User login
For MD-IQ use only
Diffuse Painful Plaques in the Setting of Chronic Lymphocytic Leukemia
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
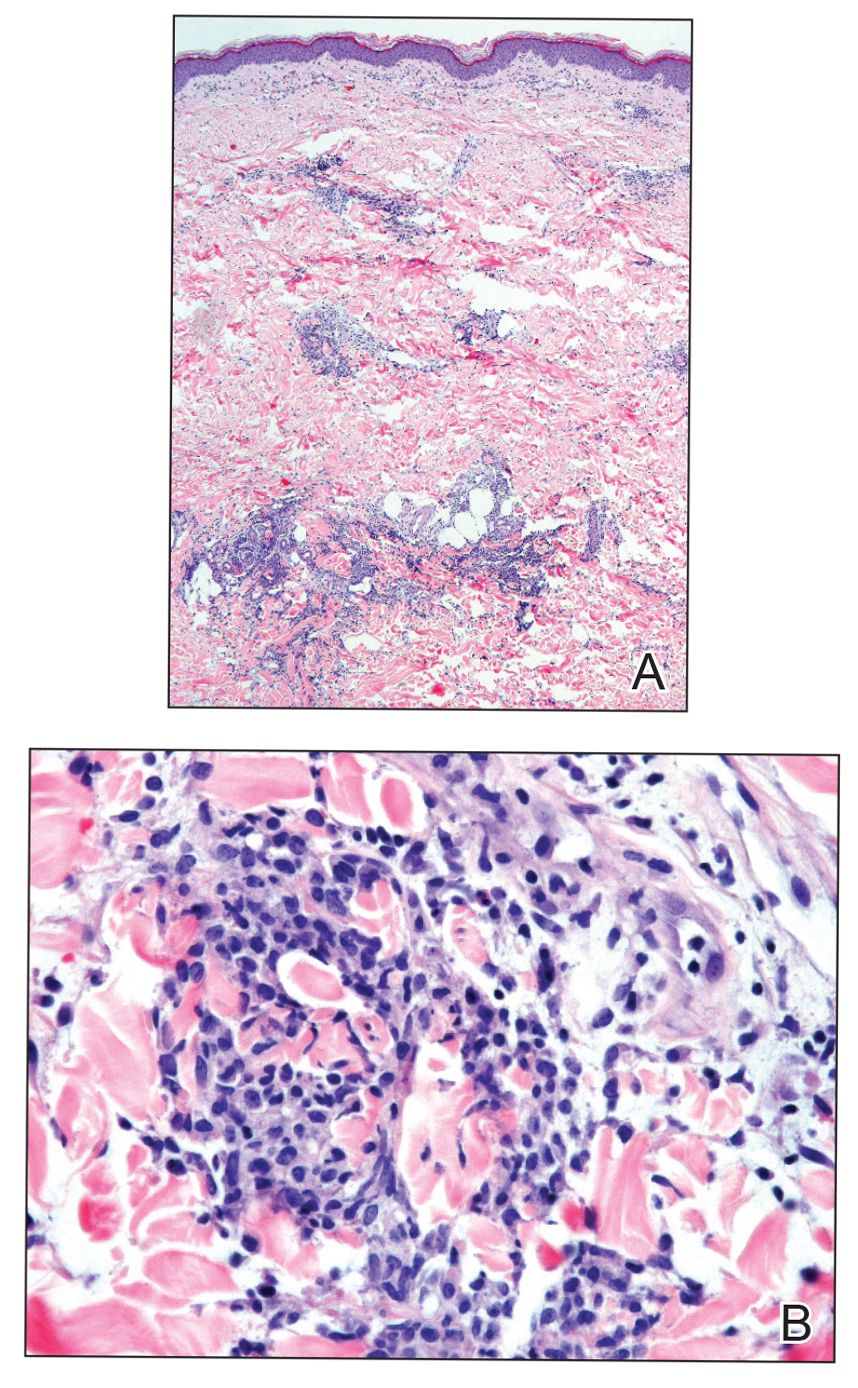
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.
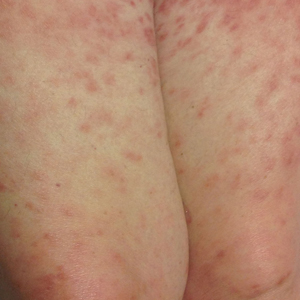
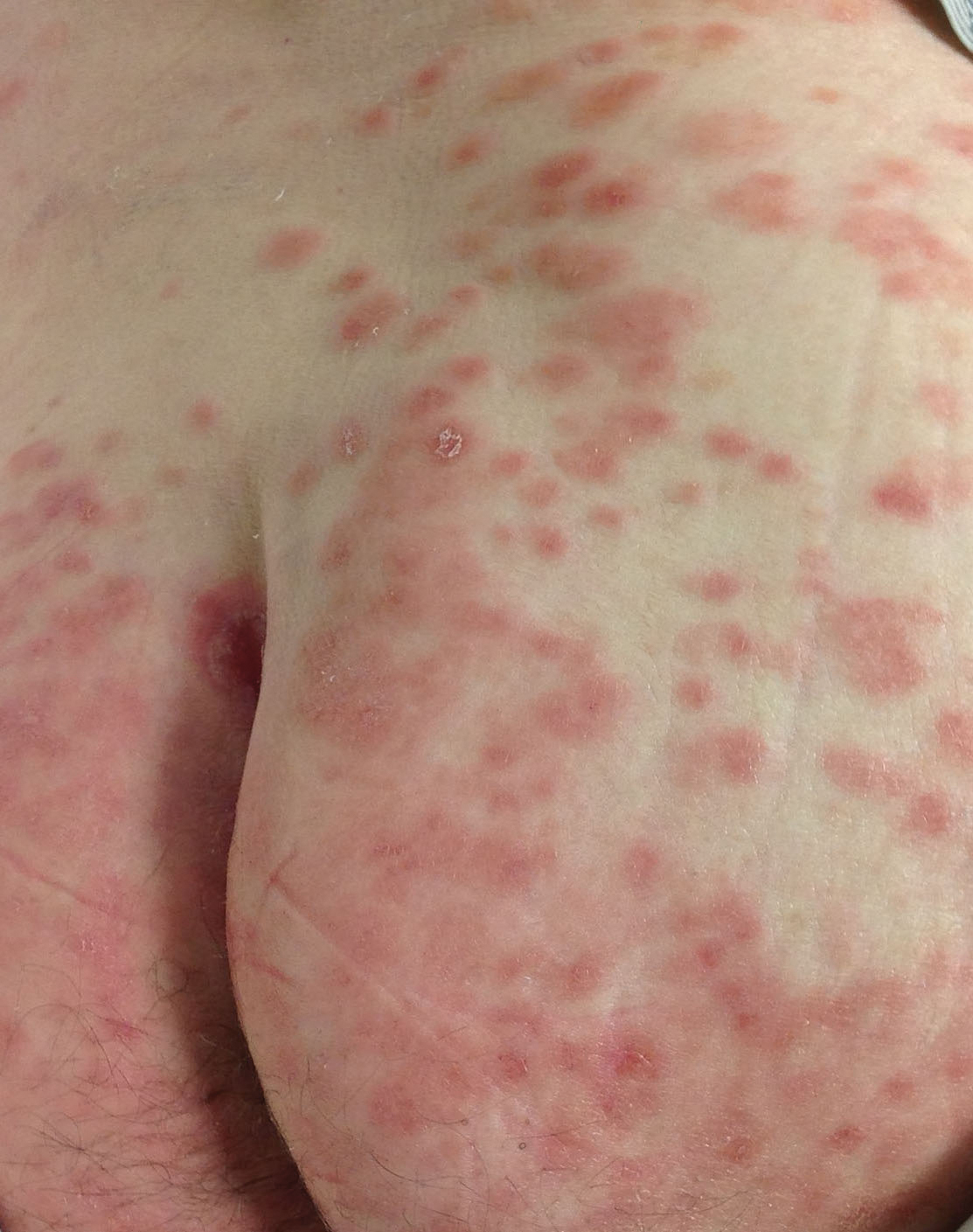
Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.

Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
The Diagnosis: Cutaneous Mycobacterium avium-intracellulare Complex Infection
Histopathologic evaluation revealed superficial and deep perivascular and periadnexal inflammation. The epidermis exhibited some vacuolar interface change and effacement with relatively sparse dyskeratotic cells. A lymphohistiocytic inflammatory infiltrate surrounded the blood vessels, nerves, and adnexal structures and extended into the subcutaneous fat (Figure). Acid-fast, Grocott-Gomori methenamine-silver, Gram, Fite, Treponema pallidum, and Alcian blue stains were performed at our institution and were all negative. Biopsies sent to the National Hansen's Disease (Leprosy) Program demonstrated scattered extracellular acid-fast organisms on Fite staining in the specimen of the forearm. Polymerase chain reaction testing for Mycobacterium leprae DNA was negative. DNA sequencing of the 16S ribosomal RNA gene matched Mycobacterium avium-intracellulare complex (MAC). In the workup of the hepatic mass, the patient incidentally was found to have large-cell transformation of chronic lymphocytic leukemia (CLL) and therefore was treated with bendamustine and rituximab as an outpatient. The patient received 1 chemotherapy infusion every 4 weeks for a total of 10 rounds. At 10-week follow-up after 2 rounds of chemotherapy, all of the skin lesions had resolved despite no antibiotic therapy for atypical infections.

Disseminated infection with MAC is relatively rare in healthy as well as immunocompromised individuals. Clinical disease most commonly is seen as an opportunistic infection in patients with AIDS who have CD4 counts less than 50/mm3 (reference range, 500-1400/mm3) or in those with preexisting lung disease.1 Cutaneous involvement has been observed in only 14% of non-AIDS patients with disseminated MAC infection.2 In another study of 76 patients with MAC infection, only 2 involved the skin or soft tissue.3 Infection of the skin without concurrent pulmonary MAC infection is rare, though trauma may cause isolated skin infection. The cutaneous presentation of MAC infection is highly variable and may include erythematous papules, pustules, panniculitis, infiltrated plaques, verrucous lesions, and draining sinuses.3 The lesions have been reported to be painful.1
Cutaneous findings occur in up to 25% of patients with CLL, either due to the seeding of leukemic cells or other secondary lesions.4 Leukemia cutis, or skin involvement by B-cell CLL, most commonly presents in the head and neck region as chronic and relapsing erythematous papules and plaques.5 It histologically presents as monomorphic lymphocytic infiltrates accentuated around periadnexal and perivascular structures, with some extending into adipose tissue.2 In our case, histopathology demonstrated a lack of monomorphous infiltrate and thus was inconsistent with leukemia cutis. Similarly, lack of pale pink deposits and lack of neutrophilic infiltrates or degenerated collagen makes amyloidosis and palisaded neutrophilic granulomatous dermatitis incorrect diagnoses, respectively.
We hypothesize that the initially undetected worsening of CLL resulted in an immunocompromised state, which facilitated this unique presentation of cutaneous MAC infection in a human immunodeficiency virus-negative patient with no clinical symptoms of active pulmonary disease. The rash was the presenting sign of both the cutaneous MAC infection and worsening CLL. Additionally, our patient's cutaneous MAC facial involvement clinically resembled the leonine facies that is classic in lepromatous leprosy. Rare reports have been published addressing this similarity.6
Treatment of MAC pulmonary disease usually includes a combination of clarithromycin or azithromycin, rifampin, and ethambutol (for nodular/bronchiectatic disease), with or without amikacin or streptomycin.7 For limited pulmonary disease in patients with adequate pulmonary reserve, surgical resection may be considered in combination with the multidrug MAC pulmonary treatment regimen for 3 months to 1 year. Patients with localized MAC disease involving only the skin, soft tissue, tendons, and joints usually are treated with surgical excision in combination with clarithromycin, rifampin, and ethambutol for 6 to 12 months.7 In our patient, we believe that chemotherapy and the subsequent reconstituted immune system likely cleared the MAC infection without targeted antibiotic treatment.
Acknowledgments
The authors would like to thank David Scollard, MD, PhD, and Barbara Stryjewska, MD, from the National Hansen's Disease (Leprosy) Association (Baton Rouge, Louisiana).
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Plaza JA, Comfere NI, Gibson LE, et al. Unusual cutaneous manifestations of chronic lymphocytic leukemia. J Am Acad Dermatol. 2009;60:772-780.
- Sivanesan SP, Khera P, Buckthal-McCuin J, et al. Cutaneous Mycobacterium avium-intracellulare complex associated with immune reconstitution inflammatory syndrome. J Am Acad Dermatol. 2010;62:E25-E26.
- Horsburgh CR, Mason UG, Farhi DC, et al. Disseminated infection with Mycobacterium avium-intracellulare. a report of 13 cases and a review of the literature. Medicine (Baltimore). 1985;64:36-48.
- Bodle EE, Cunningham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:290-296.
- Boyd AS, Robbins J. Cutaneous Mycobacterium avium intracellulare infection in an HIV+ patient mimicking histoid leprosy. Am J Dermatopathol. 2005;27:39-41.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.

Staying financially well in the time of COVID-19
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
As COVID-19 continues to threaten the United States and the world, individuals in every profession have been challenged to examine their financial situation. At Fidelity Investments, we recently conducted a national survey asking people how current events have affected their opinions and behaviors when it comes to their money. The results showed that six in 10 Americans are concerned about household finances over the next 6 months. Unfortunately, we’ve seen that even health care professionals have not been financially spared, with salaries or benefits cut or, worse, furloughs and layoffs as hospital systems struggle. I work with many physicians, including gastroenterologists, in my role as a wealth planner for Fidelity Investments and have received quite a few questions related to shoring up family finances during these difficult times.
Luckily, the financial best practices that I share in “good” times ring true even in today’s world, with a few additions given the health and economic risks created by COVID-19.
1. Review your budget. It’s one thing to know that your budget is generally balanced (the dollars you spend are less than the dollars you earn). But it’s worth taking a closer look to see just where those dollars are going. In times of uncertainty, cutting back on expenses that aren’t necessary or don’t provide meaningful value to your life can be worthwhile. If you or your family have lost income because of the pandemic, you might consider these seven simple tips to help boost your cash flow.
2. Tackle (or find relief from) student loan debt. Doctors today graduate medical school with a median debt of just under $195,000.1 Repaying these loans is daunting, particularly during the COVID-19 crisis. The recent passing of the CARES Act recognizes these difficult times: in fact, it automatically suspended required minimum loan payments and interest accrual on federal student loans until Sept. 30, 2020. This only applies to federal student loans, not private student loans. Beyond this period, if you are still struggling with payments, you may explore the possibility of refinancing, by taking out a lower-interest private loan and using that to pay off student loans (although this may extend the life of your loan). Borrowers could also consider other programs, such as REPAYE (Revised Pay As You Earn) through which your monthly payment tops out at 10% of your monthly income, or Public Service Loan Forgiveness (PSLF) if you work for a not-for-profit hospital or other qualifying employer. This program forgives the remaining balance on your direct loans after you have made 120 qualifying monthly payments while working full-time for a qualifying employer.
Additionally, borrowers could look for opportunities to reduce accrued interest, either by refinancing to a lower rate or making payments every 2 weeks rather than once each month.
3. Evaluate your emergency fund. It’s a good idea to keep 3-6 months’ of essential expenses in cash or cash-like investments. If you don’t yet have this 3- to 6-month cushion saved, now is a good time to work to reduce your expenses and stash away any extra cash.
4. Save early and often for retirement. You can borrow money to support many of life’s needs, from housing, to cars, to college. But you can’t borrow for retirement. That is why I encourage clients to put retirement savings at the top of the list, after accounting for day-to-day needs of their families. People often ask me whether it makes sense to continue saving for retirement, often a far-off goal for younger doctors, especially in these uncertain times. My answer? Yes. If you are able to save, continue to save: the earlier you begin to make contributions to your retirement account, and the longer you continue to do so, the more your retirement account(s) have the potential to grow over time.
Another question I receive is whether to take distributions from a retirement account early if you find yourself in a precarious financial situation because of the COVID-19 crisis. The CARES Act provides options allowing Americans to take a withdrawal or loan from a participating retirement plan if you, your spouse, or your dependent have a COVID-19 related illness or you’re experiencing a loss of income related to the COVID-19 pandemic. Try to look at alternative sources of income before tapping your hard-earned retirement savings. If you can find a way to continue saving and avoid drawing down your retirement accounts, your future self will thank you.
5. If you have a high-deductible health plan that offers it, explore a Health Savings Account (HSA). One of the most important factors in a solid financial plan is knowing how to pay for health care expenses, both now and as we age. HSAs are a tax-advantaged account that can be used to save money for qualified medical expenses. They are considered to provide a “triple-tax advantage” since contributions, qualified withdrawals, and investment growth are all tax-free.2 The dollars in these accounts can stay there over time, so in years with low expenses you could use these to save for health care in retirement, while in other years they can be used to pay necessary medical bills. HSAs require the participant to be enrolled in a high-deductible health plan, so you would first need to verify that your employer provides this option.
6. Be prepared to protect yourself, your practice, and your family. Typically, I encourage the medical professionals I work with to review their current insurance plans (such as disability, life, and malpractice) to determine whether they have the right levels of coverage for their situation. With COVID-19 layered on top of the usual level of risk, it’s important to consider reviewing or updating other key elements of your family’s plan, like your health care proxies and a living will.
7. Put your income to work. When your disposable income grows, and you’ve covered all of the foundational elements of a financial plan (a rainy-day fund, contingency planning for health care costs, and so on), it might be the right time to consider investing for something other than retirement. As you do that, be sure you are invested in a diversified strategy with a balance of risk and return that is comfortable for you.
Recent market volatility can bring nerves that make it difficult to stay invested. However, as long as your risk tolerance and time horizon reflect your asset allocation – the mix of stock, bonds, and cash (which a financial planner can help with) – you can take comfort in knowing that historically every severe downturn has eventually given way to further growth.
During uncertain times like these, I think the best guidance is to focus on what you can control. The considerations above are a great place to start building a financial plan to solidify you and your family’s future. A Fidelity survey found that 44% of Americans are now working to build up their emergency savings, and one-third (34%) are rethinking how they manage their money because of the COVID-19 crisis.3 Despite the stresses we all face, there is no time like the present to start or revisit your financial plan.
Footnotes
1. Barron D. Why Doctors Are Drowning in Medical School Debt. Scientific American. July 15, 2019.
2. With respect to federal taxation only. Contributions, investment earnings, and distributions may or may not be subject to state taxation. The triple tax advantages are only applicable if the money is used to pay for qualified medical expenses as described in IRS Publication 969.
3. Fidelity Market Sentiment Study presents the findings of a nationwide online survey consisting of 3,012 adults, at least 18 years of age, from which 1,591 respondents qualified as having at least one investment account. The study was fielded April 1-8, 2020, by ENGINE INSIGHTS, an independent research firm not affiliated with Fidelity Investments. The results of this survey may not be representative of all adults meeting the same criteria as those surveyed for this study. For the purposes of this study, the generations are defined as follows: Millennials (aged 24-39 years); Generation X (aged 40-55 years); Baby Boomers (aged 56-74 years).
Mr. Tudor is Vice President, Wealth Planning Consultant at Fidelity Investments.
Population health can improve postdischarge care
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
NBA star Mason Plumlee on COVID and life inside the Orlando ‘bubble’
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Welcome to the final week of HM20 Virtual!
Hospitalists, welcome to the final week of HM20 Virtual – the first virtual annual conference in the history of SHM. We hope you have enjoyed your experience thus far. What is most exciting about HM20 Virtual is how it exemplifies the agility and innovation that is in the hearts and minds of SHM and hospitalists all around the country and beyond. Because 2020 is shaping up to be anything but ordinary, SHM and its members have had to embrace changes on a scale that has surpassed anything we have seen in most of our careers. SHM is committed to keeping pace with the needs of hospitalists, including the use of virtual meetings to keep us all connected, informed, and engaged.
The full course of sessions for HM20 has a vast array of topics, aimed at quickly and concisely updating our members on core topics. These include clinical updates on common conditions, a half-dozen sessions dedicated to COVID-19, the ever-popular Rapid Fire sessions, an Update in Pediatric Top Articles, a High Value Care session, and special sessions on immigrant hospitalist issues and structural racism. Also included is the Best of Research and Innovations and the Annual Update in Hospital Medicine.
In addition, the “Simulive” sessions offer additional Q&A with the experts; our lineup this week includes many important clinical topics, including heart failure, glucose management, GI emergencies, drug allergies, endocrine emergencies, and balancing being a hospitalist and a parent. (Particularly challenging with COVID-19!) Of note, if you are unable to join any of these sessions live, all 3 weeks of the Simulive sessions will be available on demand after Aug. 31.
We are hopeful this new virtual format will exceed expectations for our members. Our dedicated HM20 faculty and SHM staff have worked tirelessly to make HM20 Virtual a success, and for that, we owe them our gratitude and appreciation. Since this is our “first rodeo,” we are very eager for your feedback on all aspects of this new format. The more we learn now, the better off we will all be with future offerings, so please be candid and honest. With so much change and uncertainty, we hope you continue to find SHM to be a place of consistency and stability, and the “source of truth” for all things hospital medicine.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Hospitalists, welcome to the final week of HM20 Virtual – the first virtual annual conference in the history of SHM. We hope you have enjoyed your experience thus far. What is most exciting about HM20 Virtual is how it exemplifies the agility and innovation that is in the hearts and minds of SHM and hospitalists all around the country and beyond. Because 2020 is shaping up to be anything but ordinary, SHM and its members have had to embrace changes on a scale that has surpassed anything we have seen in most of our careers. SHM is committed to keeping pace with the needs of hospitalists, including the use of virtual meetings to keep us all connected, informed, and engaged.
The full course of sessions for HM20 has a vast array of topics, aimed at quickly and concisely updating our members on core topics. These include clinical updates on common conditions, a half-dozen sessions dedicated to COVID-19, the ever-popular Rapid Fire sessions, an Update in Pediatric Top Articles, a High Value Care session, and special sessions on immigrant hospitalist issues and structural racism. Also included is the Best of Research and Innovations and the Annual Update in Hospital Medicine.
In addition, the “Simulive” sessions offer additional Q&A with the experts; our lineup this week includes many important clinical topics, including heart failure, glucose management, GI emergencies, drug allergies, endocrine emergencies, and balancing being a hospitalist and a parent. (Particularly challenging with COVID-19!) Of note, if you are unable to join any of these sessions live, all 3 weeks of the Simulive sessions will be available on demand after Aug. 31.
We are hopeful this new virtual format will exceed expectations for our members. Our dedicated HM20 faculty and SHM staff have worked tirelessly to make HM20 Virtual a success, and for that, we owe them our gratitude and appreciation. Since this is our “first rodeo,” we are very eager for your feedback on all aspects of this new format. The more we learn now, the better off we will all be with future offerings, so please be candid and honest. With so much change and uncertainty, we hope you continue to find SHM to be a place of consistency and stability, and the “source of truth” for all things hospital medicine.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Hospitalists, welcome to the final week of HM20 Virtual – the first virtual annual conference in the history of SHM. We hope you have enjoyed your experience thus far. What is most exciting about HM20 Virtual is how it exemplifies the agility and innovation that is in the hearts and minds of SHM and hospitalists all around the country and beyond. Because 2020 is shaping up to be anything but ordinary, SHM and its members have had to embrace changes on a scale that has surpassed anything we have seen in most of our careers. SHM is committed to keeping pace with the needs of hospitalists, including the use of virtual meetings to keep us all connected, informed, and engaged.
The full course of sessions for HM20 has a vast array of topics, aimed at quickly and concisely updating our members on core topics. These include clinical updates on common conditions, a half-dozen sessions dedicated to COVID-19, the ever-popular Rapid Fire sessions, an Update in Pediatric Top Articles, a High Value Care session, and special sessions on immigrant hospitalist issues and structural racism. Also included is the Best of Research and Innovations and the Annual Update in Hospital Medicine.
In addition, the “Simulive” sessions offer additional Q&A with the experts; our lineup this week includes many important clinical topics, including heart failure, glucose management, GI emergencies, drug allergies, endocrine emergencies, and balancing being a hospitalist and a parent. (Particularly challenging with COVID-19!) Of note, if you are unable to join any of these sessions live, all 3 weeks of the Simulive sessions will be available on demand after Aug. 31.
We are hopeful this new virtual format will exceed expectations for our members. Our dedicated HM20 faculty and SHM staff have worked tirelessly to make HM20 Virtual a success, and for that, we owe them our gratitude and appreciation. Since this is our “first rodeo,” we are very eager for your feedback on all aspects of this new format. The more we learn now, the better off we will all be with future offerings, so please be candid and honest. With so much change and uncertainty, we hope you continue to find SHM to be a place of consistency and stability, and the “source of truth” for all things hospital medicine.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Weight-loss benefit in diabetes is similar via surgery or diet
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
The metabolic benefits achieved with weight loss for patients with type 2 diabetes appear to be similar whether achieved via diet or gastric bypass surgery, according to new research.
The findings, from a small study of 22 people with type 2 diabetes and obesity, were published online August 19 in the New England Journal of Medicine. The study was conducted by Mihoko Yoshino, MD, PhD, of Washington University, St. Louis, and colleagues.
Among 11 patients in each group who experienced comparable degrees of weight loss (about 18%), there were very similar benefits in multiorgan insulin sensitivity, beta-cell function, 24-hour plasma glucose and insulin profiles, and body composition.
“The results from our study underscore the profound effect that marked weight loss can have on metabolic function in people with diabetes,” Dr. Yoshino and colleagues wrote.
“The similar findings in participants in the two groups challenge the current belief that upper gastrointestinal bypass has clinically meaningful effects on key metabolic factors involved in glucose homeostasis and the pathogenesis of diabetes that are independent of weight loss,” they added.
However, they acknowledged, “the difficulty in achieving successful long-term weight loss with lifestyle therapy often renders gastric bypass surgery far more effective than diet therapy for most patients with obesity and type 2 diabetes.”
“This study confirms the pathogenic nature of obesity in driving insulin resistance and, ultimately, type 2 diabetes; furthermore, it delivers a straightforward and important message for both clinicians and patients – reducing adipose tissue volume, by whatever means, will improve blood glucose control in persons with type 2 diabetes,” stated the authors of an accompanying editorial.
Asked to comment, Matthew M. Hutter, MD, president of the American Society for Metabolic and Bariatric Surgery and professor of surgery at Harvard Medical School and Massachusetts General Hospital, both in Boston, said in an interview that the study was “very elegant” and “well designed.”
However, he pointed out, “the reality is diet alone has been shown over and over that it’s not sustainable. For a very small few it is, but for the vast majority, that’s not the case.”
Dr. Hutter also noted that the research was conducted in a laboratory setting and that the diet group was given prepackaged food. Therefore, the study “doesn’t look at how effective [dieting] is in the real world.”
Bariatric surgery, on the other hand, “is very effective for treating type 2 diabetes. It’s a good long-term solution.”
No significant differences in multiple parameters
The nonrandomized prospective cohort study began with 33 adults with obesity and type 2 diabetes, of whom 18 received weekly diet education sessions and prepackaged food, and 15 underwent Roux-en-Y gastric bypass procedures. Seven in the diet group and four in the surgery group were withdrawn from the study because of a failure to achieve the target of 16%-18% loss of body weight.
Of those remaining in the study, mean weight loss at around 4 months was 17.8% of body weight for the 11 in the diet group and 18.7% for the 11 in the surgery group. For the diet group, the mean age was 54 years and the mean duration of diabetes was 9.1 years; for the surgery group, the mean age was 49 years and the mean duration of diabetes 9.6 years.
A three-stage hyperinsulinemic euglycemic pancreatic clamp was used to control both portal and systemic plasma insulin concentrations to provide a reliable assessment of hepatic, muscle, and adipose tissue insulin sensitivity across a physiological range of plasma insulin concentrations. In both treatment groups, similar improvements were seen in hepatic, adipose tissue, and skeletal muscle insulin sensitivity
Both groups experienced a similar drop in the use of diabetes medications. Four individuals in the diet group and two in the surgery group achieved hemoglobin A1c levels below 6.0% without any medications.
After ingestion of identical mixed meals, 4-hour areas under the curve (AUC) for plasma glucose and insulin were lower after weight loss than before for both groups, although the decrease in glucose was greater in the diet group. Postprandial glucose peaks were greater in the surgery group as well, owing to an increase in the rate of glucose delivery into the circulation.
Weight loss was associated with decreased total AUC for glucose, free fatty acids, insulin, and insulin secretion to similar degrees in both groups. However, there were differences in several factors that have been attributed to the effects of bariatric surgery independently of weight loss. These include a greater decrease in 24-hour plasma branched-chain amino acid and C3 and C5 acylcarnitine concentrations after weight loss in the surgery group than in the diet group.
Plasma bile acid levels after weight loss dropped from baseline in the diet group but rose in the surgery group. Although microbiome changes occurred in both groups, they were more pronounced with weight loss following surgery.
Study limitations: Small, not randomized, long diabetes duration
The authors acknowledged some of the study’s limitations, including the fact that it wasn’t randomized and there was a large number of dropouts, although they say that the method used to assess the primary outcome can detect small differences even in a few patients.
The editorialists – endocrinologist Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough, and pediatric nephrologist and deputy editor for the New England Journal of Medicine Julie R. Ingelfinger, MD, of Massachusetts General Hospital, Boston – pointed out that today more sleeve gastrectomies are performed than Roux-en-Y bypass procedures.
However, Dr. Hutter said that gastric bypass procedures are still being performed, that both procedures are effective and safe, and that for patients with diabetes the bypass tends to be somewhat more effective than sleeve gastrectomy.
He also qualified: “What phase you’re in is critical. Here, the 18% [weight loss] is still early in the track for bariatric surgery. They could still be losing weight after that. The diet groups could have been in the plateau or regain phase. Those [numbers] could look very different with time.”
In addition, he noted that the relatively long average duration of diabetes in the study participants makes the success of bariatric surgery less likely.
“The pancreas burns out, and the tissues become less insulin sensitive. I would have wanted to stratify the patients into early versus later disease, but you would need a larger sample size to do that,” he said.
Dr. Hutter noted: “I thought the study was very interesting. It just amazes me that 60 years after the gastric bypass procedure was invented, we’re still trying to figure out how it works.”
Dr. Hutter is a consultant for Vicarious Surgical and has received honoraria from Ethicon, Medtronic, and Olympus.
A version of this article originally appeared on Medscape.com.
Are aging physicians a burden?
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
The evaluation of physicians with alleged cognitive decline
The evaluation of physicians with alleged cognitive decline
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
As forensic evaluators, we are often asked to review and assess the cognition of aging colleagues. The premise often involves a minor mistake, a poor choice of words, or a lapse in judgment. A physician gets reported for having difficulty using a new electronic form, forgetting the dose of a brand new medication, or getting upset in a public setting. Those behaviors often lead to mandatory psychiatric evaluations. Those requirements are often perceived by the provider as an insult, and betrayal by peers despite many years of dedicated work.
Interestingly, we have noticed many independent evaluators and hospital administrators using this opportunity to send many of our colleagues to pasture. There seems to be an unspoken rule among some forensic evaluators that physicians should represent some form of apex of humanity, beyond reproach, and beyond any fault. Those evaluators will point to any mistake on cognitive scales as proof that the aging physician is no longer safe to practice.1 Forgetting that Jill is from Illinois in the Saint Louis University Mental Status Examination test or how to copy a three-dimensional cube on the Montreal Cognitive Assessment can cost someone their license.2 We are also aware of some evaluators even taking the step further and opining that physicians not only need to score adequately but also demonstrate cognition significantly above average to maintain their privileges.
There is certainly significant appeal in setting a high bar for physicians. In many ways, physicians are characterized in society by their astuteness, intelligence, and high ethical standards. Patients place their lives in the hands of physicians and should trust that those physicians have the cognitive tools to heal them. It could almost seem evident that physicians should have high IQs, score perfectly on screening tools for dementia, and complete a mandatory psychiatric evaluation without any reproach. Yet the reality is often more complex.
We have two main concerns about the idea that we should be intransigent with aging physicians. The first one is the vast differential diagnosis for minor mistakes. An aging physician refusing to comply with a new form or yelling at a clerk once when asked to learn a new electronic medical record are inappropriate though not specific assessments for dementia. Similarly, having significant difficulty learning a new electronic medical record system more often is a sign of ageism rather than cognitive impairment. Subsequently, when arriving for their evaluation, forgetting the date is a common sign of anxiety. A relatable analogy would be to compare the mistake with a medical student forgetting part of the anatomy while questioning by an attending during surgery. Imagine such medical students being referred to mandatory psychiatric evaluation when failing to answer a question during rounds.
In our practice, the most common reason for those minor mistakes during our clinical evaluation is anxiety. After all, patients who present for problems completely unrelated to cognitive decline make similar mistakes. Psychological stressors in physicians require no introduction. The concept is so prevalent and pervasive that it has its own name, “burnout.” Imagine having dedicated most of one’s life to a profession then being enumerated a list of complaints, having one’s privileges put on hold, then being told to complete an independent psychiatric evaluation. If burnout is in part caused by a lack of control, unclear job expectations, rapidly changing models of health care, and dysfunctional workplace dynamics, imagine the consequence of such a referral.
The militant evaluator will use jargon to vilify the reviewed physician. If the physician complains too voraciously, he will be described as having signs of frontotemporal dementia. If the physician comes with a written list of rebuttals, he will be described as having memory problems requiring aids. If the physician is demoralized and quiet, he will be described as being withdrawn and apathetic. If the physician refuses to use or has difficulty with new forms or electronic systems, he will be described as having “impaired executive function,” an ominous term that surely should not be associated with a practicing physician.
The second concern arises from problems with the validity and use of diagnoses like mild cognitive impairment (MCI). MCI is considered to be a transition stage when one maintains “normal activities of daily living, and normal general cognitive function.”3 The American Psychiatric Association Textbook of Psychiatry mentions that there are “however, many cases of nonprogressive MCI.” Should a disorder with generally normal cognition and unclear progression to a more severe disorder require one to be dispensed of their privileges? Should any disorder trump an assessment of functioning?
It is our experience that many if not most physicians’ practice of medicine is not a job but a profession that defines who they are. As such, their occupational habits are an overly repeated and ingrained series of maneuvers analogous to so-called muscle memory. This kind of ritualistic pattern is precisely the kind of cognition that may persist as one starts to have some deficits. This requires the evaluator to be particularly sensitive and cognizant that one may still be able to perform professionally despite some mild but notable deficits. While it is facile to diagnose someone with MCI and justify removing their license, a review of their actual clinical skills is, despite being more time consuming, more pertinent to the evaluation.
In practice, we find that many cases lie in a gray area, which is hard to define. Physicians may come to our office for an evaluation after having said something odd at work. Maybe they misdosed a medication on one occasion. Maybe they wrote the wrong year on a chart. However, if the physician was 30 years old, would we consider any one of those incidents significant? As a psychiatrist rather than a physician practicing the specialty in review, it is particularly hard and sometimes unwise to condone or sanction individual incidents.
Evaluators find solace in neuropsychological testing. However the relevance to the safety of patients is unclear. Many of those tests end up being a simple proxy for age. A physicians’ ability to sort words or cards at a certain speed might correlate to cognitive performance but has unclear significance to the ability to care for patients. Using such tests becomes a de facto age limit on the practice of medicine. It seems essential to expand and refine our repertoire of evaluation tools for the assessment of physicians. As when we perform capacity evaluation in the hospital, we enlist the assistance of the treating team in understanding the questions being asked for a patient, medical boards could consider creating independent multidisciplinary teams where psychiatry has a seat along with the relevant specialties of the evaluee. Likewise, the assessment would benefit from a broad review of the physicians’ general practice rather than the more typical review of one or two incidents.
We are promoting a more individualized approach by medical boards to the many issues of the aging physician. Retiring is no longer the dream of older physicians, but rather working in the suitable position where their contributions, clinical experience, and wisdom are positive contributions to patient care. Furthermore, we encourage medical boards to consider more nuanced decisions. A binary approach fits few cases that we see. Surgeons are a prime example of this. A surgeon in the early stages of Parkinsonism may be unfit to perform surgery but very capable of continuing to contribute to the well-being of patients in other forms of clinical work, including postsurgical care that doesn’t involve physical dexterity. Similarly, medical boards could consider other forms of partial restrictions, including a ban on procedures, a ban on hospital privileges, as well as required supervision or working in teams. Accumulated clinical wisdom allows older physicians to be excellent mentors and educators for younger doctors. There is no simple method to predict which physicians may have the early stages of a progressive dementia, and which may have a stable MCI. A yearly reevaluation if there are no further complaints, is the best approach to determine progression of cognitive problems.
Few crises like the current COVID-19 pandemic can better remind us of the importance of the place of medicine in society. Many states have encouraged retired physicians to contribute their knowledge and expertise, putting themselves in particular risk because of their age. It is a good time to be reminded that we owe them significant respect and care when deciding to remove their license. We are encouraged by the diligent efforts of medical boards in supervising our colleagues but warn against zealot evaluators who use this opportunity to force physicians into retirement. We also encourage medical boards to expand their tools and approaches when facing such cases, as mislabeled cognitive diagnoses can be an easy scapegoat of a poor understanding of the more important psychological and biological factors in the evaluation.
References
1. Tariq SH et al. Am J Geriatr Psychiatry. 2006;14:900-10.
2. Nasreddine Z. mocatest.org. Version 2004 Nov 7.
3. Hales RE et al. The American Psychiatric Publishing Textbook of Psychiatry. Washington: American Psychiatric Association Publishing, 2014.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings in chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Dr. Abrams is a forensic psychiatrist and attorney in San Diego. He is an expert in addictionology, behavioral toxicology, psychopharmacology and correctional mental health. He holds a teaching positions at the University of California, San Diego. Among his writings are chapters about competency in national textbooks. Dr. Abrams has no disclosures.
Dear 2020, where do we go from here?
The first few months of 2020 have shone a light on the challenges we face in this new decade as a health care industry and society. As the new decade dawned, we glimpsed at just the tip of the iceberg of social injustice and longstanding inequality in our society as the COVID-19 pandemic gripped our world. The evident health disparities revealed what we have always known: that our health care system is a microcosm of our society, and that this crisis laid bare the systematic bias present in our everyday lives.
The events of early 2020 have allowed hospitalists to take our rightful place among the few who can and will be the problem solvers of the most complex puzzles. Any discussion of the year 2020 would be incomplete without talking about COVID-19, the first modern pandemic. The rapid global spread, severity, and transmissibility of the novel coronavirus presented unique clinical and operational challenges.
Hospitalists in my communities not only stepped up to care for our most acutely ill, but also our critically ill COVID-19 patients. We were in lockstep with our emergency medicine and critical care medicine colleagues to ensure that patients – COVID-19 positive or negative – received the right care at the right time in the right place. We partnered with our disaster and emergency preparedness colleagues, some of us members or leaders within our hospital, system, regional, state, or national emergency operations centers.
As further evidence of health disparities emerged in the outcomes of care of patients with COVID-19 and the homicide of George Floyd raised the alarm (again) that racism is alive and well in this country, hospitalists grieved, kneeled, and then stood with our colleagues, patients, and fellow humans to advocate for change. At the front lines, we ensured that crisis standards of care action plans would not disadvantage any person for whom we may care during acute illness. Behind closed doors and in open forums, we spoke in defense of the most vulnerable and wrote about how each and every person can throw a wrench into the existing system of bias and discrimination to produce lasting, real change for the better.
I am proud to be a hospitalist, a member of this club, with game changers like Kimberly Manning, Samir Shah, Tracy Cardin, Jason Persoff, Charlie Wray, Chris Moriates, and Vineet Arora – to name just a few. Even more so, I am grateful to be a new member of the Society of Hospital Medicine’s board of directors, where I find myself in the company of admired colleagues as we chart the course of SHM into the new decade. With such a jarring launch, we face a daunting task. In the short term, the board must guide SHM in weathering the economic storm kicked off by COVID-19 and the new social distanced norms we all practice. In the long run, we have to stay the ambitious and steep course of excellence and accomplishment set by our founders.
If we as a community of hospitalists intend to lead our field – and health care in general – each one of us must individually commit to the following pursuits:
1. Maintaining excellence in our clinical practice. First and foremost, our impact on patients happens at the bedside. Honing our clinical skills, staying up to date on the latest, breaking changes in best practices in caring for hospitalized patients and establishing the kind of relationship with their patients that we would wish for ourselves must be a core function. With the staggering volume of knowledge and the rapidity with which new information is constantly added to that existing body, this may seem like an impossibly daunting task. Thankfully, SHM recognizes this vital need and provides resources to allow each one of us to succeed in this endeavor. The Journal of Hospital Medicine brings us the best and most relevant evidence for our practice, ensuring that studies are rigorously performed and reviewed and that the outcomes produced are the ones that we are after. We can maintain board certification with a focused practice in hospital medicine by utilizing the multimodal study tools available through Spark. And, when we are once again able to gather together as a community, the annual conference will provide the best education about hospital medicine available. In the meantime, feel free to explore HM20 Virtual, featuring select offerings from the original HM20 course schedule and the opportunity to earn CME.
2. Guide our future hospitalist colleagues to be 21st-century practitioners. Medical students and residents are entering our profession in a very dynamic time. The competencies they must have in order to succeed as hospitalists in 2020 and onward are different than they were when I went through training. COVID-19 has shown us that hospitalists must be “digital doctors” – they must be facile in utilizing virtual health tools, be capable of harnessing the power of health information technology in the electronic medical record to provide care, and also be able to incorporate and interpret the incredible amount of information in health care “big data.” It is our responsibility today to prepare and coach our trainees so that they may be successful tomorrow.
3. Change the system to ensure that each patient gets the safest, most equitable care we can provide. Each one of us can be at the top of our game, but if we practice in a health care system that has gaps, we may still fail in providing the safest, highest-quality care possible. It is each of our responsibility to use every patient interaction to discover the systemic forces, including the social and cultural biases, that can lead to patient harm. In that, it is our duty to protect the most vulnerable, to redesign systems such that every person can be healthy. Only through this work of improvement do we have hope to eliminate the health disparities that exist.
4. Advocate for our patients. We each have seen the incredible impact that the Affordable Care Act has had on health care delivery in the day-to-day interactions we have with our patients. Yet it is not enough. We still have room to improve the American health care system to allow better access to care, more timely provision of care, and better outcomes for our communities. Sometimes, this takes a change in policy. For each of us, it starts with being aware of how our state policy can impact how care is delivered to our patients. In addition to your own personal advocacy work, you can join forces with SHM’s Advocacy & Public Policy team to use our society to amplify your voice.
The year 2020 began with eye-opening crises that exposed the depth and breadth of the work we have before us in hospital medicine. We have an important role to play in the next decade – surely to be the most interesting time to be a hospitalist.
Dr. Tad-y is a hospitalist and director of GME quality and safety programs at the University of Colorado at Denver, Aurora. She is a member of the SHM board of directors.
The first few months of 2020 have shone a light on the challenges we face in this new decade as a health care industry and society. As the new decade dawned, we glimpsed at just the tip of the iceberg of social injustice and longstanding inequality in our society as the COVID-19 pandemic gripped our world. The evident health disparities revealed what we have always known: that our health care system is a microcosm of our society, and that this crisis laid bare the systematic bias present in our everyday lives.
The events of early 2020 have allowed hospitalists to take our rightful place among the few who can and will be the problem solvers of the most complex puzzles. Any discussion of the year 2020 would be incomplete without talking about COVID-19, the first modern pandemic. The rapid global spread, severity, and transmissibility of the novel coronavirus presented unique clinical and operational challenges.
Hospitalists in my communities not only stepped up to care for our most acutely ill, but also our critically ill COVID-19 patients. We were in lockstep with our emergency medicine and critical care medicine colleagues to ensure that patients – COVID-19 positive or negative – received the right care at the right time in the right place. We partnered with our disaster and emergency preparedness colleagues, some of us members or leaders within our hospital, system, regional, state, or national emergency operations centers.
As further evidence of health disparities emerged in the outcomes of care of patients with COVID-19 and the homicide of George Floyd raised the alarm (again) that racism is alive and well in this country, hospitalists grieved, kneeled, and then stood with our colleagues, patients, and fellow humans to advocate for change. At the front lines, we ensured that crisis standards of care action plans would not disadvantage any person for whom we may care during acute illness. Behind closed doors and in open forums, we spoke in defense of the most vulnerable and wrote about how each and every person can throw a wrench into the existing system of bias and discrimination to produce lasting, real change for the better.
I am proud to be a hospitalist, a member of this club, with game changers like Kimberly Manning, Samir Shah, Tracy Cardin, Jason Persoff, Charlie Wray, Chris Moriates, and Vineet Arora – to name just a few. Even more so, I am grateful to be a new member of the Society of Hospital Medicine’s board of directors, where I find myself in the company of admired colleagues as we chart the course of SHM into the new decade. With such a jarring launch, we face a daunting task. In the short term, the board must guide SHM in weathering the economic storm kicked off by COVID-19 and the new social distanced norms we all practice. In the long run, we have to stay the ambitious and steep course of excellence and accomplishment set by our founders.
If we as a community of hospitalists intend to lead our field – and health care in general – each one of us must individually commit to the following pursuits:
1. Maintaining excellence in our clinical practice. First and foremost, our impact on patients happens at the bedside. Honing our clinical skills, staying up to date on the latest, breaking changes in best practices in caring for hospitalized patients and establishing the kind of relationship with their patients that we would wish for ourselves must be a core function. With the staggering volume of knowledge and the rapidity with which new information is constantly added to that existing body, this may seem like an impossibly daunting task. Thankfully, SHM recognizes this vital need and provides resources to allow each one of us to succeed in this endeavor. The Journal of Hospital Medicine brings us the best and most relevant evidence for our practice, ensuring that studies are rigorously performed and reviewed and that the outcomes produced are the ones that we are after. We can maintain board certification with a focused practice in hospital medicine by utilizing the multimodal study tools available through Spark. And, when we are once again able to gather together as a community, the annual conference will provide the best education about hospital medicine available. In the meantime, feel free to explore HM20 Virtual, featuring select offerings from the original HM20 course schedule and the opportunity to earn CME.
2. Guide our future hospitalist colleagues to be 21st-century practitioners. Medical students and residents are entering our profession in a very dynamic time. The competencies they must have in order to succeed as hospitalists in 2020 and onward are different than they were when I went through training. COVID-19 has shown us that hospitalists must be “digital doctors” – they must be facile in utilizing virtual health tools, be capable of harnessing the power of health information technology in the electronic medical record to provide care, and also be able to incorporate and interpret the incredible amount of information in health care “big data.” It is our responsibility today to prepare and coach our trainees so that they may be successful tomorrow.
3. Change the system to ensure that each patient gets the safest, most equitable care we can provide. Each one of us can be at the top of our game, but if we practice in a health care system that has gaps, we may still fail in providing the safest, highest-quality care possible. It is each of our responsibility to use every patient interaction to discover the systemic forces, including the social and cultural biases, that can lead to patient harm. In that, it is our duty to protect the most vulnerable, to redesign systems such that every person can be healthy. Only through this work of improvement do we have hope to eliminate the health disparities that exist.
4. Advocate for our patients. We each have seen the incredible impact that the Affordable Care Act has had on health care delivery in the day-to-day interactions we have with our patients. Yet it is not enough. We still have room to improve the American health care system to allow better access to care, more timely provision of care, and better outcomes for our communities. Sometimes, this takes a change in policy. For each of us, it starts with being aware of how our state policy can impact how care is delivered to our patients. In addition to your own personal advocacy work, you can join forces with SHM’s Advocacy & Public Policy team to use our society to amplify your voice.
The year 2020 began with eye-opening crises that exposed the depth and breadth of the work we have before us in hospital medicine. We have an important role to play in the next decade – surely to be the most interesting time to be a hospitalist.
Dr. Tad-y is a hospitalist and director of GME quality and safety programs at the University of Colorado at Denver, Aurora. She is a member of the SHM board of directors.
The first few months of 2020 have shone a light on the challenges we face in this new decade as a health care industry and society. As the new decade dawned, we glimpsed at just the tip of the iceberg of social injustice and longstanding inequality in our society as the COVID-19 pandemic gripped our world. The evident health disparities revealed what we have always known: that our health care system is a microcosm of our society, and that this crisis laid bare the systematic bias present in our everyday lives.
The events of early 2020 have allowed hospitalists to take our rightful place among the few who can and will be the problem solvers of the most complex puzzles. Any discussion of the year 2020 would be incomplete without talking about COVID-19, the first modern pandemic. The rapid global spread, severity, and transmissibility of the novel coronavirus presented unique clinical and operational challenges.
Hospitalists in my communities not only stepped up to care for our most acutely ill, but also our critically ill COVID-19 patients. We were in lockstep with our emergency medicine and critical care medicine colleagues to ensure that patients – COVID-19 positive or negative – received the right care at the right time in the right place. We partnered with our disaster and emergency preparedness colleagues, some of us members or leaders within our hospital, system, regional, state, or national emergency operations centers.
As further evidence of health disparities emerged in the outcomes of care of patients with COVID-19 and the homicide of George Floyd raised the alarm (again) that racism is alive and well in this country, hospitalists grieved, kneeled, and then stood with our colleagues, patients, and fellow humans to advocate for change. At the front lines, we ensured that crisis standards of care action plans would not disadvantage any person for whom we may care during acute illness. Behind closed doors and in open forums, we spoke in defense of the most vulnerable and wrote about how each and every person can throw a wrench into the existing system of bias and discrimination to produce lasting, real change for the better.
I am proud to be a hospitalist, a member of this club, with game changers like Kimberly Manning, Samir Shah, Tracy Cardin, Jason Persoff, Charlie Wray, Chris Moriates, and Vineet Arora – to name just a few. Even more so, I am grateful to be a new member of the Society of Hospital Medicine’s board of directors, where I find myself in the company of admired colleagues as we chart the course of SHM into the new decade. With such a jarring launch, we face a daunting task. In the short term, the board must guide SHM in weathering the economic storm kicked off by COVID-19 and the new social distanced norms we all practice. In the long run, we have to stay the ambitious and steep course of excellence and accomplishment set by our founders.
If we as a community of hospitalists intend to lead our field – and health care in general – each one of us must individually commit to the following pursuits:
1. Maintaining excellence in our clinical practice. First and foremost, our impact on patients happens at the bedside. Honing our clinical skills, staying up to date on the latest, breaking changes in best practices in caring for hospitalized patients and establishing the kind of relationship with their patients that we would wish for ourselves must be a core function. With the staggering volume of knowledge and the rapidity with which new information is constantly added to that existing body, this may seem like an impossibly daunting task. Thankfully, SHM recognizes this vital need and provides resources to allow each one of us to succeed in this endeavor. The Journal of Hospital Medicine brings us the best and most relevant evidence for our practice, ensuring that studies are rigorously performed and reviewed and that the outcomes produced are the ones that we are after. We can maintain board certification with a focused practice in hospital medicine by utilizing the multimodal study tools available through Spark. And, when we are once again able to gather together as a community, the annual conference will provide the best education about hospital medicine available. In the meantime, feel free to explore HM20 Virtual, featuring select offerings from the original HM20 course schedule and the opportunity to earn CME.
2. Guide our future hospitalist colleagues to be 21st-century practitioners. Medical students and residents are entering our profession in a very dynamic time. The competencies they must have in order to succeed as hospitalists in 2020 and onward are different than they were when I went through training. COVID-19 has shown us that hospitalists must be “digital doctors” – they must be facile in utilizing virtual health tools, be capable of harnessing the power of health information technology in the electronic medical record to provide care, and also be able to incorporate and interpret the incredible amount of information in health care “big data.” It is our responsibility today to prepare and coach our trainees so that they may be successful tomorrow.
3. Change the system to ensure that each patient gets the safest, most equitable care we can provide. Each one of us can be at the top of our game, but if we practice in a health care system that has gaps, we may still fail in providing the safest, highest-quality care possible. It is each of our responsibility to use every patient interaction to discover the systemic forces, including the social and cultural biases, that can lead to patient harm. In that, it is our duty to protect the most vulnerable, to redesign systems such that every person can be healthy. Only through this work of improvement do we have hope to eliminate the health disparities that exist.
4. Advocate for our patients. We each have seen the incredible impact that the Affordable Care Act has had on health care delivery in the day-to-day interactions we have with our patients. Yet it is not enough. We still have room to improve the American health care system to allow better access to care, more timely provision of care, and better outcomes for our communities. Sometimes, this takes a change in policy. For each of us, it starts with being aware of how our state policy can impact how care is delivered to our patients. In addition to your own personal advocacy work, you can join forces with SHM’s Advocacy & Public Policy team to use our society to amplify your voice.
The year 2020 began with eye-opening crises that exposed the depth and breadth of the work we have before us in hospital medicine. We have an important role to play in the next decade – surely to be the most interesting time to be a hospitalist.
Dr. Tad-y is a hospitalist and director of GME quality and safety programs at the University of Colorado at Denver, Aurora. She is a member of the SHM board of directors.
Telemedicine checklist may smooth visits with older patients
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
During the pandemic, physicians have raced to set up or expand telemedicine, uncovering both advantages and shortcomings.
Although many of the suggestions, published online in Annals of Internal Medicine, are useful for all patients, Carrie Nieman, MD, MPH, and Esther S. Oh, MD, PhD, developed the list with older patients in mind.
“I have a number of patients into their 90s and with hearing loss, and we have had very successful video-based telemedicine visits,” Dr. Nieman, with the Cochlear Center for Hearing and Public Health at Johns Hopkins Bloomberg School of Public Health in Baltimore said in an interview. “Age should not be considered synonymous with inability or unwillingness to use technology.”
Their recommendations included the following:
- Assume some degree of hearing loss, which affects about two-thirds of adults aged 70 years and older.
- Ask patients to wear headphones or a headset or confirm that they are wearing their hearing aids and are in a quiet location.
- Use a headset.
- When possible, use video and have the camera focused on your face.
- Use captioning when available and provide a written summary of key points and instructions.
- Pay attention to cues, such as nodding along or looking to a loved one, that suggest a patient may not be following the conversation.
“If cognitive impairment is suspected, several screening tools can be used over the telephone to identify individuals who may need more comprehensive, in-person assessment,” wrote Dr. Nieman and Dr. Oh, who is with the division of geriatric medicine and gerontology at Johns Hopkins University School of Medicine. For example, data suggest that a modified version of the Mini–Mental State Examination and the Delirium Symptom Interview could be useful tools. “A formal diagnosis of dementia is not recommended solely based on a telephone-based cognitive screening,” however, Dr. Nieman and Dr. Oh said.
For patients with hearing loss, video visits avoid a current limitation of in-person visits: face masks that hinder patients’ ability to read lips and other visual cues. “For many of us, we rely on these types of cues more than we think,” Dr. Nieman said in an interview.
“When you have doubts about whether you and your patient are on the same page, check in with the patient,” Dr. Nieman said. “When appropriate, having a loved one or a care partner join an encounter, or at least a portion of the encounter, can be helpful to both the patient and the provider.”
Many older patients unprepared
Millions of older patients may not have been ready for the rapid shift to telemedicine brought on by COVID-19, a recent study in JAMA Internal Medicine suggests. Between 32% and 38% of older adults in the United States may not have been ready for video visits, largely because of inexperience with technology. Approximately 20% could have difficulty with telephone visits because of problems hearing or communicating or because of dementia.
Kenneth Lam, MD, of the division of geriatrics at the University of California, San Francisco (UCSF), and colleagues arrived at these estimates after analyzing data from more than 4,500 participants in the National Health and Aging Trends Study that was conducted in 2018. The study is nationally representative of Medicare beneficiaries 65 years or older.
The aim of the study “was to call attention to what clinicians were already experiencing on the front lines,” Dr. Lam said. In an interview, he imagined two scenarios based on his colleagues’ accounts of telemedicine visits.
In one case, a 72-year-old woman logs into Zoom Health on her iPad without any trouble. “She explains she just pushed on the URL and everything loaded up and you have a great visit,” Dr. Lam said. “This is likely to be the case for over 50% of the older people you see; I share this picture to combat ageism, which is, truthfully, just inaccurate stereotyping of older people and gets in the way of actionable, data-driven policies.
“However, for around one in three older adults (and closer to three out of every four of those over the age of 85), you will book an appointment and they will say they don’t have an email address or a computer or know how to go online,” Dr. Lam said. “Or suppose they decide to try it out. ... Come appointment time, you log on and they pick up, but now their sound doesn’t work. They keep saying they can see you but they can’t hear you. ... They accidentally hang up. You place another call, and they ask if you can switch to a phone conversation instead.”
By phone, the physician can address concerns about the patient’s blood pressure, which the patient has been measuring daily. “But when it comes to looking at the swelling in their legs, you’re out of luck, and you’ve been on this call for 45 minutes,” Dr. Lam said.
Have a backup plan
Making sure patients are prepared and having a backup plan can help, said Kaitlin Willham, MD, of UCSF and the San Francisco VA Medical Center.
She says older patients fall into a wide range of categories in terms of skills and access to equipment. Knowing which category a patient falls into and having relevant support available to troubleshoot are important.
During the pandemic, Dr. Willham has conducted many more telemedicine visits with patients who are at their place of residence, whether a private home or a residential care facility. “Even outside of the current crisis, there are benefits to home video visits,” Dr. Willham said. “A home video visit can provide a more holistic view of the patient than an office visit, allowing the clinician to see how the person lives, what they might be challenged by. It allows the clinician to identify areas of intervention and, if there is a care partner, involving that person in the plan. If the visit starts without major technical or communication barriers, they are generally very well received.”
For patients with problems hearing for whom headphones or amplification devices are not available, “using a landline for the audio portion of the visit can help, as can having someone with the patient reiterate what was said,” Dr. Willham suggested. “Many video platforms also enable the clinician to type messages or share a screen with a live document. These options can work well when there is very severe or complete lack of hearing.”
Sometimes an in-person visit is the right way to go, even when technical hurdles can be overcome.
“Although many older adults are willing and able to learn to use telemedicine, an equitable health system should recognize that for some, such as those with dementia and social isolation, in-person visits are already difficult and telemedicine may be impossible,” Dr. Lam and coauthors wrote. “For these patients, clinics and geriatric models of care such as home visits are essential.”
Dr. Nieman, Dr. Oh, and one of Dr. Lam’s coauthors have received funding from the National Institutes of Health. Dr. Oh also has received funding from the Roberts Family Fund. Dr. Nieman serves as a board member of the nonprofit organization Access HEARS and is on the board of trustees of the Hearing Loss Association of America.
A version of this article originally appeared on Medscape.com.
Erratum
Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images
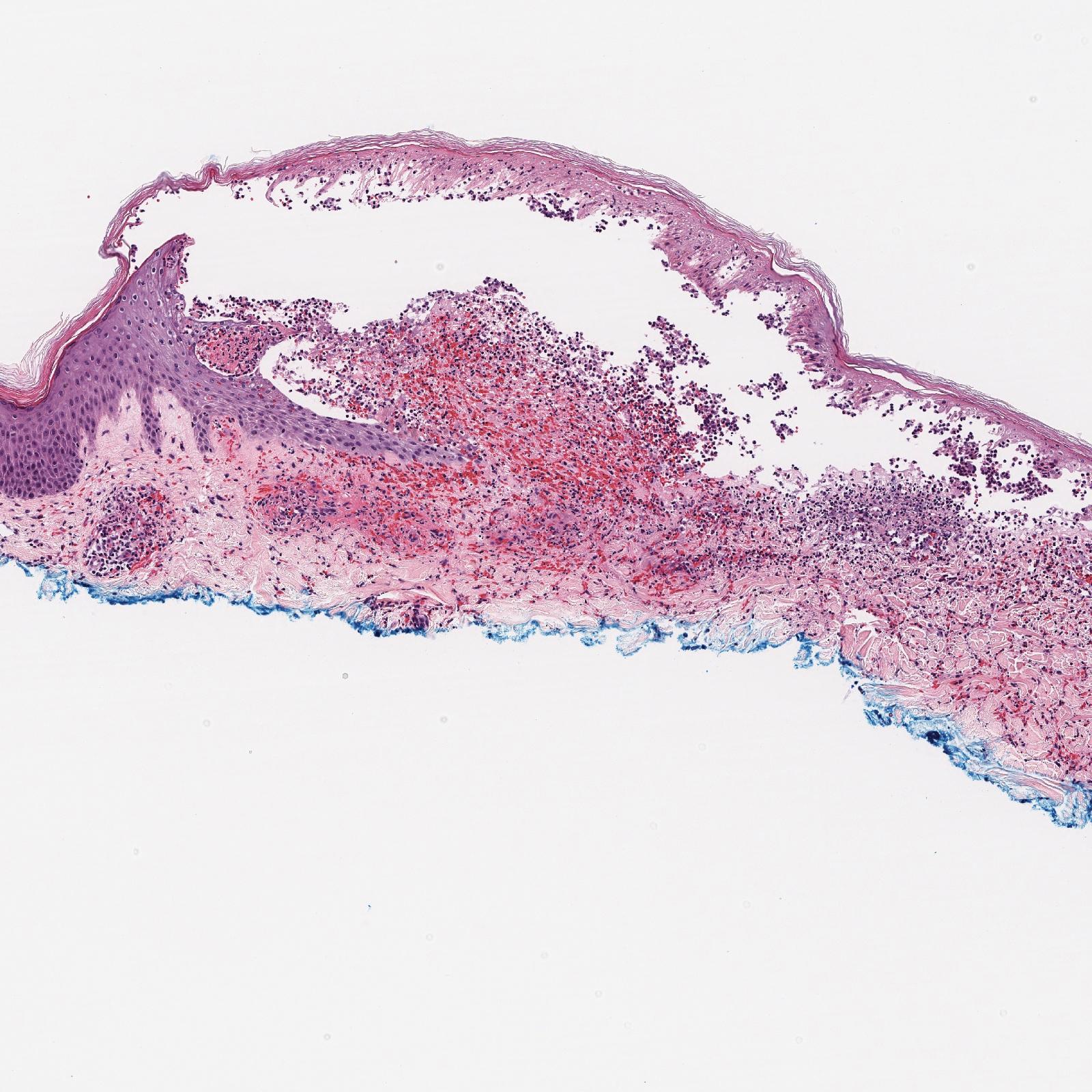
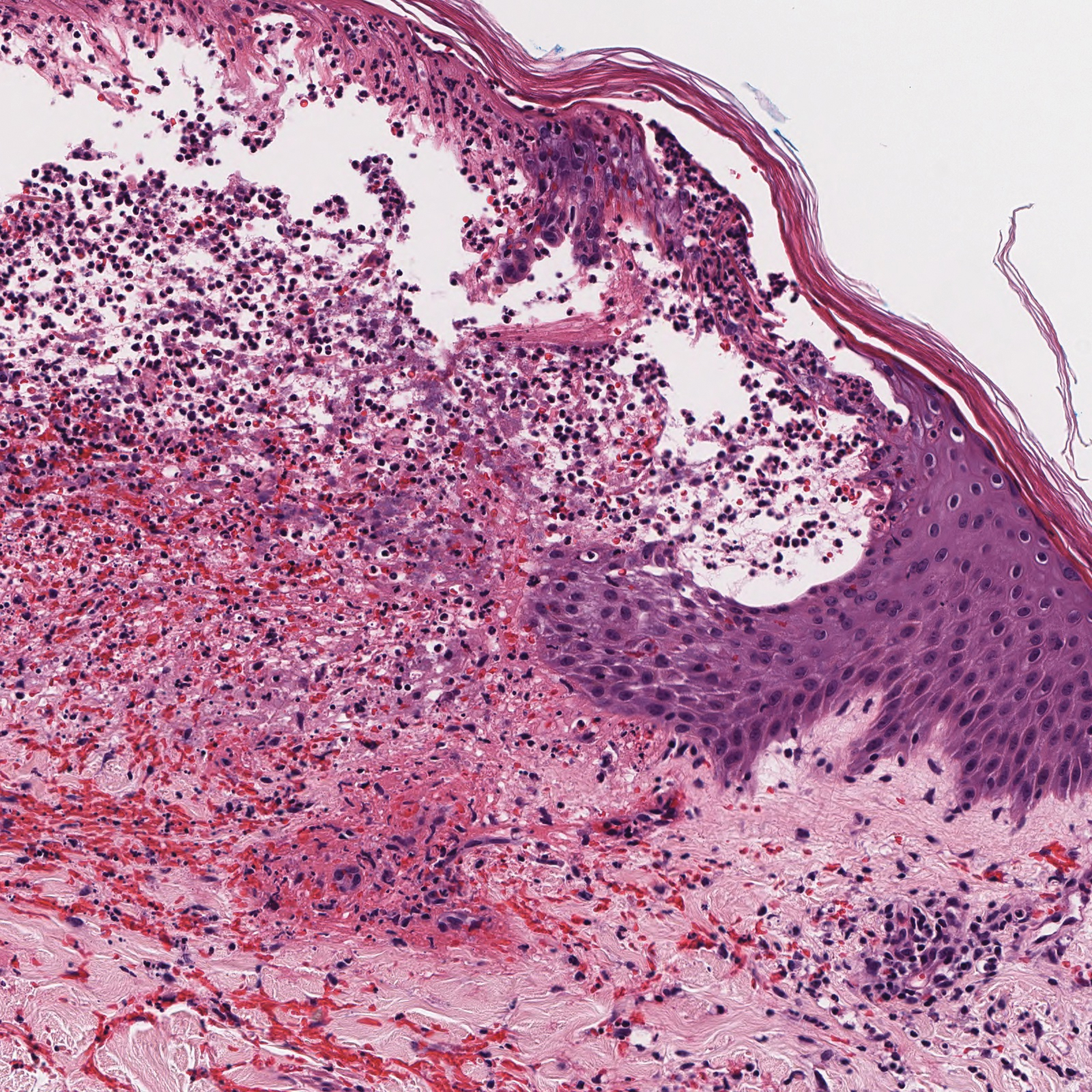
Discussion Images




Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images


Discussion Images




Grandhi R, Shamloul N, Powell M. Purpuric bullae on the lower extremities. Cutis. 2020;105:282, 286-287.
In the article above from the June 2020 issue, the images were incorrect. The correct images appear below. The article has been corrected online at www.mdedge.com/dermatology. We apologize for the error.
Quiz Images


Discussion Images