User login
For MD-IQ use only
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
The Long, Controversial Search for a ‘Cancer Microbiome’
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
Last year, the controversy heightened when experts questioned a high-profile study — a 2020 analysis claiming that the tumors of 33 different cancers had their own unique microbiomes — on whether the “signature” of these bacterial compositions could help diagnose cancer.
The incident renewed the spotlight on “tumor microbiomes” because of the bold claims of the original paper and the strongly worded refutations of those claims. The broader field has focused primarily on ways the body’s microbiome interacts with cancers and cancer treatment.
This controversy has highlighted the challenges of making headway in a field where researchers may not even have the tools yet to puzzle-out the wide-ranging implications the microbiome holds for cancer diagnosis and treatment.
But it is also part of a provocative question within that larger field: whether tumors in the body, far from the natural microbiome in the gut, have their own thriving communities of bacteria, viruses, and fungi. And, if they do, how do those tumor microbiomes affect the development and progression of the cancer and the effectiveness of cancer therapies?
Cancer Controversy
The evidence is undeniable that some microbes can directly cause certain cancers and that the human gut microbiome can influence the effectiveness of certain therapies. Beyond that established science, however, the research has raised as many questions as answers about what we do and don’t know about microbiota and cancer.
The only confirmed microbiomes are on the skin and in the gut, mouth, and vagina, which are all areas with an easy direct route for bacteria to enter and grow in or on the body. A series of papers in recent years have suggested that other internal organs, and tumors within them, may have their own microbiomes.
“Whether microbes exist in tumors of internal organs beyond body surfaces exposed to the environment is a different matter,” said Ivan Vujkovic-Cvijin, PhD, an assistant professor of biomedical sciences and gastroenterology at Cedars-Sinai Medical Center in Los Angeles, whose lab studies how human gut microbes affect inflammatory diseases. “We’ve only recently had the tools to study that question on a molecular level, and the reported results have been conflicting.”
For example, research allegedly identified microbiota in the human placenta nearly one decade ago. But subsequent research contradicted those claims and showed that the source of the “placental microbiome” was actually contamination. Subsequent similar studies for other parts of the body faced the same scrutiny and, often, eventual debunking.
“Most likely, our immune system has undergone selective pressure to eliminate everything that crosses the gut barrier because there’s not much benefit to the body to have bacteria run amok in our internal organs,” Dr. Vujkovic-Cvijin said. “That can only disrupt the functioning of our tissues, to have an external organism living inside them.”
The controversy that erupted last summer, surrounding research from the lab of Rob Knight, PhD, at the University of California, San Diego, centered on a slightly different but related question: Could tumors harbor their own microbiomes?
This news organization spoke with two of the authors who published a paper contesting Dr. Knight’s findings: Steven Salzberg, PhD, a professor of biomedical engineering at John Hopkins Medicine, Baltimore, Maryland, and Abraham Gihawi, PhD, a research fellow at Norwich Medical School at the University of East Anglia in the United Kingdom.
Dr. Salzberg described two major problems with Dr. Knight’s study.
“What they found were false positives because of contamination in the database and flaws in their methods,” Dr. Salzberg said. “I can’t prove there’s no cancer microbiome, but I can say the cancer microbiomes that they reported don’t exist because the species they were finding aren’t there.”
Dr. Knight disagrees with Dr. Salzberg’s findings, noting that Dr. Salzberg and his co-authors did not examine the publicly available databases used in his study. In a written response, he said that his team’s examination of the database revealed that less than 1% of the microbial genomes overlapped with human ones and that removing them did not change their findings.
Dr. Knight also noted that his team could still “distinguish cancer types by their microbiome” even after running their analysis without the technique that Dr. Salzberg found fault with.
Dr. Salzberg said that the database linked above is not the one Dr. Knight’s study used, however. “The primary database in their study was never made public (it’s too large, they said), and it has/had about 69,000 genomes,” Dr. Salzberg said by email. “But even if we did, this is irrelevant. He’s trying to distract from the primary errors in their study,” which Dr. Salzberg said Dr. Knight’s team has not addressed.
The critiques Dr. Salzberg raised have been leveled at other studies investigating microbiomes specifically within tumors and independent of the body’s microbiome.
For example, a 2019 study in Nature described a fungal microbiome in pancreatic cancer that a Nature paper 4 years later directly contradicted, citing flaws that invalidated the original findings. A different 2019 study in Cell examined pancreatic tumor microbiota and patient outcomes, but it’s unclear whether the microorganisms moved from the gut to the pancreas or “constitute a durably colonized community that lives inside the tumor,” which remains a matter of debate, Dr. Vujkovic-Cvijin said.
A 2020 study in Science suggested diverse microbial communities in seven tumor types, but those findings were similarly called into question. That study stated that “bacteria were first detected in human tumors more than 100 years ago” and that “bacteria are well-known residents in human tumors,” but Dr. Salzberg considers those statements misleading.
It’s true that bacteria and viruses have been detected in tumors because “there’s very good evidence that an acute infection caused by a very small number of viruses and bacteria can cause a tumor,” Dr. Salzberg said. Human papillomavirus, for example, can cause six different types of cancer. Inflammation and ulcers caused by Helicobacter pylori may progress to stomach cancer, and Fusobacterium nucleatum and Enterococcus faecalis have been shown to contribute to colorectal cancer. Those examples differ from a microbiome; this “a community of bacteria and possibly other microscopic bugs, like fungi, that are happily living in the tumor” the same way microbes reside in our guts, he said.
Dr. Knight said that many bacteria his team identified “have been confirmed independently in subsequent work.” He acknowledged, however, that more research is needed.
Several of the contested studies above were among a lengthy list that Dr. Knight provided, noting that most of the disagreements “have two sides to them, and critiques from one particular group does not immediately invalidate a reported finding.”
Yet, many of the papers Dr. Knight listed are precisely the types that skeptics like Dr. Salzberg believe are too flawed to draw reliable conclusions.
“I think many agree that microbes may exist within tumors that are exposed to the environment, like tumors of the skin, gut, and mouth,” Dr. Vujkovic-Cvijin said. It’s less clear, however, whether tumors further from the body’s microbiome harbor any microbes or where they came from if they do. Microbial signals in organs elsewhere in the body become faint quickly, he said.
Underdeveloped Technology
Though Dr. Salzberg said that the concept of a tumor microbiome is “implausible” because there’s no easy route for bacteria to reach internal organs, it’s unclear whether scientists have the technology yet to adequately answer this question.
For one thing, samples in these types of studies are typically “ultra-low biomass samples, where the signal — the amount of microbes in the sample — is so low that it’s comparable to how much would be expected to be found in reagents and environmental contamination through processing,” Dr. Vujkovic-Cvijin explained. Many polymerases used to amplify a DNA signal, for example, are made in bacteria and may retain trace amounts identified in these studies.
Dr. Knight agreed that low biomass is a challenge in this field but is not an unsurmountable one.
Another challenge is that study samples, as with Dr. Knight’s work, were collected during routine surgeries without the intent to find a microbial signal. Simply using a scalpel to cut through the skin means cutting through a layer of bacteria, and surgery rooms are not designed to eliminate all bacteria. Some work has even shown there is a “hospital microbiome,” so “you can easily have that creep into your signal and mistake it for tumor-resident bacteria,” Dr. Vujkovic-Cvijin said.
Dr. Knight asserted that the samples are taken under sterile conditions, but other researchers do not think the level of sterility necessary for completely clean samples is possible.
“Just because it’s in your sample doesn’t mean it was in your tumor,” Dr. Gihawi said.
Even if scientists can retrieve a reliable sample without contamination, analyzing it requires comparing the genetic material to existing databases of microbial genomes. Yet, contamination and misclassification of genetic sequences can be problems in those reference genomes too, Dr. Gihawi explained.
Machine learning algorithms have a role in interpreting data, but “we need to be careful of what we use them for,” he added.
“These techniques are in their infancy, and we’re starting to chase them down, which is why we need to move microbiome research in a way that can be used clinically,” Dr. Gihawi said.
Influence on Cancer Treatment Outcomes
Again, however, the question of whether microbiomes exist within tumors is only one slice of the much larger field looking at microbiomes and cancer, including its influence on cancer treatment outcomes. Although much remains to be learned, less controversy exists over the thousands of studies in the past two decades that have gradually revealed how the body’s microbiome can affect both the course of a cancer and the effectiveness of different treatments.
The growing research showing the importance of the gut microbiome in cancer treatments is not surprising given its role in immunity more broadly. Because the human immune system must recognize and defend against microbes, the microbiome helps train it, Dr. Vujkovic-Cvijin said.
Some bacteria can escape the gut — a phenomenon called bacterial translocation — and may aid in fighting tumors. To grow large enough to be seen on imaging, tumors need to evolve several abilities, such as growing enough vascularization to receive blood flow and shutting down local immune responses.
“Any added boost, like immunotherapy, has a chance of breaking through that immune forcefield and killing the tumor cells,” Dr. Vujkovic-Cvijin said. Escaped gut bacteria may provide that boost.
“There’s a lot of evidence that depletion of the gut microbiome impairs immunotherapy and chemotherapy. The thinking behind some of those studies is that gut microbes can cross the gut barrier and when they do, they activate the immune system,” he said.
In mice engineered to have sterile guts, for example, the lack of bacteria results in less effective immune systems, Dr. Vujkovic-Cvijin pointed out. A host of research has shown that antibiotic exposure during and even 6 months before immunotherapy dramatically reduces survival rates. “That’s pretty convincing to me that gut microbes are important,” he said.
Dr. Vujkovic-Cvijin cautioned that there continues to be controversy on understanding which bacteria are important for response to immunotherapy. “The field is still in its infancy in terms of understanding which bacteria are most important for these effects,” he said.
Dr. Knight suggested that escaped bacteria may be the genesis of the ones that he and other researchers believe exist in tumors. “Because tumor microbes must come from somewhere, it is to be expected that some of those microbes will be co-opted from body-site specific commensals.”
It’s also possible that metabolites released from gut bacteria escape the gut and could theoretically affect distant tumor growth, Dr. Gihawi said. The most promising avenue of research in this area is metabolites being used as biomarkers, added Dr. Gihawi, whose lab published research on a link between bacteria detected in men’s urine and a more aggressive subset of prostate cancers. But that research is not far enough along to develop lab tests for clinical use, he noted.
No Consensus Yet
Even before the controversy erupted around Dr. Knight’s research, he co-founded the company Micronoma to develop cancer tests based on his microbe findings. The company has raised $17.5 million from private investors as of August 2023 and received the US Food and Drug Administration’s Breakthrough Device designation, allowing the firm to fast-track clinical trials testing the technology. The recent critiques have not changed the company’s plans.
It’s safe to say that scientists will continue to research and debate the possibility of tumor microbiomes until a consensus emerges.
“The field is evolving and studies testing the reproducibility of tumor-resident microbial signals are essential for developing our understanding in this area,” Dr. Vujkovic-Cvijin said.
Even if that path ultimately leads nowhere, as Dr. Salzberg expects, research into microbiomes and cancer has plenty of other directions to go.
“I’m actually quite an optimist,” Dr. Gihawi said. “I think there’s a lot of scope for some really good research here, especially in the sites where we know there is a strong microbiome, such as the gastrointestinal tract.”
A version of this article appeared on Medscape.com.
The AGA Future Leaders Program: A Mentee-Mentor Triad Perspective
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.
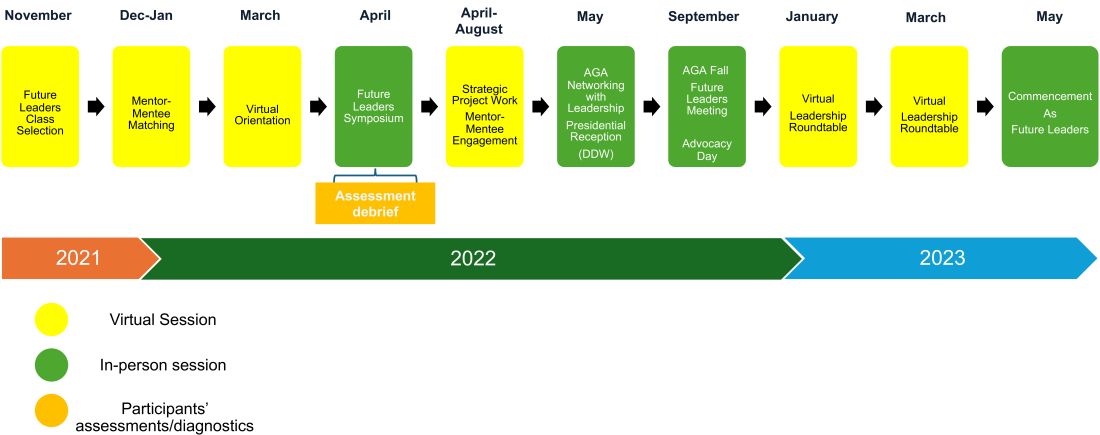
We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Navigating the Search for a Financial Adviser
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?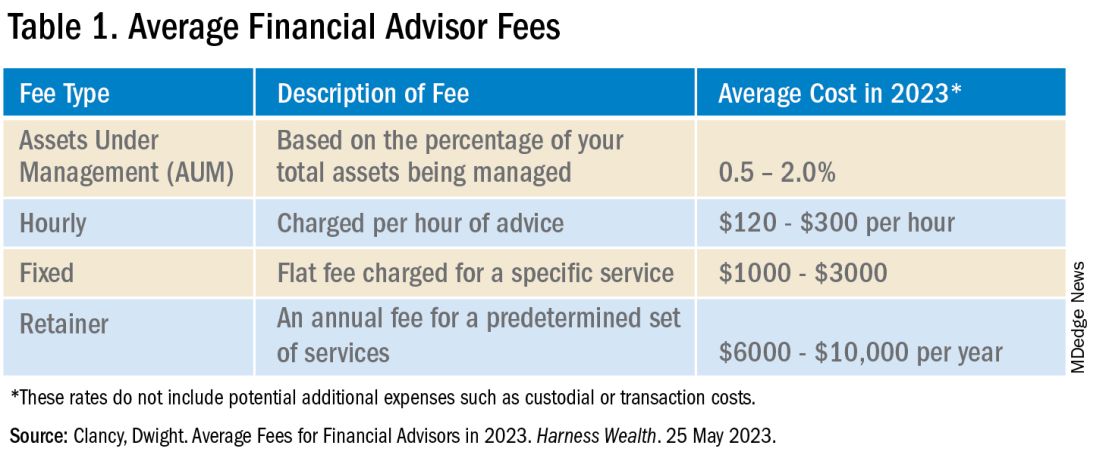
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
Achieving Promotion for Junior Faculty in Academic Medicine: An Interview With Experts
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
A Simplified Approach to Pelvic Floor Dysfunction
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).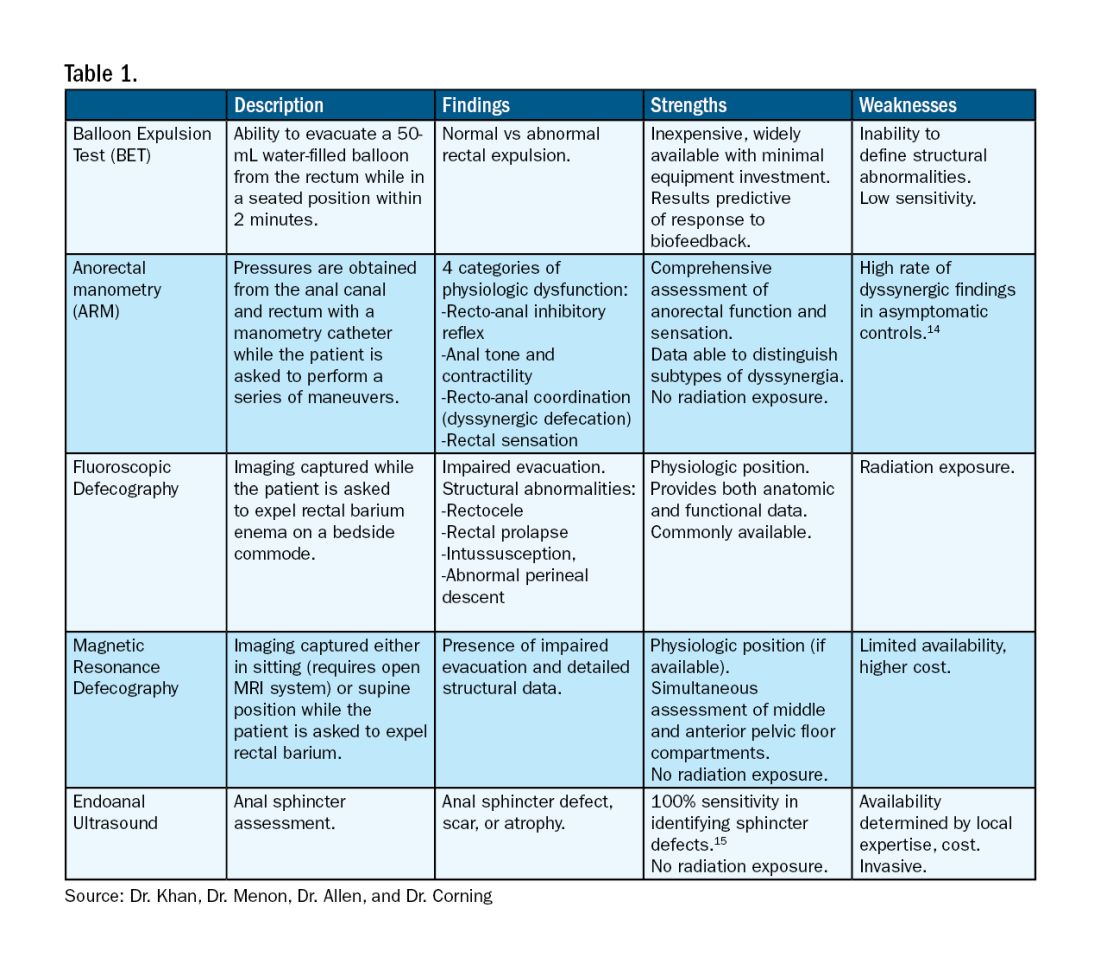
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Defining Your ‘Success’
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
GI Doc Aims to Lift Barriers to CRC Screening for Black Patients
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
In gastroenterology, a good bedside manner is a vital attribute. Visiting with an anxious patient before a colonoscopy, Adjoa Anyane-Yeboa, MD, MPH, knew what to say to calm him down.
“I could tell he was really nervous about the procedure, even though he wasn’t letting on,” said Dr. Anyane-Yeboa, a gastroenterologist with Massachusetts General Hospital in Boston. She put him at ease by cracking jokes and making him smile during the consent process. After it was over, he thanked her for making him feel more comfortable.
“I will have it done again, and I’ll come back to you next time,” said the patient.
GI doctors perform colonoscopies all day, every day, “so we sometimes forget how nervous people are. But it’s nice to be able to connect with people and put them at ease,” she said.
Interacting with patients gives her joy. Addressing health disparities is her long-term goal. Dr. Anyane-Yeboa’s research has focused on the barriers to colorectal cancer screening in the Black population, as well as disparities in inflammatory bowel disease (IBD).
“I think there’s a lot that still needs to be done around colorectal cancer screening,” she said.
In an interview, she talks more in depth about her research and her ongoing work to increase public knowledge and awareness about colorectal cancer screening.
Q: Why did you choose GI?
Dr. Anyane-Yeboa: When I got to residency, GI was the rotation that was the most fun. I was the most excited to read about it, the most excited to go to work the next day.
I remember people saying, “You should look at the people who are in the field and look at their personalities, and then think about which personalities match you best.” In residency I considered hematology, cardiology, and GI. The cardiologists were so serious, so intense, talking about research methods all the time. Whereas, the GI folks were joking, laughing, making fart jokes. I felt like these were my people, lighthearted and easy-going. And I genuinely enjoyed going to work every day and learning about the disorders of the GI tract. I still do to this day.
Q: Let’s discuss your research with IBD in Black populations and colorectal cancer screening.
Dr. Anyane-Yeboa: My two main areas of work are in IBD and minority populations, predominantly Black populations, and in colorectal cancer screening in minority populations, and again, mostly in historically marginalized populations.
With colon cancer, we know that there are disparities with incidence in mortality. Black individuals have had the second highest incidence in mortality from colorectal cancer. For me, being a Black female physician and seeing people who look like me, time and time again, being diagnosed with colorectal cancer and dying is really what drives me, because in GI, colon cancer screening is our bread and butter.
Some of the work that I’m doing now around colorectal cancer is in predominantly Black community health centers, working on increasing colorectal cancer screening rates in this population, and figuring out what the barriers are to screening and how we can address them, and what are some strategies that will work in a health center setting to get people screened.
Q: One study of yours surveyed unscreened Black individuals age ≥ 45 and found age-specific barriers to CRC screening in this population, as well as a lack of targeted messaging to incentivize screening.
Dr. Anyane-Yeboa: That mixed method study was done in partnership with the National Colorectal Cancer Roundtable and American Cancer Society.
In that study, we found that the most common barrier to screening was self-procrastination or delay of screening, meaning, “I’m going to get screened, just not right now.” It’s not a priority. What was unique about this is we looked at it from age breakdown, so 45-49, 50-54, 55-plus. With the younger 45-49 group, we don’t know as much about how to get them screened. We also saw that healthcare providers weren’t starting conversations about screening with these younger newly eligible patients.
We also described effective messages to get people screened in that paper as well.
Q: What changes would you like to see going forward with screening? What still needs to happen?
Dr. Anyane-Yeboa: In some of the other work that I’ve done, particularly with the health centers and younger populations interviewed in focus groups, I’m seeing that those who are younger don’t really know much about colorectal cancer screening. Those who do know about it have seen commercials about popular stool-based testing brands, and that’s how they’ve learned about screening.
What I would like to see is ways to increase the knowledge and awareness about colorectal cancer screening and colorectal cancer on a broad scale, on a more national, public-facing scale. Because I’m realizing that if they’re healthy young folks who aren’t going to the physician, who don’t have a primary care provider, then they might not even really hear about colorectal cancer screening. We need ways to educate the general public so individuals can advocate for themselves around screening.
I also want to see more providers discussing screening with all patients, starting from those 45-49, and younger if they have a family history. Providers should screen every single patient that they see. We know that every single person should be screened at 45 and older, and not all providers, surprisingly, are discussing it with their patients.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
Dr. Anyane-Yeboa: Saturday morning is my favorite time of the week. I’m either catching up on my TV shows, or I might be on a walk with my dog, particularly in the afternoon. I live near an arboretum, so I usually walk through there on the weekend afternoons. I also might be trying out a new restaurant with my friends. I love traveling, so I might also be sightseeing in another country.
Lightning Round
Texting or talking?
Texting
Favorite junk food?
Cookies
Cat or dog person?
Both; love cats, have a dog
If you weren’t a gastroenterologist, what would you be?
Fashion boutique owner
Best place you’ve traveled to?
Morocco
How many cups of coffee do you drink per day?
Two
Favorite ice cream?
Don’t eat ice cream, only cookies
Favorite sport?
Tennis
Optimist or pessimist?
Optimist (glass half full)
Converging on Our Nation’s Capital
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Recurrent Soft Tissue Rosai Dorfman Disease of Right Medial Thigh Lipoma With Lymph Node Involvement
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531