, and although planned comparisons did not reach the threshold for statistical significance, reductions in anxiety approached significance in the THC plus D-limonene group compared with the THC alone condition (P = .08). The combination group also reported significantly lower subjective ratings of unpleasant drug effects than the THC alone group (P = .03).
In particular, a main effect of treatment was found for the anxious/nervous category on the DEQ (P < .01), as well as the “paranoid” (P < .01) and heart racing (P < .0001) categories.
On the other hand, ratings of anxious/nervous and paranoid categories were significantly lower in the 30-mg THC plus 15-mg D-limonene vs the 30-mg THC alone condition (P < .05, for all).
As for cognition, following drug administration, a significant main effect of treatment was observed for the DSST (P < .05), but no significant differences between THC and THC plus D-limonene combination conditions or between D-limonene alone and placebo were detected.
There were no differences within each THC dose and between D-limonene alone versus placebo conditions. Moreover, there were no main effects of treatment found for SBP or DBP.
The combination condition produced significantly greater concentrations of THC than the THC alone condition (P < .05).
“This study provides the first evidence that there are chemical constituents found naturally in the cannabis plant that can reduce some of the adverse effects of using delta-9-THC,” Dr. Vandrey said.
Although the exact mechanism by which vaporized D-limonene counters the anxiogenic effects of THC is unclear, “our best guess is that D-limonene is producing an anxiolytic effect on its own that is not mediated by cannabinoid receptors,” Dr. Vandrey said.
Significant Impact
Commenting on the research, Joshua Lile, PhD, professor, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, noted that the study seems to be the first of its kind to study the influence of terpene on THC response.
The research “makes a significant impact on our field,” and is “among the few controlled clinical studies that have demonstrated interactions between THC and other cannabis constituents, supporting the validity of the ‘entourage’ effect,” said Dr. Lile, who was not involved with the current research.
“This work is particularly important, given the unfounded claims sometimes made by the cannabis industry regarding the effects of different cannabis products,” he added.
Also commenting on the study, Ziva Cooper, PhD, professor and director of the UCLA Center for Cannabis and Cannabinoids, University of California Los Angeles, said the findings “have direct implications for improving the safety of cannabis, whether it’s being used for medical or nonmedical purposes, especially in people and patients who do not have experience with cannabis, a group that is at high risk for experiencing anxiety after using cannabis.”
In addition, “an important aspect to this study is that the effects of limonene in reducing anxiety attributed to delta-9-THC were observed at higher concentrations (or doses) than those usually present in the plant,” Dr. Copper said. “This calls for further investigation into new cannabis formulations specifically designed to leverage the potential protective effects of the terpene.”
This research was supported by the National Institute on Drug Abuse. Dr. Vandrey served as a consultant or received honoraria from Mira1a Therapeutics, Inc.; Jazz Pharmaceuticals; Charlotte’s Web; Syqe Medical Ltd.; and WebMD. The other authors’ disclosures are listed on the original paper. Dr. Lile declared no relevant financial relationships. Dr. Cooper reported receiving study drug from Canopy Growth Corp and True Terpenes, study-related materials from Storz & Bickel, and research support from the National Institute on Drug Abuse, National Center for Complementary and Integrative Health, California Department of Cannabis Control, Center for Medicinal Cannabis Research, and California Highway Patrol.
A version of this article appeared on Medscape.com.
, new data from a small study suggested.
Participants who inhaled vaporized D-limonene and THC reported significantly greater decreases in anxiogenic effects than did people who received either component alone or a placebo. Reductions were greater as the dose of the D-limonene was increased.
Investigators noted that the findings could have implications for the use of medicinal or recreational cannabis, which has increased in recent years due to state legalization efforts.
“People use cannabis to help reduce anxiety, depression, and posttraumatic stress disorder, but since THC levels vary widely, if a person overshoots their tolerance of THC, cannabis can induce anxiety rather than relieve it,” senior investigator Ryan Vandrey, PhD, professor of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, said in a news release.
“Our study demonstrates that D-limonene can modulate the effects of THC in a meaningful way and make THC more tolerable to people using it for both therapeutic and non-therapeutic purposes,” he added.
The study was published online in Drug and Alcohol Dependence.
Entourage Theory
Cannabis legalization has opened the door to an increased range of medicinal and nonmedicinal uses, but its benefits can be limited by the anxiety and panic some people experience with its use, investigators noted.
Many cannabis plants have been bred to contain higher concentrations of THC, with some dispensaries selling cannabis with more than 20%-30% THC. The plants often include cannabidiol, “minor” cannabinoids, and terpenes, such as D-limonene.
Prior studies pointed to THC as the cause of acute behavioral and psychoactive effects some cannabis users experience. However, a new, untested theory, the “cannabis entourage effect theory,” suggested other components in cannabis, including D-limonene, may contribute to the anxiogenic symptoms.
“We were motivated by scientific publications that hypothesized D-limonene can attenuate the acute anxiogenic effects of cannabis, but for which empirical data did not exist,” Dr. Vandrey said.
Investigators designed a small double-blind, within-subjects crossover study of 20 healthy adults (median age, 26 years; 50% men). About half of participants were Caucasian/non-Hispanic, 30% African American/non-Hispanic, 10% Caucasian/Hispanic, and 10% Asian/non-Hispanic.
All participants completed nine outpatient drug administration sessions, during which they inhaled vaporized D-limonene alone (1 or 5 mg), THC alone (15 or 30 mg), the same doses of THC and D-limonene together, or placebo.
Primary outcomes included subjective drug effects, measured with the Drug Effect Questionnaire (DEQ) and the 20-item state subscale of the State-Trait Anxiety Inventory (STAI-S). Investigators also measured cognitive/psychomotor performance with the Digit Symbol Substitution Task (DSST) and the Paced Serial Addition Task.
Vital signs such as heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), and plasma D-limonene and THC concentrations were also tracked.
Participants’ responses were measured at baseline and then an additional nine times after initial exposure over the course of each 6-hour test session. Blood and urine samples were collected from participants before, during, and after each session.
First Evidence
There were no significant differences in outcomes between the D-limonene alone and placebo groups.
Receipt of 15- and 30-mg doses of THC alone was associated with subjective reports of acute cannabis exposure, including cognitive and physiological effects.
A treatment effect was observed for “anxious/nervous” (P < .01), “paranoid” (P < .01), and “heart racing” (P < .0001).
In planned comparisons, ratings of anxiety-like subjective effects qualitatively decreased as D-limonene dose increased, and concurrent administration of 30-mg THC plus 15-mg D-limonene significantly reduced ratings of “anxious/nervous” and “paranoid” on the DEQ compared to 30 mg of THC alone (P < .05).
Findings were similar on the composite score of the STAI-S, and although planned comparisons did not reach the threshold for statistical significance, reductions in anxiety approached significance in the THC plus D-limonene group compared with the THC alone condition (P = .08). The combination group also reported significantly lower subjective ratings of unpleasant drug effects than the THC alone group (P = .03).
In particular, a main effect of treatment was found for the anxious/nervous category on the DEQ (P < .01), as well as the “paranoid” (P < .01) and heart racing (P < .0001) categories.
On the other hand, ratings of anxious/nervous and paranoid categories were significantly lower in the 30-mg THC plus 15-mg D-limonene vs the 30-mg THC alone condition (P < .05, for all).
As for cognition, following drug administration, a significant main effect of treatment was observed for the DSST (P < .05), but no significant differences between THC and THC plus D-limonene combination conditions or between D-limonene alone and placebo were detected.
There were no differences within each THC dose and between D-limonene alone versus placebo conditions. Moreover, there were no main effects of treatment found for SBP or DBP.
The combination condition produced significantly greater concentrations of THC than the THC alone condition (P < .05).
“This study provides the first evidence that there are chemical constituents found naturally in the cannabis plant that can reduce some of the adverse effects of using delta-9-THC,” Dr. Vandrey said.
Although the exact mechanism by which vaporized D-limonene counters the anxiogenic effects of THC is unclear, “our best guess is that D-limonene is producing an anxiolytic effect on its own that is not mediated by cannabinoid receptors,” Dr. Vandrey said.
Significant Impact
Commenting on the research, Joshua Lile, PhD, professor, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, noted that the study seems to be the first of its kind to study the influence of terpene on THC response.
The research “makes a significant impact on our field,” and is “among the few controlled clinical studies that have demonstrated interactions between THC and other cannabis constituents, supporting the validity of the ‘entourage’ effect,” said Dr. Lile, who was not involved with the current research.
“This work is particularly important, given the unfounded claims sometimes made by the cannabis industry regarding the effects of different cannabis products,” he added.
Also commenting on the study, Ziva Cooper, PhD, professor and director of the UCLA Center for Cannabis and Cannabinoids, University of California Los Angeles, said the findings “have direct implications for improving the safety of cannabis, whether it’s being used for medical or nonmedical purposes, especially in people and patients who do not have experience with cannabis, a group that is at high risk for experiencing anxiety after using cannabis.”
In addition, “an important aspect to this study is that the effects of limonene in reducing anxiety attributed to delta-9-THC were observed at higher concentrations (or doses) than those usually present in the plant,” Dr. Copper said. “This calls for further investigation into new cannabis formulations specifically designed to leverage the potential protective effects of the terpene.”
This research was supported by the National Institute on Drug Abuse. Dr. Vandrey served as a consultant or received honoraria from Mira1a Therapeutics, Inc.; Jazz Pharmaceuticals; Charlotte’s Web; Syqe Medical Ltd.; and WebMD. The other authors’ disclosures are listed on the original paper. Dr. Lile declared no relevant financial relationships. Dr. Cooper reported receiving study drug from Canopy Growth Corp and True Terpenes, study-related materials from Storz & Bickel, and research support from the National Institute on Drug Abuse, National Center for Complementary and Integrative Health, California Department of Cannabis Control, Center for Medicinal Cannabis Research, and California Highway Patrol.
A version of this article appeared on Medscape.com.
From Drug and Alcohol Dependence