User login
For MD-IQ use only
How to truly connect with your patients
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
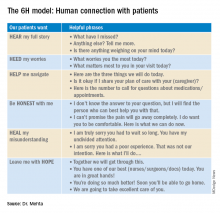
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
Introducing the ‘6H model’
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
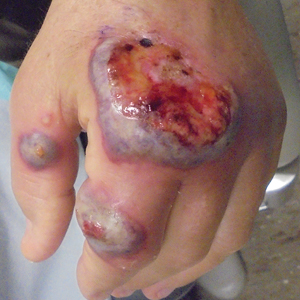
Nonhealing Ulcerative Hand Wound
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
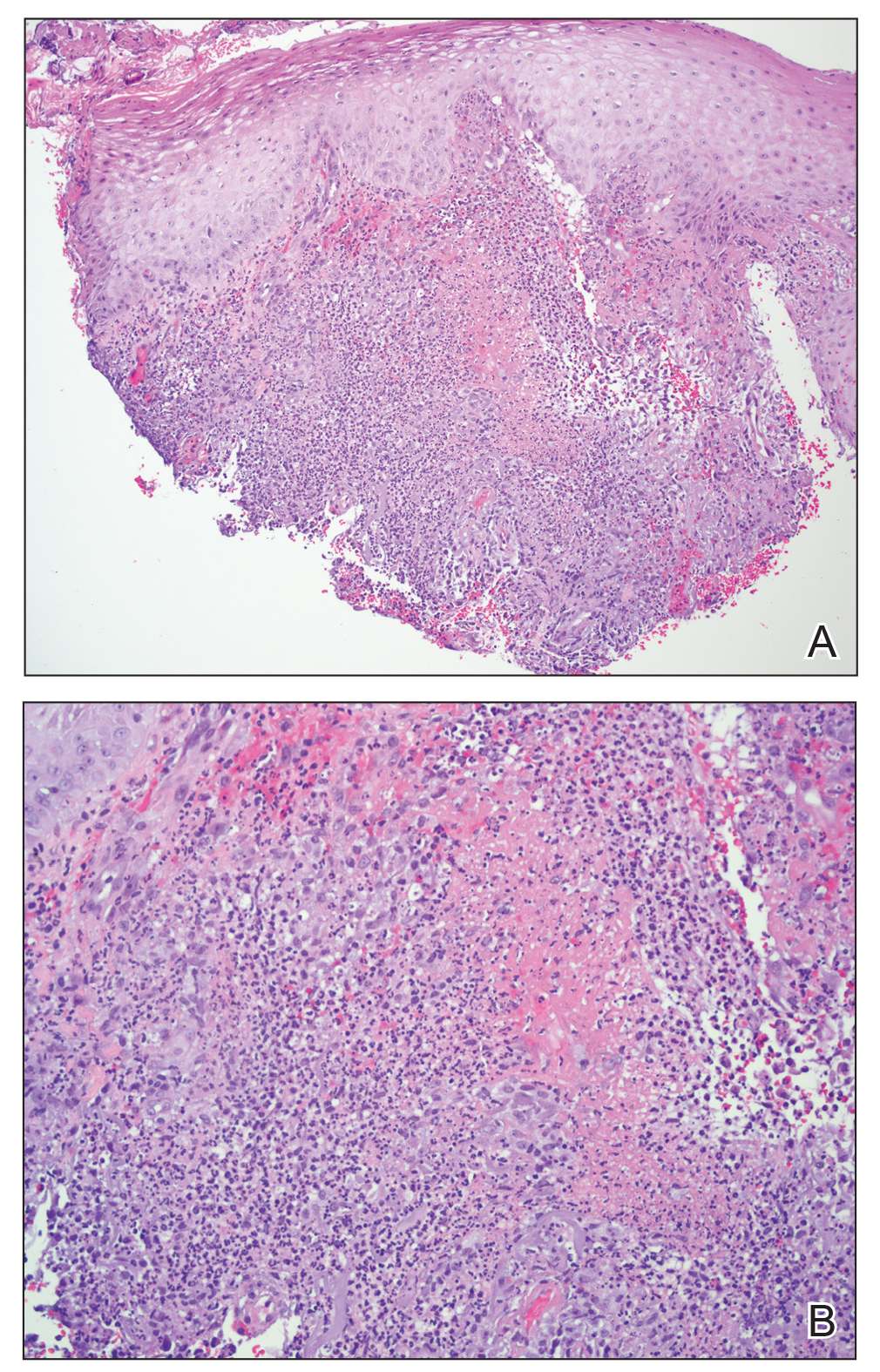
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.
Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
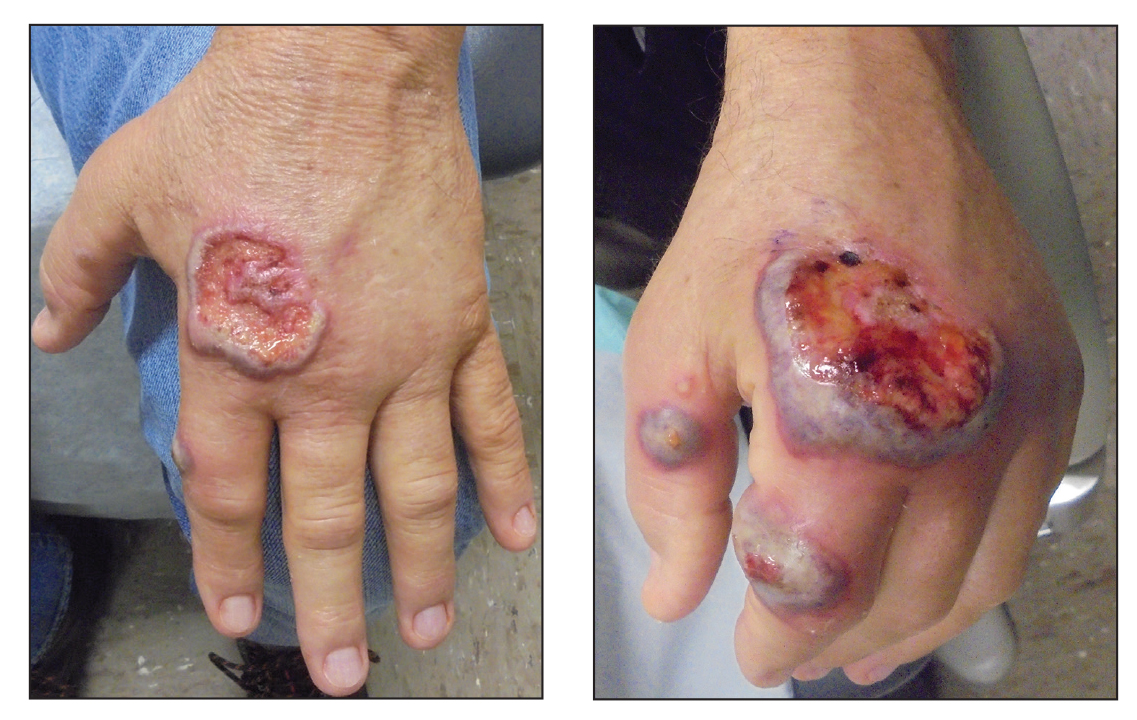
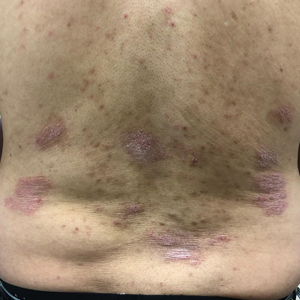
A 63-year-old man presented with an expanding wound on the dorsal aspect of the left hand after striking it on a wall. He sustained a small laceration that progressively became more edematous and developed a violaceous border. He presented to the emergency department the following day and was prescribed bacitracin with no improvement in the lesion. He returned to the emergency department after the symptoms worsened and was subsequently prescribed a 10-day course of oral trimethoprim-sulfamethoxazole (1600/320 mg) twice daily. Physical examination at a follow-up visit 11 days after the initial injury revealed an expanding, 4.3×5.0-cm, ulcerated wound with surrounding erythema and serosanguineous drainage (left). He was started on a 10-day course of amoxicillin–clavulanic acid (1750/250 mg) twice daily and underwent debridement the same day. On postoperative day 2 (13 days following the onset of symptoms), the wound had not improved, and 2 new 1-cm bullae on the left first and second fingers had progressed (right). Erythrocyte sedimentation rate (33 mm/h [reference range, 0–10 mm/h]) and C-reactive protein (3.701 mg/dL [reference range, 0–0.747 mg/dL]) were elevated; however, other laboratory studies, including a complete blood cell count, were within reference range. He remained afebrile, and a review of systems was normal. Punch biopsy specimens were obtained.
PHM20 Virtual: Common incidental findings seen on pediatric imaging
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
Valproate-Induced Lower Extremity Swelling
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
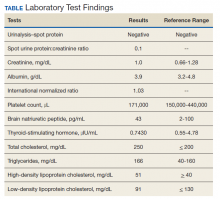
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
Bilateral lower extremity edema is a common condition with a broad differential diagnosis. New, severe peripheral edema implies a more nefarious underlying etiology than chronic venous insufficiency and should prompt a thorough evaluation for underlying conditions, such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, hypoalbuminemia, or lymphatic or venous obstruction. We present a case of a patient with sudden onset new bilateral lower extremity edema due to a rare adverse drug reaction (ADR) from valproate.
Case Presentation
A 63-year-old male with a history of seizures, bipolar disorder type I, and memory impairment due to traumatic brain injury (TBI) from a gunshot wound 24 years prior presented to the emergency department for witnessed seizure activity in the community. The patient had been incarcerated for the past 20 years, throughout which he had been taking the antiepileptic drugs (AEDs) phenytoin and divalproex and did not have any seizure activity. No records prior to his incarceration were available for review.
The patient recently had been released from prison and was nonadherent with his AEDs, leading to a witnessed seizure. This episode was described as preceded by an electric sensation, followed by rhythmic shaking of the right upper extremity without loss of consciousness. His regimen prior to admission included divalproex 1,000 mg daily and phenytoin 200 mg daily. His only other medication was folic acid.
Neurology was consulted on admission. An awake and asleep 4-hour electroencephalogram showed intermittent focal slowing of the right frontocentral region and frequent epileptiform discharges in the right prefrontal region during sleep, corresponding to areas of chronic right anterior frontal and temporal encephalomalacia seen on brain imaging. His seizures were thought likely to be secondary to prior head trauma. While the described seizure activity involving the right upper extremity was not consistent with the location of his prior TBI, neurology considered that he might have simple partial seizures with multiple foci or that his seizure event prior to admission was not accurately described. The neurology consult recommended switching from phenytoin 200 mg daily to lacosamide 100 mg twice daily on admission. His prior dose of divalproex 1,000 mg daily also was resumed for its antiepileptic effect and the added benefit of mood stabilization, as the patient reported elevated mood and decreased need for sleep on admission.
Eight days after changing his AED regimen, the patient was found to have new onset bilateral grade 1+ pitting edema to the level of his shins. He had no history of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysuria, or changes in his urination. Although medical records from his incarceration were not available for review, the patient reported that he had never had peripheral edema.
On physical examination, the patient had no periorbital edema, jugular venous pressure of 8 cm H2O, negative hepatojugular reflex, unremarkable cardiac and lung examination, and grade 2+ posterior tibial and dorsalis pedis pulses bilaterally. He underwent extensive laboratory evaluation for potential underlying causes, including nephrotic syndrome, cirrhosis, hypothyroidism, and CHF (Table). Valproate levels were initially subtherapeutic on admission (< 10 µg/mL, reference range 50-125 µg/mL) then rose to within therapeutic range (54 µg/mL-80 µg/mL throughout admission) after neurology recommended increasing the dose from 1,000 mg daily to 1,500 mg daily. His measured valproate levels were never supratherapeutic.
An electrocardiogram showed normal sinus rhythm unchanged from admission. Transthoracic echocardiogram showed normal left ventricular (LV) size and estimated LV ejection fraction of 55 to 60%. Abdominal ultrasound showed no evidence of cirrhosis and normal portal vein flow. Ultrasound of the lower extremities showed no deep venous thrombosis or valvular insufficiency. The patient was prescribed compression stockings. However, due to memory impairment, he was relatively nonadherent, and his lower extremity edema worsened to grade 3+ over several days. Due to the progressive swelling with no identified cause, a computed tomographic venogram of the abdomen and pelvis was performed to determine whether an inferior vena cava (IVC) thrombus was present. This study was unremarkable and did not show any external IVC compression.
After extensive evaluation did not reveal any other cause, the temporal course of events suggested an association between the patient’s peripheral edema and resumption of divalproex. His swelling remained stable. Discontinuation of divalproex was considered, but the patient’s mood remained euthymic, and he had no further seizure activity while on this medication, so the benefit of continuation was felt to outweigh any risks of switching to another agent.
Discussion
Valproate and its related forms, such as divalproex, often are used in the treatment of generalized or partial seizures, psychiatric disorders, and the prophylaxis of migraine headaches. Common ADRs include gastrointestinal symptoms, sedation, and dose-related thrombocytopenia, among many others. Rare ADRs include fulminant hepatitis, pancreatitis, hyperammonemia, and peripheral edema.1 There have been case reports of valproate-induced peripheral edema, which seems to be an idiosyncratic ADR that occurs after long-term administration of the medication.2,3 Early studies reported valproate-related edema in the context of valproate-induced hepatic injury.4 However, in more recent case reports, valproate-related edema has been found in patients without hepatotoxicity or supratherapeutic drug levels.1,2
The exact mechanism by which valproate causes peripheral edema is unknown. It has been reported that medications affecting the γ-aminobutyric acid (GABA) system such as benzodiazepines, for example, can cause this rare ADR.5 Unlike benzodiazepines, valproate has an indirect effect on the GABA system, through increasing availability of GABA.6 GABA receptors have been identified on peripheral tissues, suggesting that GABAergic medications also may have an effect on regional vascular resistance.7 This mechanism was proposed by prior case reports but has yet to be proven in studies.2
In this case, initiation of lacosamide temporally coinciding with development of the patient’s edema leads one to question whether lacosamide may have caused this ADR. Other medications commonly used in seizure management (such as benzodiazepines and gabapentin) have been reported to cause new onset peripheral edema.5,8 To date, however, there are no reported cases of peripheral edema due to lacosamide. While there are known interactions between various AEDs that may impact drug levels of valproate, there are no reported drug-drug interactions between lacosamide and valproate.9
Conclusions
Our case adds to the small but growing body of literature that suggests peripheral edema is a rare but clinically significant ADR of valproate. With its broad differential diagnosis, new onset peripheral edema is a concern that often warrants an extensive evaluation for underlying causes. Clinicians should be aware of this ADR as use of valproate becomes increasingly common so that an extensive workup is not always performed on patients with peripheral edema.
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
1. Prajapati H, Kansal D, Negi R. Magnesium valproate-induced pedal edema on chronic therapy: a rare adverse drug reaction. Indian J Pharmacol. 2017;49(5):399. doi:10.4103/ijp.IJP_239_17
2. Lin ST, Chen CS, Yen CF, Tsei JH, Wang SY. Valproate-related peripheral oedema: a manageable but probably neglected condition. Int J Neuropsychopharmacol. 2009;12(7):991-993. doi:10.1017/S1461145709000509
3. Ettinger A, Moshe S, Shinnar S. Edema associated with long‐term valproate therapy. Epilepsia. 1990;31(2):211-213. doi:10.1111/j.1528-1167.1990.tb06308.x
4. Zimmerman HJ, Ishak KG. Valproate‐induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2(5):591S-597S. doi:10.1002/hep.1840020513
5. Mathew T, D’Souza D, Nadimpally US, Nadig R. Clobazam‐induced pedal edema: “an unrecognized side effect of a common antiepileptic drug.” Epilepsia. 2016;57(3): 524-525. doi:10.1111/epi.13316
6. Bourin M, Chenu F, Hascoët M. The role of sodium channels in the mechanism of action of antidepressants and mood stabilizers. Curr Drug Targets. 2009;10(11):1052-1060. doi:10.2174/138945009789735138
7. Takemoto Y. Effects of gamma‐aminobutyric acid on regional vascular resistances of conscious spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22(suppl):S102-Sl04. doi:10.1111/j.1440-1681.1995.tb02839.x
8. Bidaki R, Sadeghi Z, Shafizadegan S, et al. Gabapentin induces edema, hyperesthesia and scaling in a depressed patient; a diagnostic challenge. Adv Biomed Res. 2016;5:1. doi:10.4103/2277-9175.174955
9. Cawello W, Nickel B, Eggert‐Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459-471. doi:10.1177/0091270009347675
Ten-Year Outcomes of a Systems-Based Approach to Longitudinal Amputation Care in the US Department of Veteran Affairs
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
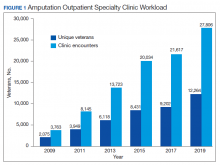
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
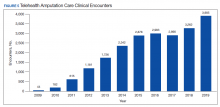
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
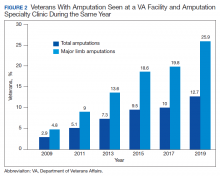
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
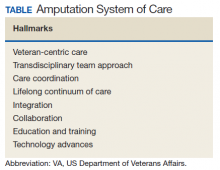
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
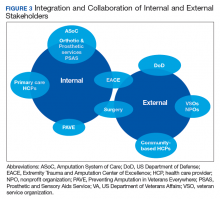
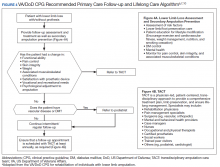
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
The US Department of Veterans Affairs (VA) established a formal Amputation System of Care (ASoC) in 2008 with the goal of enhancing the quality and consistency of amputation rehabilitation care for veterans with limb loss.1,2 Throughout its history, the VA has placed a high priority on the care that is provided to veterans with limb amputation.1,3 Amputations have medical, physical, social, and psychological ramifications for the veteran and his or her family. Therefore, management of veterans with limb loss requires a comprehensive, coordinated, transdisciplinary program of services throughout the continuum of care. This includes offering the latest practices in medical interventions, artificial limbs, assistive technologies, and rehabilitation strategies to restore function and thereby optimize quality of life.
Amputation System of Care
The ASoC is an integrated system within the Veterans Health Administration (VHA) that provides specialized expertise in amputation rehabilitation incorporating the latest practices in medical management, rehabilitation therapies, artificial limbs, and assistive technologies. The system facilitates patient-centered, gender-sensitive, lifelong care and care coordination across the entire health continuum from acute inpatient hospitalization through a spectrum of inpatient, residential, and outpatient rehabilitation care settings. Through the provision of quality rehabilitation and prosthetic limb care, the ASoC strives to minimize disability and enable the highest level of social, vocational, and recreational success for veterans with an amputation.1-3
The policy and procedures for the ASoC have been detailed in prior VA Handbooks and in the ASoC Directive.1 This article highlights the background, population served, and organizational structure of the ASoC by detailing the outcomes and accomplishments of this systems-based approach to longitudinal amputation care between 2009 and 2019. Four core areas of activities and accomplishments are highlighted: (1) learning organization creation; (2) trust in VA care; (3) system modernization; and (4) customer service. This analysis and description of the VA amputation care program serves as a model of amputation care that can be used in the civilian sector. There also is potential for the ASoC to serve as a care model example for other populations within the VA.
Organizational Structure
The ASoC is an integrated, national health care delivery system in which each VA medical center (VAMC) has a specific designation that reflects the level of expertise and accessibility across the system based on an individual veteran’s needs and the specific capabilities of each VAMC.1-3 The organizational structure for the ASoC is similar to the Polytrauma System of Care in that facilities are divided into 4 tiers.1,4
For the ASoC, the 4 tiers are Regional Amputation Centers (RAC) at 7 VAMCs, Polytrauma Amputation Network Sites (PANS) at 18 VAMCs, Amputation Clinic Teams (ACT) at 106 VAMCs, and Amputation Points of Contact (APoC) at 22 VAMCs. The RAC locations provide the highest level of specialized expertise in clinical care and prosthetic limb technology and have rehabilitation capabilities to manage the most complicated cases. Like the RAC facilities, PANS provide a full range of clinical and ancillary services to veterans within their catchment area and serve as referral locations for veterans with needs that are more complex. ACT sites have a core amputation specialty team that provides regular follow-up and address ongoing care needs. ACT sites may or may not have full ancillary services, such as surgical subspecialties or an in-house prosthetics laboratory. APoC facilities have at least 1 person on staff who serves as the point of contact for consultation, assessment, and referral of a veteran with an amputation to a facility capable of providing the level of services required.1
The VA also places a high priority on both primary and secondary amputation prevention. The Preventing Amputations in Veterans Everywhere (PAVE) program and the ASoC coordinate efforts in order to address the prevention of an initial amputation, the rehabilitation of veterans who have had an amputation, and the prevention of a second amputation in those with an amputation.1,5
Population Served
The ASoC serves veterans with limb loss regardless of the etiology. This includes care of individuals with complex limb trauma and those with other injuries or disease processes resulting in a high likelihood of requiring a limb amputation. In 2019, the VA provided care to 96,519 veterans with amputation, and about half (46,214) had at least 1 major limb amputation, which is defined as an amputation at or proximal to the wrist or ankle.6 The majority of veterans with amputation treated within the VA have limb loss resulting from disease processes, such as diabetes mellitus (DM) and peripheral vascular disease (PVD). Amputations caused by these diseases generally occur in the older veteran population and are associated with comorbidities, such as cardiovascular disease, hypertension, and end-stage renal disease. Veterans with amputation due to trauma, including conflict-related injuries, are commonly younger at the time of their amputation. Although the number of conflict-related amputations is small compared with the number of amputations associated with disease processes, both groups require high-quality, comprehensive, lifelong care.
Between 2009 and 2019, the number of veterans with limb loss receiving care in the VA increased 34%.6 With advances in vascular surgery and limb-sparing procedures, minor amputations are more common than major limb amputations and more below-knee rather than above-knee amputations have been noted over the same time. However, the high prevalence of DM in the overall veteran population places about 1.8 million veterans at risk for amputation, and it is anticipated that the volume of limb loss in the veteran population will continue to grow and possibly accelerate.5
Performance Metrics
During this same period, the amputation specialty clinic encounter to unique ratio (a measure of how frequently patients return to the clinic each year) rose from 1.8 in 2009 to 2.3 in 2019 for both the total amputation population and for those with major limb amputation. When looking more specifically at the RAC facilities, the encounter to unique ratio increased from 1.5 to 3.0 over the same time, reflecting the added benefit of having dedicated resources for the amputation specialty program.6
Comparing the percentage of veterans with amputation who are seen in the VA for any service with those who also are seen in the amputation specialty clinic in the same year is a performance metric that reflects the penetration of amputation specialty services across the system. Between 2009 and 2019, this increased from 2.9 to 12.7% for the overall amputation population and from 4.8 to 26% for those with major limb amputation (Figure 2). This metric improved to a greater extent in RAC facilities; 44% of veterans with major limb amputation seen at a RAC were also seen in the amputation specialty clinic in 2019.6
System Hallmarks
One of the primary hallmarks of the ASoC is the interdisciplinary team approach addressing all aspects of management across the continuum of care (Table). The core team consists of a physician, therapist, and prosthetist, and may include a variety of other disciplines based on a veteran’s individual needs. This model promotes veteran-centric care. Comprehensive management of veterans with limb loss includes addressing medical considerations such as residual limb skin health to the prescription of artificial limbs and the provision of therapy services for prosthetic limb gait training.1,2
Lifelong care for veterans living with limb loss is another hallmark of the ASoC. The provision of care coordination across the continuum of care from acute hospitalization following an amputation to long-term follow-up in the outpatient setting for veteran’s lifespan is essential. Care coordination is provided across the system of care, which assures that a veteran with limb loss can obtain the required services through consultation or referral to a RAC or PANS as needed. Care coordination for the ASoC is facilitated by amputation rehabilitation coordinators at each of the RAC and PANS designated VAMCs.
Integration of services and resource collaboration are additional key aspects of the ASoC (Figure 3). In order to be successful, care of the veteran facing potential amputation or living with the challenges postamputation must be well-integrated into the broader care of the individual. Many veterans who undergo amputation have significant medical comorbidities, including a high prevalence of DM and peripheral vascular disease. Management of these conditions in collaboration with primary care and other medical specialties promotes the achievement of rehabilitation goals. Integration of surgical services and amputation prevention strategies is critical. Another essential element of the system is maintaining amputation specialty care team contact with all veterans with limb loss on at least an annual basis. A clinical practice guideline published in 2017 on lower Limb amputation rehabilitation emphasizes this need for an annual contact and includes a management and referral algorithm to assist primary care providers in the management of veterans with amputation (Figure 4).7
Collaboration with external partners has been an important element in the system of care development. The VA has partnered extensively with the US Department of Defense (DoD) to transition service members with amputation from the military health care system to the VA. The VA and DoD also have collaborated through the development and provision of joint provider trainings, clinical practice guidelines, incentive funding programs, and patient education materials. Congress authorized the Extremity Trauma and Amputation Center of Excellence (EACE) in 2009 with the mission to serve as the joint DoD and VA lead element focused on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations. The EACE has several lines of effort, including clinical affairs, research, and global outreach focused on building partnerships and fostering collaboration to optimize quality of life for those with extremity trauma and amputation. The Amputee Coalition, the largest nonprofit consumer-based amputee advocacy organization in the US, has been an important strategic partner for the dissemination of guideline recommendations and patient education as well as the development and provision of peer support services.
Methods
The ASoC created a learning organization to develop and maintain a knowledgeable and highly skilled clinical workforce through the identification of best practices related to amputation rehabilitation and the use of innovative education delivery models. During the past 10 years, the ASoC conducted 9 national, live health care provider training events in conjunction with the DoD. In conjunction with the EACE, the ASoC holds 6 national Grand Rounds sessions each year. Dissemination of information and trainings across both the VA and DoD has been facilitated through a national listserv referred to as the Federal Amputation Interest Group (FAIG), which has > 800 members. Since 2009, the VA, in collaboration with the DoD, has produced 3 clinical practice guidelines (CPGs) related to amputation care. The Lower Limb Amputation CPG was published in 2007 and updated in 2017, and a CPG and associated clinician resources focused on upper extremity amputation were published in 2014.7,8 In addition to these formal, comprehensive, and evidence-driven guidelines, the ASoC has developed other clinical support documents covering a range of topics from prosthesis prescription candidacy determination to osseointegration. In conjunction with the EACE, The ASoC also has published guidance for clinical implementation of new technologies such as the Mobius Bionics LUKE arm and Dynamic Response Ankle-Foot Orthoses.
The ASoC strives to improve the psychosocial welfare of veterans with amputation and enhance trust in VA amputation care services through sharing results on the quality and timeliness of care. The Commission on Accreditation for Rehabilitation Facilities (CARF) provides an international, independent, peer-reviewed system of accreditation that is widely recognized by federal agencies, state governments, major insurers, and professional organizations.1,2 CARF offers amputation specialty accreditation for inpatient and outpatient programs that signifies the attainment of a distinguished level of expertise and the provision of a comprehensive spectrum of services related to amputation care and rehabilitation. During its development, the ASoC established the expectation that each of the RAC and PANS designated VAMCs would attain and maintain CARF amputation specialty accreditation. The ASoC has achieved 100% success on this metric.
In addition, the ASoC has completed many other initiatives focused on enhancing trust in VA amputation care services. These include assuring compliance with implementation of the Mission Act as it relates to the provision of amputation care and prosthetic limb delivery so that any services provided in the community are well integrated and at the direction of the amputation specialty team. The ASoC has maintained a strong relationship with the Amputee Coalition to provide veterans with high-quality patient education materials as well as integrated peer support services.
ASoC virtual and face-to-face training events incorporate suicide prevention training for providers. Special focus has been placed on care provision for Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn veterans with conflict-related multiple limb amputations. Although relatively small, this cohort is recognized as a unique and important population due to their unique care needs and increased risk for secondary complications. In 2019, 83% of these individuals were contacted to assure their amputation care needs were being adequately addressed.
Discussion
Over the past 10 years, the ASoC has built a modern, high-performance network of care to best serve veterans with amputation. Maturation of the system has included the addition of 3 new PANS locations to improve access to services as well as to better support geographic regions near large DoD military treatment facilities. The number of ACT designated VAMCs also has grown from 101 to 106 locations. The regional organization of sites has been modified to enhance the availability of referral and consultative services across the system. In addition, the ASoC has supported the development of an upper extremity amputation specialty program for consultation or referral to a highly specialized team of providers well versed in the significant technology advances that have taken place with upper extremity prostheses.9
One of the key components to high-performance network development is attaining a clear picture of the clinical demands and service delivery needs of the population served. The Amputee Data Repository was developed with the support of the VHA Support Service Center (VSSC) in order to better understand and track the population of veterans with amputation.6 The development and implementation of the Amputee Data Repository took place over several years, and the product was officially released into publication in 2015. The overall goals of this resource are to provide a data system for the ASoC to identify clinical care volumes and patterns of treatment; better understand the demographics of the veteran amputee population; assess the effectiveness of new treatment strategies; and utilize data analysis outcomes to influence clinical practice. The acquisition and analysis of this information will provide justification for the modification of clinical practice and will enhance the quality of care for all veterans with amputation.
Although the ASoC focuses primarily on the provision of clinical services, the system has been leveraged to support research activities and the advancement of artificial limb technologies. For example, ASoC providers and investigators supported the clinical research required to test and optimize the development of the DEKA arm. These research efforts resulted in the US Food and Drug Administration approval and commercialization of this device. Once the device became commercially available as the LUKE arm, the ASoC developed a clinical implementation strategy that assured availability and appropriate prescription and training with the new technology. The VA also has supported research and program development in osseointegration with further investigations and clinical implementation being planned.
Telehealth
The goal of the ASoC is to provide timely access and greater choice to specialty amputation rehabilitation services for veterans as determined by their clinical needs. One key strategy used to achieve this goal has been the expansion of virtual communication tools to enhance access to clinical expertise. Telehealth (Virtual Care) amputation services afford the opportunity to provide specialized clinical expertise to veterans who otherwise may not have access to this level of service or consultation.1,2 For others, virtual care services reduce the need for travel. The ASoC has leveraged these services effectively to enhance specialty amputation care for veterans in rural areas. Over time, the scope of virtual care services has expanded to provide virtual peer support services as well as care in the veteran’s home.
Another unique example is the use of virtual care to see veterans when they are being provided services by a community prosthetist. This service improves the timeliness of care and reduces the travel burden for the veteran. Between 2009 and 2019, total virtual care encounters to provide amputation-related services grew from 44 encounters to 3,905 encounters (Figure 5). In 2019, 13.8% of veterans seen in a VA outpatient amputation specialty clinic had at least 1 virtual encounter in the same year.6
In addition to the expansion of virtual care and building capacity through increasing the number of amputation specialty clinics and providers, the ASoC has used a host of other strategies to improve care access. The development of provider expertise in amputation care has been achieved through the methods of extensive provider training. Implementation of Patient Self-Referral Direct Scheduling allows veterans to access the outpatient amputation specialty clinic without a referral and without having to be seen by their primary care provider. This initiative provides easier and more timely access to amputation specialty services while reducing burden on primary care services. The amputation outpatient specialty clinic was one of a few specialty programs to be an early adopter of national online scheduling. The implementation of this service is still ongoing, but this program gives veterans greater control over scheduling, canceling, and rescheduling appointments.
Conclusions
During the 10 years following its implementation, the VA ASoC has successfully enhanced the quality and consistency of care and rehabilitation services provided to veterans with limb loss through the provision of highly specialized services in the areas of medical care, rehabilitation services, and prosthetic technology. This mission has been accomplished through prioritization and implementation of key strategic initiatives in learning organization creation, trust in VA care, development of a modern, high-performance network, and customer service. Collaborative partnerships both internally within the VA and externally with key stakeholders has facilitated this development, and these will need to be enhanced for future success. Evolving trends in amputation surgery, limb transplantation, artificial limb control and suspension strategies as well as advances in assistive technology also will need to be integrated into best practices and program development.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.03(1): Amputation system of care. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7482. Published August 3, 2018. Accessed July 31, 2020.
2. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs Amputations System of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
3. Reiber GE, Smith DG. VA paradigm shift in care of veterans with limb loss. J Rehabil Res Dev. 2010;47(4):vii-x. doi:10.1682/jrrd.2010.03.0030
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1172.01: Polytrauma system of care. https://www.va.gov/OPTOMETRY/docs/VHA_Directive_1172-01_Polytrauma_System_of_Care_1172_01_D_2019-01-24.pdf. Published January 24, 2019. Accessed July 31, 2020.
5. VHA Directive 1410, Prevention of amputation in veterans everywhere (PAVE) program, https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed July 31, 2020.
6. VHA Amputee Data Repository. VHA Support Service Center. http://vssc.med.va.gov. [Nonpublic source, not verified.]
7. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPG092817.pdf. Accessed July 16, 2020.
8. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: The Management of upper extremity amputation rehabilitation.Version 1-2014. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf. Accessed July 16, 2020.
9. Resnik L, Meucci MR, Lieberman-Klinger S, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93(4):710-717. doi:10.1016/j.apmr.2011.11.010
10. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical practice guidelines: rehabilitation of lower limb amputation. Version 2.0 -2017. Pocket card. https://www.healthquality.va.gov/guidelines/Rehab/amp/VADoDLLACPGPocketCard092817.pdf. Accessed July 31, 2020.
Welcome to week 2 of HM20 Virtual!
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
Psoriasis in Patients of Color: Differences in Morphology, Clinical Presentation, and Treatment
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”
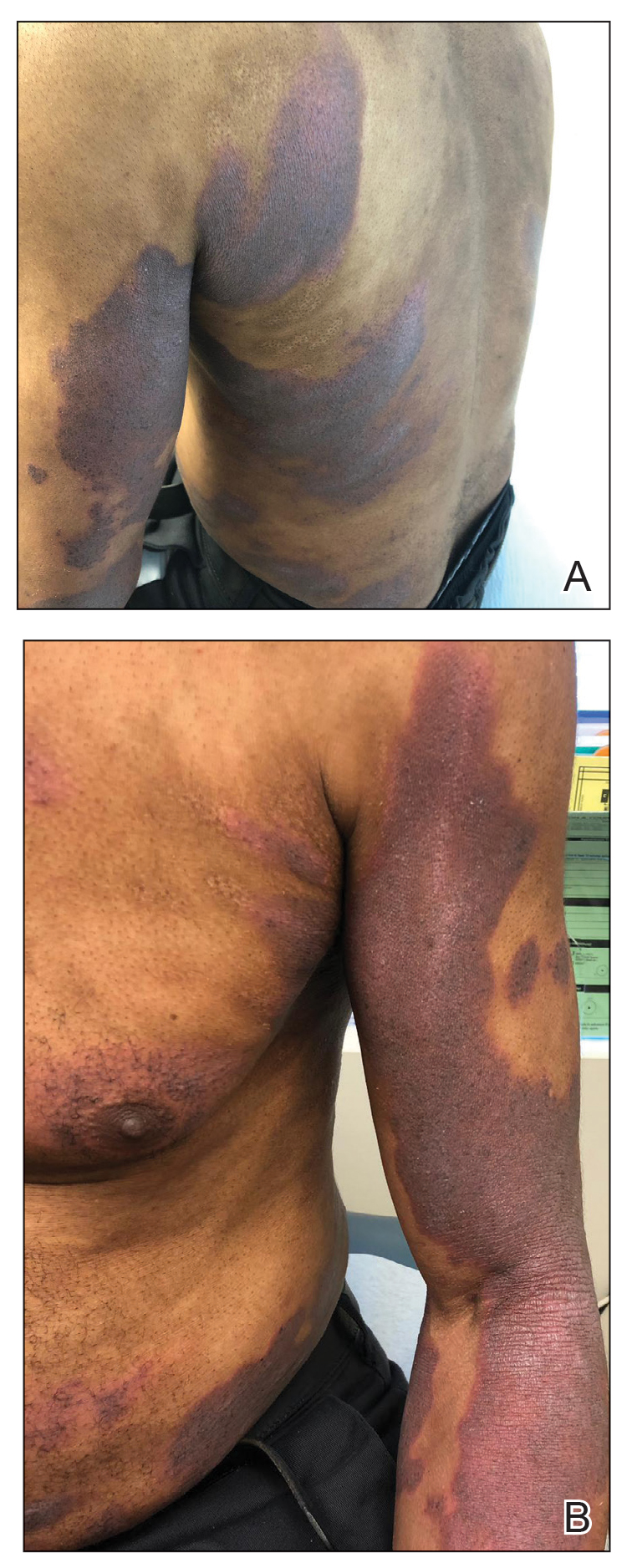
Patient 2
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”
Patient 3
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.
Comment
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.
Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”

Patient 2
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”

Patient 3
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.

Comment
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.

Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”

Patient 2
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”

Patient 3
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.

Comment
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.

Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
Practice Points
- There are key differences in psoriasis in patients with skin of color, including the morphology, clinical presentation, treatment, and psychosocial impact.
- Recognition and awareness of these differences may normalize the condition for patients, support them seeking medical attention sooner, and better inform them of all possible treatment options.
- Advocating further education on these differences in residency training and continuing medical education programs may help physicians make earlier diagnoses and personalize physician-patient conversations.
Management of Psoriasis With Biologics in Clinical Practice: An Update for 2020
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.
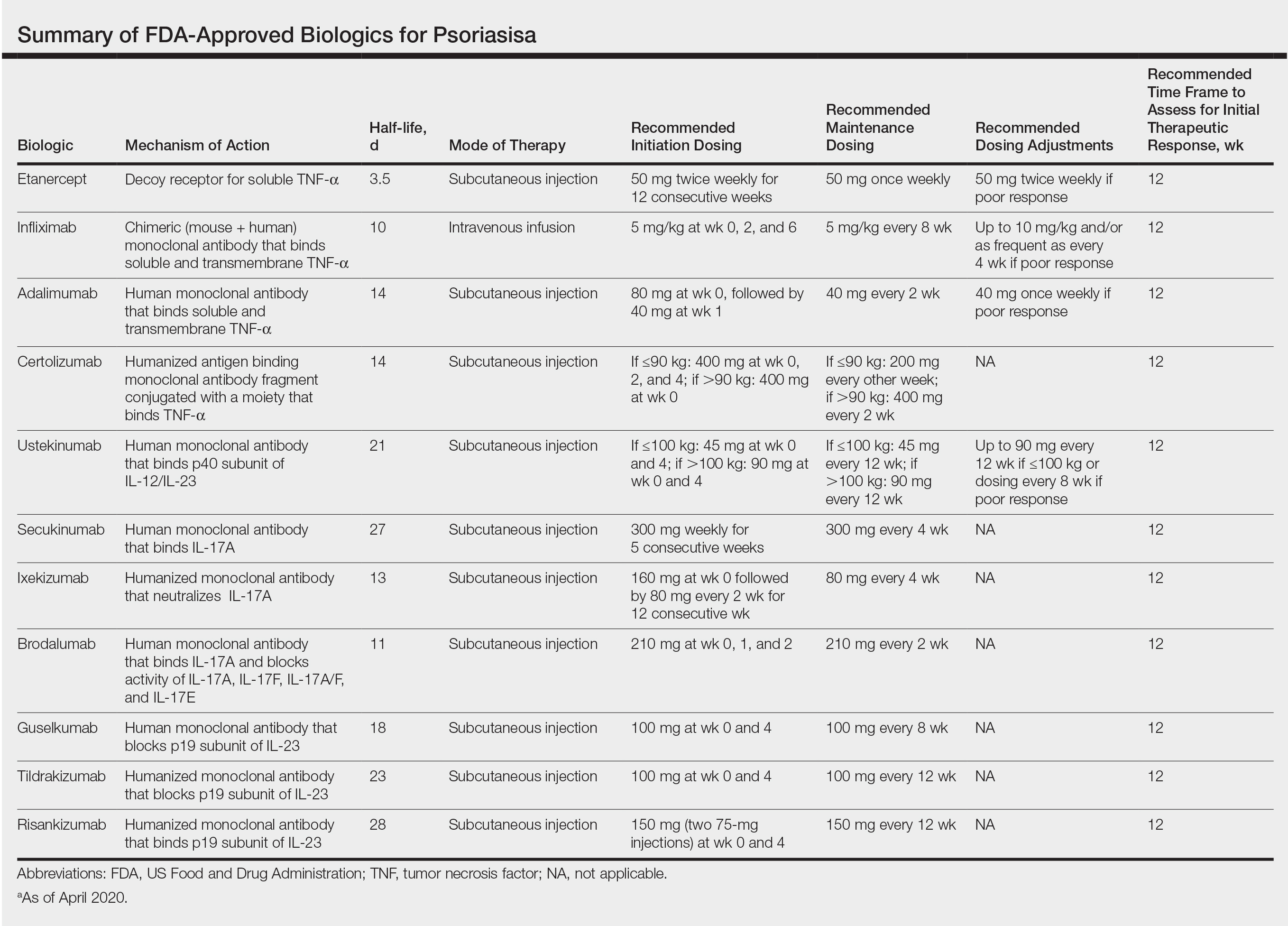
Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
Practice Points
- Inform patients about current data guiding treatment from clinical trials of biologics.
- Explain to patients that finding the treatment that is the best fit for them may require trial and error, as everyone responds to treatments differently.
- Consult with patients about misconceptions and potential fears about biologics and what the protocol is for monitoring safety during treatment.
Treatment of Psoriasis in Pregnancy
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Practice Points
- Robust safety data often are lacking for the use of topical and systemic agents to treat psoriasis in pregnancy.
- Professional society guidelines on the use of systemic agents in pregnancy vary among dermatology, gastroenterology, and rheumatology organizations.
Product News August 2020
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].