User login
For MD-IQ use only
Management of Acute Opioid Toxicity in the Outpatient Setting
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Practice Points
- Opioid overdose continues to be a major public health concern. Dermatologists may encounter opioid toxicity in their practice, and prompt recognition and treatment are crucial.
- Naloxone is a quick-acting, easy-to-use, and relatively inexpensive medication that can easily be stored and administered in dermatologists’ offices
HM20 Virtual product theaters: Aug. 18-20
Aug. 18, 2020. 12:00 p.m. – 1:00 p.m. ET
Selecting A First-Choice Therapy for Systolic HF: Meeting the Burden of Proof
Speaker:
Javed Butler, MD, MPH, MBA
Chairman, Department of Medicine
University of Mississippi Medical Center, Jackson
Program description:
What is the burden of proof that needs to be met before a therapy can be selected for the treatment of systolic heart failure (HF)? Hear from Dr. Javed Butler, chairman of the department of medicine at the University of Mississippi Medical Center, Jackson, to learn more about selecting a first-choice therapy for your patients with systolic heart failure.
In this program, Dr. Butler will discuss how aligning your therapy selection to pathophysiologic pathways for heart failure with reduced ejection fraction (HFrEF), it is possible to reduce mortality and morbidity while providing a proven safety and tolerability profile.
Regardless of your patients’ previous HF treatment history, following this program, you can feel confident selecting your first-choice therapy for your patients with HFrEF.
Sponsored by Novartis Pharmaceuticals Corporation, and the faculty will be compensated for his or her time.
Aug. 19, 2020. 12:00 p.m.– 1:00 p.m. ET
COVID-19 and Beyond: Integrating Mobile Messaging and Patient Records for Inpatient Care Team Collaboration
Speaker:
Christopher Maiona, MD
Chief Medical Officer
PatientKeeper
Program description:
In this stressful and unpredictable time for hospitalists (and all clinicians), focusing hospital investments where they have the most immediate impact on patient care is more vital than ever. Of all the technology capabilities a hospital might consider implementing today, none would be more valuable to hospitalists than MOBILITY ... because instant access to patient records and care team colleagues – anytime, anywhere, from their smartphones and tablets – will provide a direct and immediate benefit to providers and patients.
In this HM20 Virtual Product Theater, you’ll discover that adding mobility and instant communications in a manner that intuitively supports hospitalist workflow is not only possible, it’s a relatively easy lift. We will introduce the PatientKeeper Clinical Communications Suite and demonstrate how it lets providers:
- Immediately access patient records via native iOS and Android apps on smartphones and tablets
- Securely instant message care team members, consultants, practice administrators, and any other necessary hospital staff, with embedded patient context
- Share quick notes about patients with other providers using a simple “scratch pad” to capture the most salient points -- ideal for handing off to coverage and/or in a high-volume, high-throughput crisis care/triage environment
- Treat more patients, more expeditiously
Sponsored by PatientKeeper
Aug. 20, 2020. 12:00 p.m. – 1:00 p.m. ET
The PRODIGY Study and the PRODIGY Risk Prediction Tool: First Step Toward Improving Outcomes and Reducing Costs
Speakers:
Sabry Ayad, MD
Cleveland Clinic
Roop Kaw, MD
Cleveland Clinic
Objectives:
- Describe implementation strategy for continuous respiratory monitoring
- Discuss the challenges associated with predicting respiratory compromise postoperatively
- Recognize patients at risk for respiratory compromise
- Introduce evidence-based guidelines for monitoring patients for OIRD
- Identify methods to operationalize and integrate best risk stratification and monitoring practices into your facility
Sponsored by Medtronic
Aug. 18, 2020. 12:00 p.m. – 1:00 p.m. ET
Selecting A First-Choice Therapy for Systolic HF: Meeting the Burden of Proof
Speaker:
Javed Butler, MD, MPH, MBA
Chairman, Department of Medicine
University of Mississippi Medical Center, Jackson
Program description:
What is the burden of proof that needs to be met before a therapy can be selected for the treatment of systolic heart failure (HF)? Hear from Dr. Javed Butler, chairman of the department of medicine at the University of Mississippi Medical Center, Jackson, to learn more about selecting a first-choice therapy for your patients with systolic heart failure.
In this program, Dr. Butler will discuss how aligning your therapy selection to pathophysiologic pathways for heart failure with reduced ejection fraction (HFrEF), it is possible to reduce mortality and morbidity while providing a proven safety and tolerability profile.
Regardless of your patients’ previous HF treatment history, following this program, you can feel confident selecting your first-choice therapy for your patients with HFrEF.
Sponsored by Novartis Pharmaceuticals Corporation, and the faculty will be compensated for his or her time.
Aug. 19, 2020. 12:00 p.m.– 1:00 p.m. ET
COVID-19 and Beyond: Integrating Mobile Messaging and Patient Records for Inpatient Care Team Collaboration
Speaker:
Christopher Maiona, MD
Chief Medical Officer
PatientKeeper
Program description:
In this stressful and unpredictable time for hospitalists (and all clinicians), focusing hospital investments where they have the most immediate impact on patient care is more vital than ever. Of all the technology capabilities a hospital might consider implementing today, none would be more valuable to hospitalists than MOBILITY ... because instant access to patient records and care team colleagues – anytime, anywhere, from their smartphones and tablets – will provide a direct and immediate benefit to providers and patients.
In this HM20 Virtual Product Theater, you’ll discover that adding mobility and instant communications in a manner that intuitively supports hospitalist workflow is not only possible, it’s a relatively easy lift. We will introduce the PatientKeeper Clinical Communications Suite and demonstrate how it lets providers:
- Immediately access patient records via native iOS and Android apps on smartphones and tablets
- Securely instant message care team members, consultants, practice administrators, and any other necessary hospital staff, with embedded patient context
- Share quick notes about patients with other providers using a simple “scratch pad” to capture the most salient points -- ideal for handing off to coverage and/or in a high-volume, high-throughput crisis care/triage environment
- Treat more patients, more expeditiously
Sponsored by PatientKeeper
Aug. 20, 2020. 12:00 p.m. – 1:00 p.m. ET
The PRODIGY Study and the PRODIGY Risk Prediction Tool: First Step Toward Improving Outcomes and Reducing Costs
Speakers:
Sabry Ayad, MD
Cleveland Clinic
Roop Kaw, MD
Cleveland Clinic
Objectives:
- Describe implementation strategy for continuous respiratory monitoring
- Discuss the challenges associated with predicting respiratory compromise postoperatively
- Recognize patients at risk for respiratory compromise
- Introduce evidence-based guidelines for monitoring patients for OIRD
- Identify methods to operationalize and integrate best risk stratification and monitoring practices into your facility
Sponsored by Medtronic
Aug. 18, 2020. 12:00 p.m. – 1:00 p.m. ET
Selecting A First-Choice Therapy for Systolic HF: Meeting the Burden of Proof
Speaker:
Javed Butler, MD, MPH, MBA
Chairman, Department of Medicine
University of Mississippi Medical Center, Jackson
Program description:
What is the burden of proof that needs to be met before a therapy can be selected for the treatment of systolic heart failure (HF)? Hear from Dr. Javed Butler, chairman of the department of medicine at the University of Mississippi Medical Center, Jackson, to learn more about selecting a first-choice therapy for your patients with systolic heart failure.
In this program, Dr. Butler will discuss how aligning your therapy selection to pathophysiologic pathways for heart failure with reduced ejection fraction (HFrEF), it is possible to reduce mortality and morbidity while providing a proven safety and tolerability profile.
Regardless of your patients’ previous HF treatment history, following this program, you can feel confident selecting your first-choice therapy for your patients with HFrEF.
Sponsored by Novartis Pharmaceuticals Corporation, and the faculty will be compensated for his or her time.
Aug. 19, 2020. 12:00 p.m.– 1:00 p.m. ET
COVID-19 and Beyond: Integrating Mobile Messaging and Patient Records for Inpatient Care Team Collaboration
Speaker:
Christopher Maiona, MD
Chief Medical Officer
PatientKeeper
Program description:
In this stressful and unpredictable time for hospitalists (and all clinicians), focusing hospital investments where they have the most immediate impact on patient care is more vital than ever. Of all the technology capabilities a hospital might consider implementing today, none would be more valuable to hospitalists than MOBILITY ... because instant access to patient records and care team colleagues – anytime, anywhere, from their smartphones and tablets – will provide a direct and immediate benefit to providers and patients.
In this HM20 Virtual Product Theater, you’ll discover that adding mobility and instant communications in a manner that intuitively supports hospitalist workflow is not only possible, it’s a relatively easy lift. We will introduce the PatientKeeper Clinical Communications Suite and demonstrate how it lets providers:
- Immediately access patient records via native iOS and Android apps on smartphones and tablets
- Securely instant message care team members, consultants, practice administrators, and any other necessary hospital staff, with embedded patient context
- Share quick notes about patients with other providers using a simple “scratch pad” to capture the most salient points -- ideal for handing off to coverage and/or in a high-volume, high-throughput crisis care/triage environment
- Treat more patients, more expeditiously
Sponsored by PatientKeeper
Aug. 20, 2020. 12:00 p.m. – 1:00 p.m. ET
The PRODIGY Study and the PRODIGY Risk Prediction Tool: First Step Toward Improving Outcomes and Reducing Costs
Speakers:
Sabry Ayad, MD
Cleveland Clinic
Roop Kaw, MD
Cleveland Clinic
Objectives:
- Describe implementation strategy for continuous respiratory monitoring
- Discuss the challenges associated with predicting respiratory compromise postoperatively
- Recognize patients at risk for respiratory compromise
- Introduce evidence-based guidelines for monitoring patients for OIRD
- Identify methods to operationalize and integrate best risk stratification and monitoring practices into your facility
Sponsored by Medtronic
Stress-induced brain activity linked to chest pain in CAD patients
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
New SHM research on EMRs calls for ‘more caring, less clicking’
White paper offers concrete recommendations
One of the most significant shifts in hospital practice over recent decades has been the widespread adoption of electronic medical records as a replacement for conventional paper records.
While EMRs show a lot of promise – having the potential to centralize and simplify clinician notes, make information more accessible and reduce paper waste – there is strong evidence that they are not working as well as they could.
Some research suggests that these systems may decrease the working efficiency of clinicians. Now, major health care institutions are looking to understand why these systems are not working — as well as how they may be improved.
A recent white paper from the Society of Hospital Medicine’s Healthcare Information Technology Special Interest Group – titled “More Caring, Less Clicking” – reviews the current shortcomings of EMRs from a hospitalist perspective and provides recommendations for how these systems can be made more workable and efficient.
The current state of EMRs
“Numerous previous papers – including SHM’s 2017 white paper ‘Hospitalist Perspectives on Electronic Medical Records’ – have linked EMRs to decreased provider satisfaction and increased burnout related to multiple issues, including an increase in ‘screen time’ as opposed to patient ‘face-to-face’ time, and limitations in usability and interoperability,” said Rupesh Prasad, MD, SFHM, medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. “Studies have shown that most of a provider’s time spent is in areas like clinical documentation, entry of orders, and accessing patient information.”
The 2017 SHM white paper referenced by Dr. Prasad reported that 74% of hospitalists surveyed were dissatisfied with their EMR. A full one-quarter of surveyed physicians went so far as saying they would prefer switching to paper record keeping.
Other research has also found a possible link between EMRs and physician burnout and dissatisfaction. It is also not uncommon for hospitalists to spend up to 25% of their time at work using their EMR – time that should, ideally, be spent with patients.
The 2017 paper also showed that clinician notes in the United States are four times longer, on average, than notes in other countries. There are a few reasons for this – including technology design and billing requirements encouraging longer notes. Whatever the cause, however, longer notes linked to physician burnout may be partially responsible for the large amounts of time physicians spend looking at EMRs.
While EMRs may hold significant potential for hospitalists, as they are designed currently, they are simply not delivering the value many expected. The new white paper from the Healthcare Information Technology Special Interest Group outlines practical changes that could be made to EMRs to improve their use in hospitals.
The paper breaks down current issues with EMRs into five broad categories – documentation, clinical decision support, order entry, communication, and data review – to discuss how EMRs are currently failing in these areas as well as how they might be improved.
Improving EMR documentation
One of the most significant hurdles clinicians currently face lies in how EMRs currently store and display documentation. Combined with physician note-taking habits, this makes these systems much less usable than they could be. Longer notes, when displayed in current EMR UIs, mostly lead to clutter, making them harder to navigate and difficult to scan quickly for important information.
The authors identify a few different ways that future EMRs may be able to help with this problem.
EMR documentation tools will likely need to be redesigned to optimize documentation entry, standardize note formatting, and improve readability. Many electronic notes contain vestigial formatting and data left over from the design of paper notes. As a result, many of these electronic notes include information that is stored elsewhere and does not need to be explicitly included in every note. Cutting down on repetitive information storage will make important information more visible and help make patient notes easier to scan.
The paper also recommends a few other features that would make documentation more readable – like allowing clinicians to write documentation in SOAP format (subjective, objective, assessment, and plan), to facilitate critical thinking during the note-taking process, and having the EMR display that documentation in APSO format (assessment, plan, subjective, objective).
Doctors have long called for APSO or another note-taking format to replace SOAP in EMRs. Designing EMRs to rearrange SOAP notes to APSO could be a compromise that improves note readability while not requiring that clinicians learn new note-taking strategies.
The paper’s authors also recommended more extensive clinician training on writing notes. While clinicians are often taught how to write certain notes – like progress notes, histories, and physical and discharge summaries – more specific guidance is not always provided. Better training provided by institutions could help improve the quality and readability of clinician notes.
These changes, however, may not be as beneficial as possible without better institutional support for clinicians. Implementing some of the biggest changes recommended by the SHM will require some level of standardization across platforms and institution commitment to training clinicians on best use practices for EMRs. Improved responsiveness to clinician needs will require a coordinated effort with backing from both administrative and governance groups.
Expanding EMR usability
“Our white paper presents evidence-based recommendations that can be implemented at the ground level in collaboration with other stakeholders, including IT, informatics, and administration, to help improve on the current state,” Dr. Prasad said.
“We believe that hospitalists as key stakeholders in health care, have both the responsibility and are uniquely positioned to directly impact EMR functionality,” he noted. “For example, hospitalists can participate in designing appropriate, actionable alerts that would help with patient safety while also improving provider efficiency. Simple steps like limiting hard stops in order entry to would help speed up the process, and free up time for direct patient care. Availability of tools like secure text messaging would help with effective patient care team communication to improve safety and care delivery.”
EMRs often lack features like voice control and speech-to-text transcription, along with other basic accessibility features like compatibility with screen readers. Implementing these features could improve the efficiency of clinicians’ note-taking while also providing wider software usability.
EMRs are not typically designed to work with mobile devices, meaning clinicians cannot enter notes or order medications until they’ve returned to their desk or workstation.
This lack of functionality creates issues in several ways. When clinicians are unable to enter notes on the move, they will need to either keep mental notes or quickly jot down paper notes. This can effectively double the amount of note-taking that clinicians must do or introduce greater room for error. In cases where progress notes are taken throughout the day, this also means the EMR’s documentation timeline may not be accurate or usable.
Requiring clinicians to return to workstations before entering order information can also increase the risk of medication errors, which remains high despite hopes that EMRs could reduce error rates.
Adding support for cross-device and mobile EMR use could help improve the efficiency of note-taking and help cut down on error. Implementing mobile access could have a few different benefits for clinicians – like improving note-taking efficiency in hospitals, where doctors often see patients far away from their workstations.
EMRs also often lack support for certain hardware, like mobile stations and widescreen monitors, which can improve a clinician’s ability to document in real-time and are a better fit in certain work flows.
The SHM paper also recommends a few other tweaks to usability – like reducing the amount of password entry and reentry – that could make these systems easier to use and more efficient.
New features – like the use of natural language processing technology to analyze and organize information contained in clinicians’ notes – could provide further benefits and take full advantage of the advanced technologies that EMRs can integrate.
Dr. Prasad noted, however, that some of these upgrades – especially EMR compatibility with mobile devices – will require some institutional support. Bring-your-own-device policies or system-provided mobile devices will be necessary if institutions want their clinicians to be able to take advantage of mobile EMR access.
These policies will also likely require some kind of mobile device management solution to manage the security of sensitive patient data as it is accessed from personal devices. This may increase the level of necessary institutional buy-in for this support to work.
Designing EMRs with clinician needs in mind
Dr. Prasad said he and his coauthors recommend that EMR developers base more of their design on the needs of clinicians.
Currently, EMR interfaces can make important data unavailable, depending on what a clinician is trying to do. As a result, clinicians often need to rely on mental recall of important information as they navigate EMR systems.
These interfaces also typically do not support any level of user customization or process-specific interfaces, meaning every clinician is working with the same interface regardless of the tasks they need to perform or the information they need access to. Allowing for customization or implementing new process- or disease-specific interfaces could help avoid some of the problems caused by one-size-fits-all interfaces, which are not necessarily compatible with every clinician work flow.
EMR interfaces should also be designed, wherever possible, with familiar or standardized formats and the use of color coding and other techniques that can make interfaces easier to navigate quickly. Right now, many EMR systems utilize inconsistent layout design that can be cluttered with irrelevant information, slowing down interface navigation and sometimes requiring backtracking from clinicians.
Ideally, this will improve the speed of information gathering and data review, reducing the amount of time clinicians need to spend working with their EMR.
The white paper also recommends that EMR designers improve alert systems so that they are more actionable and interrupt clinicians less often – and that, when they do, they ensure that clinicians can respond to them. Designers should also reduce hard-stops or in-line alerts that halt clinicians’ work flows and require immediate responses where possible.
Increased EMR support for clinical decision support systems is one of the biggest health care trends expected to be seen throughout this decade. However, many clinicians are disappointed with the lack of flexibility and optimization of the current alerts that CDS provides. Updating and improving these knowledge-based systems will likely become essential for delivering better alerts and improving decision-making and efficiency.
Overall, EMR design should be informed by the needs of the people these products are designed to support, Dr. Prasad said. The people that work with EMRs – especially frontline staff like providers, nurses, and pharmacists that regularly interact with EMRs to provide care – should be involved early on in the EMR design process. Right now, their needs are not reflected in current EMR design. EMR companies, by working with these hospital staff members, could help improve ease of use and, ideally, prevent some of the errors associated with the current implementation of these systems.
“System designers should be able to avoid some of the most common problems of EMRs – and predict potential problems – by consistently soliciting and integrating clinician feedback during the design process and over the lifespan of a product,” Dr. Prasad said.
How EMRs can be improved
Over the past few years, EMRs have become quickly adopted by health care professionals and institutions. However, despite hopes that EMRs could significantly improve record keeping and note-taking, these systems continue to pose serious challenges for the clinicians who use them. Evidence from recent research suggests that these systems are inefficient and may contribute to physician burnout.
As a result, organizations like SHM are looking for ways that these systems can be improved.
“The growth of health IT has led to availability of large amounts of data and opportunities for applications in [artificial intelligence and machine learning,” Dr. Prasad noted. “While this has opened many avenues to help positively impact patient care and outcomes, it also poses multiple challenges like validation, customization, and governance. Hospitalists can partner with other health professions and IT leaders to work toward the common goal of improving the health of the population while also providing a positive experience to the end user.”
Another problem with current EMRs is their lack of flexibility. These systems are often not compatible with mobile devices and certain types of hardware and may be difficult or impossible to customize. They also frequently require unnecessary information during the note-taking process that results in cluttered and difficult-to-scan documentation. Improving EMR flexibility – and inviting clinicians to consult during the design process – could solve many of these problems.
New technological developments may also soon help developers improve their EMRs. In the future, as technology like natural language processing becomes more advanced and more commonly used, they may be able to make EMRs even more efficient and user friendly.
White paper offers concrete recommendations
White paper offers concrete recommendations
One of the most significant shifts in hospital practice over recent decades has been the widespread adoption of electronic medical records as a replacement for conventional paper records.
While EMRs show a lot of promise – having the potential to centralize and simplify clinician notes, make information more accessible and reduce paper waste – there is strong evidence that they are not working as well as they could.
Some research suggests that these systems may decrease the working efficiency of clinicians. Now, major health care institutions are looking to understand why these systems are not working — as well as how they may be improved.
A recent white paper from the Society of Hospital Medicine’s Healthcare Information Technology Special Interest Group – titled “More Caring, Less Clicking” – reviews the current shortcomings of EMRs from a hospitalist perspective and provides recommendations for how these systems can be made more workable and efficient.
The current state of EMRs
“Numerous previous papers – including SHM’s 2017 white paper ‘Hospitalist Perspectives on Electronic Medical Records’ – have linked EMRs to decreased provider satisfaction and increased burnout related to multiple issues, including an increase in ‘screen time’ as opposed to patient ‘face-to-face’ time, and limitations in usability and interoperability,” said Rupesh Prasad, MD, SFHM, medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. “Studies have shown that most of a provider’s time spent is in areas like clinical documentation, entry of orders, and accessing patient information.”
The 2017 SHM white paper referenced by Dr. Prasad reported that 74% of hospitalists surveyed were dissatisfied with their EMR. A full one-quarter of surveyed physicians went so far as saying they would prefer switching to paper record keeping.
Other research has also found a possible link between EMRs and physician burnout and dissatisfaction. It is also not uncommon for hospitalists to spend up to 25% of their time at work using their EMR – time that should, ideally, be spent with patients.
The 2017 paper also showed that clinician notes in the United States are four times longer, on average, than notes in other countries. There are a few reasons for this – including technology design and billing requirements encouraging longer notes. Whatever the cause, however, longer notes linked to physician burnout may be partially responsible for the large amounts of time physicians spend looking at EMRs.
While EMRs may hold significant potential for hospitalists, as they are designed currently, they are simply not delivering the value many expected. The new white paper from the Healthcare Information Technology Special Interest Group outlines practical changes that could be made to EMRs to improve their use in hospitals.
The paper breaks down current issues with EMRs into five broad categories – documentation, clinical decision support, order entry, communication, and data review – to discuss how EMRs are currently failing in these areas as well as how they might be improved.
Improving EMR documentation
One of the most significant hurdles clinicians currently face lies in how EMRs currently store and display documentation. Combined with physician note-taking habits, this makes these systems much less usable than they could be. Longer notes, when displayed in current EMR UIs, mostly lead to clutter, making them harder to navigate and difficult to scan quickly for important information.
The authors identify a few different ways that future EMRs may be able to help with this problem.
EMR documentation tools will likely need to be redesigned to optimize documentation entry, standardize note formatting, and improve readability. Many electronic notes contain vestigial formatting and data left over from the design of paper notes. As a result, many of these electronic notes include information that is stored elsewhere and does not need to be explicitly included in every note. Cutting down on repetitive information storage will make important information more visible and help make patient notes easier to scan.
The paper also recommends a few other features that would make documentation more readable – like allowing clinicians to write documentation in SOAP format (subjective, objective, assessment, and plan), to facilitate critical thinking during the note-taking process, and having the EMR display that documentation in APSO format (assessment, plan, subjective, objective).
Doctors have long called for APSO or another note-taking format to replace SOAP in EMRs. Designing EMRs to rearrange SOAP notes to APSO could be a compromise that improves note readability while not requiring that clinicians learn new note-taking strategies.
The paper’s authors also recommended more extensive clinician training on writing notes. While clinicians are often taught how to write certain notes – like progress notes, histories, and physical and discharge summaries – more specific guidance is not always provided. Better training provided by institutions could help improve the quality and readability of clinician notes.
These changes, however, may not be as beneficial as possible without better institutional support for clinicians. Implementing some of the biggest changes recommended by the SHM will require some level of standardization across platforms and institution commitment to training clinicians on best use practices for EMRs. Improved responsiveness to clinician needs will require a coordinated effort with backing from both administrative and governance groups.
Expanding EMR usability
“Our white paper presents evidence-based recommendations that can be implemented at the ground level in collaboration with other stakeholders, including IT, informatics, and administration, to help improve on the current state,” Dr. Prasad said.
“We believe that hospitalists as key stakeholders in health care, have both the responsibility and are uniquely positioned to directly impact EMR functionality,” he noted. “For example, hospitalists can participate in designing appropriate, actionable alerts that would help with patient safety while also improving provider efficiency. Simple steps like limiting hard stops in order entry to would help speed up the process, and free up time for direct patient care. Availability of tools like secure text messaging would help with effective patient care team communication to improve safety and care delivery.”
EMRs often lack features like voice control and speech-to-text transcription, along with other basic accessibility features like compatibility with screen readers. Implementing these features could improve the efficiency of clinicians’ note-taking while also providing wider software usability.
EMRs are not typically designed to work with mobile devices, meaning clinicians cannot enter notes or order medications until they’ve returned to their desk or workstation.
This lack of functionality creates issues in several ways. When clinicians are unable to enter notes on the move, they will need to either keep mental notes or quickly jot down paper notes. This can effectively double the amount of note-taking that clinicians must do or introduce greater room for error. In cases where progress notes are taken throughout the day, this also means the EMR’s documentation timeline may not be accurate or usable.
Requiring clinicians to return to workstations before entering order information can also increase the risk of medication errors, which remains high despite hopes that EMRs could reduce error rates.
Adding support for cross-device and mobile EMR use could help improve the efficiency of note-taking and help cut down on error. Implementing mobile access could have a few different benefits for clinicians – like improving note-taking efficiency in hospitals, where doctors often see patients far away from their workstations.
EMRs also often lack support for certain hardware, like mobile stations and widescreen monitors, which can improve a clinician’s ability to document in real-time and are a better fit in certain work flows.
The SHM paper also recommends a few other tweaks to usability – like reducing the amount of password entry and reentry – that could make these systems easier to use and more efficient.
New features – like the use of natural language processing technology to analyze and organize information contained in clinicians’ notes – could provide further benefits and take full advantage of the advanced technologies that EMRs can integrate.
Dr. Prasad noted, however, that some of these upgrades – especially EMR compatibility with mobile devices – will require some institutional support. Bring-your-own-device policies or system-provided mobile devices will be necessary if institutions want their clinicians to be able to take advantage of mobile EMR access.
These policies will also likely require some kind of mobile device management solution to manage the security of sensitive patient data as it is accessed from personal devices. This may increase the level of necessary institutional buy-in for this support to work.
Designing EMRs with clinician needs in mind
Dr. Prasad said he and his coauthors recommend that EMR developers base more of their design on the needs of clinicians.
Currently, EMR interfaces can make important data unavailable, depending on what a clinician is trying to do. As a result, clinicians often need to rely on mental recall of important information as they navigate EMR systems.
These interfaces also typically do not support any level of user customization or process-specific interfaces, meaning every clinician is working with the same interface regardless of the tasks they need to perform or the information they need access to. Allowing for customization or implementing new process- or disease-specific interfaces could help avoid some of the problems caused by one-size-fits-all interfaces, which are not necessarily compatible with every clinician work flow.
EMR interfaces should also be designed, wherever possible, with familiar or standardized formats and the use of color coding and other techniques that can make interfaces easier to navigate quickly. Right now, many EMR systems utilize inconsistent layout design that can be cluttered with irrelevant information, slowing down interface navigation and sometimes requiring backtracking from clinicians.
Ideally, this will improve the speed of information gathering and data review, reducing the amount of time clinicians need to spend working with their EMR.
The white paper also recommends that EMR designers improve alert systems so that they are more actionable and interrupt clinicians less often – and that, when they do, they ensure that clinicians can respond to them. Designers should also reduce hard-stops or in-line alerts that halt clinicians’ work flows and require immediate responses where possible.
Increased EMR support for clinical decision support systems is one of the biggest health care trends expected to be seen throughout this decade. However, many clinicians are disappointed with the lack of flexibility and optimization of the current alerts that CDS provides. Updating and improving these knowledge-based systems will likely become essential for delivering better alerts and improving decision-making and efficiency.
Overall, EMR design should be informed by the needs of the people these products are designed to support, Dr. Prasad said. The people that work with EMRs – especially frontline staff like providers, nurses, and pharmacists that regularly interact with EMRs to provide care – should be involved early on in the EMR design process. Right now, their needs are not reflected in current EMR design. EMR companies, by working with these hospital staff members, could help improve ease of use and, ideally, prevent some of the errors associated with the current implementation of these systems.
“System designers should be able to avoid some of the most common problems of EMRs – and predict potential problems – by consistently soliciting and integrating clinician feedback during the design process and over the lifespan of a product,” Dr. Prasad said.
How EMRs can be improved
Over the past few years, EMRs have become quickly adopted by health care professionals and institutions. However, despite hopes that EMRs could significantly improve record keeping and note-taking, these systems continue to pose serious challenges for the clinicians who use them. Evidence from recent research suggests that these systems are inefficient and may contribute to physician burnout.
As a result, organizations like SHM are looking for ways that these systems can be improved.
“The growth of health IT has led to availability of large amounts of data and opportunities for applications in [artificial intelligence and machine learning,” Dr. Prasad noted. “While this has opened many avenues to help positively impact patient care and outcomes, it also poses multiple challenges like validation, customization, and governance. Hospitalists can partner with other health professions and IT leaders to work toward the common goal of improving the health of the population while also providing a positive experience to the end user.”
Another problem with current EMRs is their lack of flexibility. These systems are often not compatible with mobile devices and certain types of hardware and may be difficult or impossible to customize. They also frequently require unnecessary information during the note-taking process that results in cluttered and difficult-to-scan documentation. Improving EMR flexibility – and inviting clinicians to consult during the design process – could solve many of these problems.
New technological developments may also soon help developers improve their EMRs. In the future, as technology like natural language processing becomes more advanced and more commonly used, they may be able to make EMRs even more efficient and user friendly.
One of the most significant shifts in hospital practice over recent decades has been the widespread adoption of electronic medical records as a replacement for conventional paper records.
While EMRs show a lot of promise – having the potential to centralize and simplify clinician notes, make information more accessible and reduce paper waste – there is strong evidence that they are not working as well as they could.
Some research suggests that these systems may decrease the working efficiency of clinicians. Now, major health care institutions are looking to understand why these systems are not working — as well as how they may be improved.
A recent white paper from the Society of Hospital Medicine’s Healthcare Information Technology Special Interest Group – titled “More Caring, Less Clicking” – reviews the current shortcomings of EMRs from a hospitalist perspective and provides recommendations for how these systems can be made more workable and efficient.
The current state of EMRs
“Numerous previous papers – including SHM’s 2017 white paper ‘Hospitalist Perspectives on Electronic Medical Records’ – have linked EMRs to decreased provider satisfaction and increased burnout related to multiple issues, including an increase in ‘screen time’ as opposed to patient ‘face-to-face’ time, and limitations in usability and interoperability,” said Rupesh Prasad, MD, SFHM, medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. “Studies have shown that most of a provider’s time spent is in areas like clinical documentation, entry of orders, and accessing patient information.”
The 2017 SHM white paper referenced by Dr. Prasad reported that 74% of hospitalists surveyed were dissatisfied with their EMR. A full one-quarter of surveyed physicians went so far as saying they would prefer switching to paper record keeping.
Other research has also found a possible link between EMRs and physician burnout and dissatisfaction. It is also not uncommon for hospitalists to spend up to 25% of their time at work using their EMR – time that should, ideally, be spent with patients.
The 2017 paper also showed that clinician notes in the United States are four times longer, on average, than notes in other countries. There are a few reasons for this – including technology design and billing requirements encouraging longer notes. Whatever the cause, however, longer notes linked to physician burnout may be partially responsible for the large amounts of time physicians spend looking at EMRs.
While EMRs may hold significant potential for hospitalists, as they are designed currently, they are simply not delivering the value many expected. The new white paper from the Healthcare Information Technology Special Interest Group outlines practical changes that could be made to EMRs to improve their use in hospitals.
The paper breaks down current issues with EMRs into five broad categories – documentation, clinical decision support, order entry, communication, and data review – to discuss how EMRs are currently failing in these areas as well as how they might be improved.
Improving EMR documentation
One of the most significant hurdles clinicians currently face lies in how EMRs currently store and display documentation. Combined with physician note-taking habits, this makes these systems much less usable than they could be. Longer notes, when displayed in current EMR UIs, mostly lead to clutter, making them harder to navigate and difficult to scan quickly for important information.
The authors identify a few different ways that future EMRs may be able to help with this problem.
EMR documentation tools will likely need to be redesigned to optimize documentation entry, standardize note formatting, and improve readability. Many electronic notes contain vestigial formatting and data left over from the design of paper notes. As a result, many of these electronic notes include information that is stored elsewhere and does not need to be explicitly included in every note. Cutting down on repetitive information storage will make important information more visible and help make patient notes easier to scan.
The paper also recommends a few other features that would make documentation more readable – like allowing clinicians to write documentation in SOAP format (subjective, objective, assessment, and plan), to facilitate critical thinking during the note-taking process, and having the EMR display that documentation in APSO format (assessment, plan, subjective, objective).
Doctors have long called for APSO or another note-taking format to replace SOAP in EMRs. Designing EMRs to rearrange SOAP notes to APSO could be a compromise that improves note readability while not requiring that clinicians learn new note-taking strategies.
The paper’s authors also recommended more extensive clinician training on writing notes. While clinicians are often taught how to write certain notes – like progress notes, histories, and physical and discharge summaries – more specific guidance is not always provided. Better training provided by institutions could help improve the quality and readability of clinician notes.
These changes, however, may not be as beneficial as possible without better institutional support for clinicians. Implementing some of the biggest changes recommended by the SHM will require some level of standardization across platforms and institution commitment to training clinicians on best use practices for EMRs. Improved responsiveness to clinician needs will require a coordinated effort with backing from both administrative and governance groups.
Expanding EMR usability
“Our white paper presents evidence-based recommendations that can be implemented at the ground level in collaboration with other stakeholders, including IT, informatics, and administration, to help improve on the current state,” Dr. Prasad said.
“We believe that hospitalists as key stakeholders in health care, have both the responsibility and are uniquely positioned to directly impact EMR functionality,” he noted. “For example, hospitalists can participate in designing appropriate, actionable alerts that would help with patient safety while also improving provider efficiency. Simple steps like limiting hard stops in order entry to would help speed up the process, and free up time for direct patient care. Availability of tools like secure text messaging would help with effective patient care team communication to improve safety and care delivery.”
EMRs often lack features like voice control and speech-to-text transcription, along with other basic accessibility features like compatibility with screen readers. Implementing these features could improve the efficiency of clinicians’ note-taking while also providing wider software usability.
EMRs are not typically designed to work with mobile devices, meaning clinicians cannot enter notes or order medications until they’ve returned to their desk or workstation.
This lack of functionality creates issues in several ways. When clinicians are unable to enter notes on the move, they will need to either keep mental notes or quickly jot down paper notes. This can effectively double the amount of note-taking that clinicians must do or introduce greater room for error. In cases where progress notes are taken throughout the day, this also means the EMR’s documentation timeline may not be accurate or usable.
Requiring clinicians to return to workstations before entering order information can also increase the risk of medication errors, which remains high despite hopes that EMRs could reduce error rates.
Adding support for cross-device and mobile EMR use could help improve the efficiency of note-taking and help cut down on error. Implementing mobile access could have a few different benefits for clinicians – like improving note-taking efficiency in hospitals, where doctors often see patients far away from their workstations.
EMRs also often lack support for certain hardware, like mobile stations and widescreen monitors, which can improve a clinician’s ability to document in real-time and are a better fit in certain work flows.
The SHM paper also recommends a few other tweaks to usability – like reducing the amount of password entry and reentry – that could make these systems easier to use and more efficient.
New features – like the use of natural language processing technology to analyze and organize information contained in clinicians’ notes – could provide further benefits and take full advantage of the advanced technologies that EMRs can integrate.
Dr. Prasad noted, however, that some of these upgrades – especially EMR compatibility with mobile devices – will require some institutional support. Bring-your-own-device policies or system-provided mobile devices will be necessary if institutions want their clinicians to be able to take advantage of mobile EMR access.
These policies will also likely require some kind of mobile device management solution to manage the security of sensitive patient data as it is accessed from personal devices. This may increase the level of necessary institutional buy-in for this support to work.
Designing EMRs with clinician needs in mind
Dr. Prasad said he and his coauthors recommend that EMR developers base more of their design on the needs of clinicians.
Currently, EMR interfaces can make important data unavailable, depending on what a clinician is trying to do. As a result, clinicians often need to rely on mental recall of important information as they navigate EMR systems.
These interfaces also typically do not support any level of user customization or process-specific interfaces, meaning every clinician is working with the same interface regardless of the tasks they need to perform or the information they need access to. Allowing for customization or implementing new process- or disease-specific interfaces could help avoid some of the problems caused by one-size-fits-all interfaces, which are not necessarily compatible with every clinician work flow.
EMR interfaces should also be designed, wherever possible, with familiar or standardized formats and the use of color coding and other techniques that can make interfaces easier to navigate quickly. Right now, many EMR systems utilize inconsistent layout design that can be cluttered with irrelevant information, slowing down interface navigation and sometimes requiring backtracking from clinicians.
Ideally, this will improve the speed of information gathering and data review, reducing the amount of time clinicians need to spend working with their EMR.
The white paper also recommends that EMR designers improve alert systems so that they are more actionable and interrupt clinicians less often – and that, when they do, they ensure that clinicians can respond to them. Designers should also reduce hard-stops or in-line alerts that halt clinicians’ work flows and require immediate responses where possible.
Increased EMR support for clinical decision support systems is one of the biggest health care trends expected to be seen throughout this decade. However, many clinicians are disappointed with the lack of flexibility and optimization of the current alerts that CDS provides. Updating and improving these knowledge-based systems will likely become essential for delivering better alerts and improving decision-making and efficiency.
Overall, EMR design should be informed by the needs of the people these products are designed to support, Dr. Prasad said. The people that work with EMRs – especially frontline staff like providers, nurses, and pharmacists that regularly interact with EMRs to provide care – should be involved early on in the EMR design process. Right now, their needs are not reflected in current EMR design. EMR companies, by working with these hospital staff members, could help improve ease of use and, ideally, prevent some of the errors associated with the current implementation of these systems.
“System designers should be able to avoid some of the most common problems of EMRs – and predict potential problems – by consistently soliciting and integrating clinician feedback during the design process and over the lifespan of a product,” Dr. Prasad said.
How EMRs can be improved
Over the past few years, EMRs have become quickly adopted by health care professionals and institutions. However, despite hopes that EMRs could significantly improve record keeping and note-taking, these systems continue to pose serious challenges for the clinicians who use them. Evidence from recent research suggests that these systems are inefficient and may contribute to physician burnout.
As a result, organizations like SHM are looking for ways that these systems can be improved.
“The growth of health IT has led to availability of large amounts of data and opportunities for applications in [artificial intelligence and machine learning,” Dr. Prasad noted. “While this has opened many avenues to help positively impact patient care and outcomes, it also poses multiple challenges like validation, customization, and governance. Hospitalists can partner with other health professions and IT leaders to work toward the common goal of improving the health of the population while also providing a positive experience to the end user.”
Another problem with current EMRs is their lack of flexibility. These systems are often not compatible with mobile devices and certain types of hardware and may be difficult or impossible to customize. They also frequently require unnecessary information during the note-taking process that results in cluttered and difficult-to-scan documentation. Improving EMR flexibility – and inviting clinicians to consult during the design process – could solve many of these problems.
New technological developments may also soon help developers improve their EMRs. In the future, as technology like natural language processing becomes more advanced and more commonly used, they may be able to make EMRs even more efficient and user friendly.
Wellness for the Dermatology Resident
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident Pearls
- Resident wellness is an important issue affecting resident physicians of all specialties, including dermatology.
- To improve wellness, changes can be made by targeting the following 4 areas of well-being: professional, physical, psychological, and social.
iResident: Virtual care on hospital medicine teaching services during a pandemic
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.
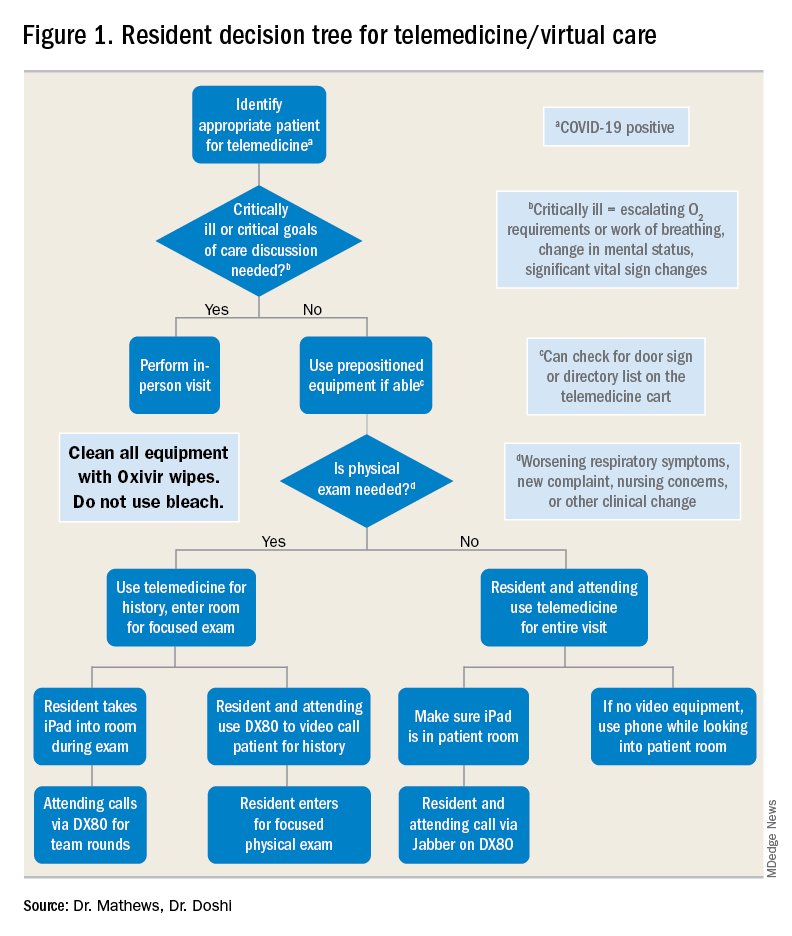
For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.

For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.

For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
Most clinicians undertreat childhood lichen sclerosus
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
FROM SPD 2020
A dedicated mobility technician improves inpatient mobility
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have shown improved hospital outcomes in patients who ambulate regularly. Many assisted mobility protocols aimed at ambulating patients multiple times daily are nurse centered. However, implementation is difficult because of the large number of nursing duties and difficulty finding time away from other competing responsibilities.
Study design: Single-blind randomized controlled trial.
Setting: Single-center 1,440-bed tertiary care hospital.
Synopsis: This study randomized 102 moderately impaired adult inpatients aged 60 years and older with Activity Measures for Post-Acute Care mobility scores of 16-20 to either dedicated regular ambulation sessions with mobility technicians or usual care with hospital nurse–driven protocol. Patients who achieved greater than 400 steps were more likely to discharge to home rather than post–acute care (71% vs. 46%; P = .01). Assisted ambulation did not decrease length of stay or affect the discharge disposition, but it did increase the total daily number of steps taken by patients (1,182 vs. 726; P = .02, per-protocol analysis) and the patients’ mobility scores (18.90 vs. 18.27, P = .04).
Bottom line: A dedicated mobility technician to provide assisted ambulation for older inpatients can improve patient mobility.
Citation: Hamilton AC et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: A pilot randomized controlled trial. J Hosp Med. 2019;14:272-7.
Dr. Nelson is a hospitalist at Ochsner Health System, New Orleans.
Vitamin D fails to prevent late-life depression, boost mood
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Coming soon: The 2020 SoHM Report!
On behalf of SHM’s Practice Analysis Committee, I am excited to announce the scheduled September 2020 release of the 2020 State of Hospital Medicine Report (SoHM)!
For reasons all too familiar, this year’s SoHM survey process was unlike any in SHM’s history. We were still collecting survey responses from a few stragglers in early March when the entire world shut down almost overnight to flatten the curve of a deadly pandemic. Hospital medicine group (HMG) leaders were suddenly either up to their eyeballs trying to figure out how to safely care for huge influxes of COVID-19 patients that overwhelmed established systems of care or were trying to figure out how to staff in a low-volume environment with few COVID patients, a relative trickle of ED admissions, and virtually no surgical care. And everywhere, hospitals and their HMGs were quickly stressed in ways that would have been unimaginable just a couple of months earlier – financially, operationally, epidemiologically, and culturally.
SHM offices closed, with all staff working from home. And the talented people who would normally have been working diligently on the survey data were suddenly redirected to focus on COVID-related issues, including tracking government announcements that were changing daily and providing needed resources to SHM members. By the time they could raise their heads and begin thinking about survey data, we were months behind schedule.
I need to give a huge shout-out to our survey manager extraordinaire Josh Lapps, SHM’s Director of Policy and Practice Management, and his survey support team including Luke Heisinger and Kim Schonberger. Once they were able to turn their focus back to the SoHM, they worked like demons to catch up. And in addition to the work of preparing the SoHM for publication, they helped issue and analyze a follow-up survey to investigate how HMGs adjusted their staffing and operations in response to COVID! As I write this, we appear to be back on schedule for a September SoHM release date, with the COVID supplemental survey report to follow soon after. Thanks also to PAC committee members who, despite their own stresses, rose to the challenge of participating in calls and planning the supplemental survey.
Despite the pandemic, HMGs found survey participation valuable. When all was said and done, we had a respectable number of respondent groups: 502 this year vs. 569 in 2018. Although the number of respondent groups is down, the average group size has increased, so that an all-time high of 10,122 employed/contracted full-time equivalent (FTE) hospitalists (plus 484 locum tenens FTEs) are represented in the data set. The respondents continue to be very diverse, representing all practice models and every state – and even a couple of other countries. One notable change is a significant increase in pediatric HM group participation, thanks to a recruitment charge led by PAC member Sandra Gage, associate division chief of hospital medicine at Phoenix Children’s Hospital, and supported by the inclusion of several new pediatric HM-specific questions to better capture unique attributes of these hospital medicine practices.
We had more multisite respondents than ever, and the multisite respondents overwhelmingly used the new “retake” feature in the online version of the survey. I’m happy to report that we received consistent positive feedback about our new electronic survey platform, and thanks to its capabilities data analysis has been significantly automated, enhancing both efficiency and data reliability.
The survey content is more wide ranging than ever. In addition to the usual topics such as scope of services, staffing and scheduling, compensation models, evaluation and management code distribution, and HM group finances, the 2020 report will include the afore-referenced information about HM groups serving children, expanded information on nurse practitioner (NPs)/physician assistant (PA) roles, and data on diversity in HM physician leadership. The follow-up COVID survey will be published separately as a supplement, available only to purchasers of the SoHM report.
Multiple options for SoHM report purchase. All survey participants will receive access to the online version of the survey. Others may purchase the hard copy report, online access, or both. The report has a colorful, easy-to-read layout, and many of the tables have been streamlined to make them easier to read. I encourage you to sign up to preorder your copy of the SoHM Report today at www.hospitalmedicine.org/sohm; you’ll almost certainly discover a treasure trove of worthwhile information.
Use the report to assess how your practice compares to other practices, but always keep in mind that surveys don’t tell you what should be; they only tell you what currently is the case – or at least, what was during the survey period. New best practices not yet reflected in survey data are emerging all the time, and that is probably more true today in the new world affected by this pandemic than ever before. And while the ways others do things won’t always be right for your group’s unique situation and needs, it always helps to know how you compare with others. Whether you are partners or employees, you and your colleagues “own” the success of your hospital medicine practice and, armed with the best available data, are the best judges of what is right for you.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
On behalf of SHM’s Practice Analysis Committee, I am excited to announce the scheduled September 2020 release of the 2020 State of Hospital Medicine Report (SoHM)!
For reasons all too familiar, this year’s SoHM survey process was unlike any in SHM’s history. We were still collecting survey responses from a few stragglers in early March when the entire world shut down almost overnight to flatten the curve of a deadly pandemic. Hospital medicine group (HMG) leaders were suddenly either up to their eyeballs trying to figure out how to safely care for huge influxes of COVID-19 patients that overwhelmed established systems of care or were trying to figure out how to staff in a low-volume environment with few COVID patients, a relative trickle of ED admissions, and virtually no surgical care. And everywhere, hospitals and their HMGs were quickly stressed in ways that would have been unimaginable just a couple of months earlier – financially, operationally, epidemiologically, and culturally.
SHM offices closed, with all staff working from home. And the talented people who would normally have been working diligently on the survey data were suddenly redirected to focus on COVID-related issues, including tracking government announcements that were changing daily and providing needed resources to SHM members. By the time they could raise their heads and begin thinking about survey data, we were months behind schedule.
I need to give a huge shout-out to our survey manager extraordinaire Josh Lapps, SHM’s Director of Policy and Practice Management, and his survey support team including Luke Heisinger and Kim Schonberger. Once they were able to turn their focus back to the SoHM, they worked like demons to catch up. And in addition to the work of preparing the SoHM for publication, they helped issue and analyze a follow-up survey to investigate how HMGs adjusted their staffing and operations in response to COVID! As I write this, we appear to be back on schedule for a September SoHM release date, with the COVID supplemental survey report to follow soon after. Thanks also to PAC committee members who, despite their own stresses, rose to the challenge of participating in calls and planning the supplemental survey.
Despite the pandemic, HMGs found survey participation valuable. When all was said and done, we had a respectable number of respondent groups: 502 this year vs. 569 in 2018. Although the number of respondent groups is down, the average group size has increased, so that an all-time high of 10,122 employed/contracted full-time equivalent (FTE) hospitalists (plus 484 locum tenens FTEs) are represented in the data set. The respondents continue to be very diverse, representing all practice models and every state – and even a couple of other countries. One notable change is a significant increase in pediatric HM group participation, thanks to a recruitment charge led by PAC member Sandra Gage, associate division chief of hospital medicine at Phoenix Children’s Hospital, and supported by the inclusion of several new pediatric HM-specific questions to better capture unique attributes of these hospital medicine practices.
We had more multisite respondents than ever, and the multisite respondents overwhelmingly used the new “retake” feature in the online version of the survey. I’m happy to report that we received consistent positive feedback about our new electronic survey platform, and thanks to its capabilities data analysis has been significantly automated, enhancing both efficiency and data reliability.
The survey content is more wide ranging than ever. In addition to the usual topics such as scope of services, staffing and scheduling, compensation models, evaluation and management code distribution, and HM group finances, the 2020 report will include the afore-referenced information about HM groups serving children, expanded information on nurse practitioner (NPs)/physician assistant (PA) roles, and data on diversity in HM physician leadership. The follow-up COVID survey will be published separately as a supplement, available only to purchasers of the SoHM report.
Multiple options for SoHM report purchase. All survey participants will receive access to the online version of the survey. Others may purchase the hard copy report, online access, or both. The report has a colorful, easy-to-read layout, and many of the tables have been streamlined to make them easier to read. I encourage you to sign up to preorder your copy of the SoHM Report today at www.hospitalmedicine.org/sohm; you’ll almost certainly discover a treasure trove of worthwhile information.
Use the report to assess how your practice compares to other practices, but always keep in mind that surveys don’t tell you what should be; they only tell you what currently is the case – or at least, what was during the survey period. New best practices not yet reflected in survey data are emerging all the time, and that is probably more true today in the new world affected by this pandemic than ever before. And while the ways others do things won’t always be right for your group’s unique situation and needs, it always helps to know how you compare with others. Whether you are partners or employees, you and your colleagues “own” the success of your hospital medicine practice and, armed with the best available data, are the best judges of what is right for you.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
On behalf of SHM’s Practice Analysis Committee, I am excited to announce the scheduled September 2020 release of the 2020 State of Hospital Medicine Report (SoHM)!
For reasons all too familiar, this year’s SoHM survey process was unlike any in SHM’s history. We were still collecting survey responses from a few stragglers in early March when the entire world shut down almost overnight to flatten the curve of a deadly pandemic. Hospital medicine group (HMG) leaders were suddenly either up to their eyeballs trying to figure out how to safely care for huge influxes of COVID-19 patients that overwhelmed established systems of care or were trying to figure out how to staff in a low-volume environment with few COVID patients, a relative trickle of ED admissions, and virtually no surgical care. And everywhere, hospitals and their HMGs were quickly stressed in ways that would have been unimaginable just a couple of months earlier – financially, operationally, epidemiologically, and culturally.
SHM offices closed, with all staff working from home. And the talented people who would normally have been working diligently on the survey data were suddenly redirected to focus on COVID-related issues, including tracking government announcements that were changing daily and providing needed resources to SHM members. By the time they could raise their heads and begin thinking about survey data, we were months behind schedule.
I need to give a huge shout-out to our survey manager extraordinaire Josh Lapps, SHM’s Director of Policy and Practice Management, and his survey support team including Luke Heisinger and Kim Schonberger. Once they were able to turn their focus back to the SoHM, they worked like demons to catch up. And in addition to the work of preparing the SoHM for publication, they helped issue and analyze a follow-up survey to investigate how HMGs adjusted their staffing and operations in response to COVID! As I write this, we appear to be back on schedule for a September SoHM release date, with the COVID supplemental survey report to follow soon after. Thanks also to PAC committee members who, despite their own stresses, rose to the challenge of participating in calls and planning the supplemental survey.
Despite the pandemic, HMGs found survey participation valuable. When all was said and done, we had a respectable number of respondent groups: 502 this year vs. 569 in 2018. Although the number of respondent groups is down, the average group size has increased, so that an all-time high of 10,122 employed/contracted full-time equivalent (FTE) hospitalists (plus 484 locum tenens FTEs) are represented in the data set. The respondents continue to be very diverse, representing all practice models and every state – and even a couple of other countries. One notable change is a significant increase in pediatric HM group participation, thanks to a recruitment charge led by PAC member Sandra Gage, associate division chief of hospital medicine at Phoenix Children’s Hospital, and supported by the inclusion of several new pediatric HM-specific questions to better capture unique attributes of these hospital medicine practices.
We had more multisite respondents than ever, and the multisite respondents overwhelmingly used the new “retake” feature in the online version of the survey. I’m happy to report that we received consistent positive feedback about our new electronic survey platform, and thanks to its capabilities data analysis has been significantly automated, enhancing both efficiency and data reliability.
The survey content is more wide ranging than ever. In addition to the usual topics such as scope of services, staffing and scheduling, compensation models, evaluation and management code distribution, and HM group finances, the 2020 report will include the afore-referenced information about HM groups serving children, expanded information on nurse practitioner (NPs)/physician assistant (PA) roles, and data on diversity in HM physician leadership. The follow-up COVID survey will be published separately as a supplement, available only to purchasers of the SoHM report.
Multiple options for SoHM report purchase. All survey participants will receive access to the online version of the survey. Others may purchase the hard copy report, online access, or both. The report has a colorful, easy-to-read layout, and many of the tables have been streamlined to make them easier to read. I encourage you to sign up to preorder your copy of the SoHM Report today at www.hospitalmedicine.org/sohm; you’ll almost certainly discover a treasure trove of worthwhile information.
Use the report to assess how your practice compares to other practices, but always keep in mind that surveys don’t tell you what should be; they only tell you what currently is the case – or at least, what was during the survey period. New best practices not yet reflected in survey data are emerging all the time, and that is probably more true today in the new world affected by this pandemic than ever before. And while the ways others do things won’t always be right for your group’s unique situation and needs, it always helps to know how you compare with others. Whether you are partners or employees, you and your colleagues “own” the success of your hospital medicine practice and, armed with the best available data, are the best judges of what is right for you.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.