User login
For MD-IQ use only
Botulinum Toxin for the Treatment of Intractable Raynaud Phenomenon
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
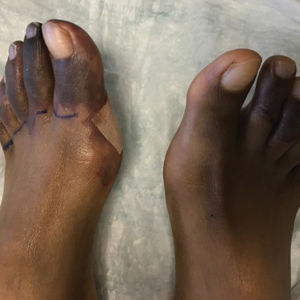
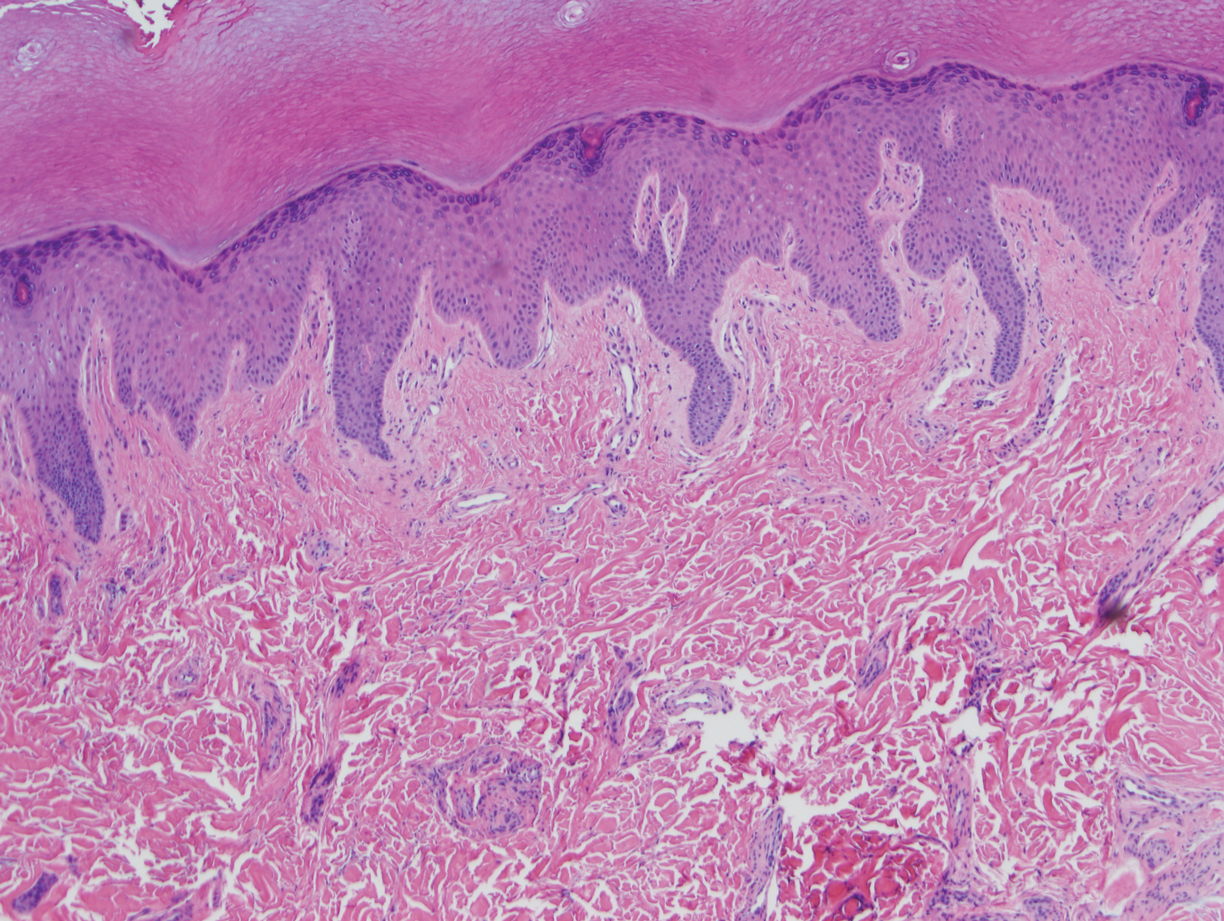
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.
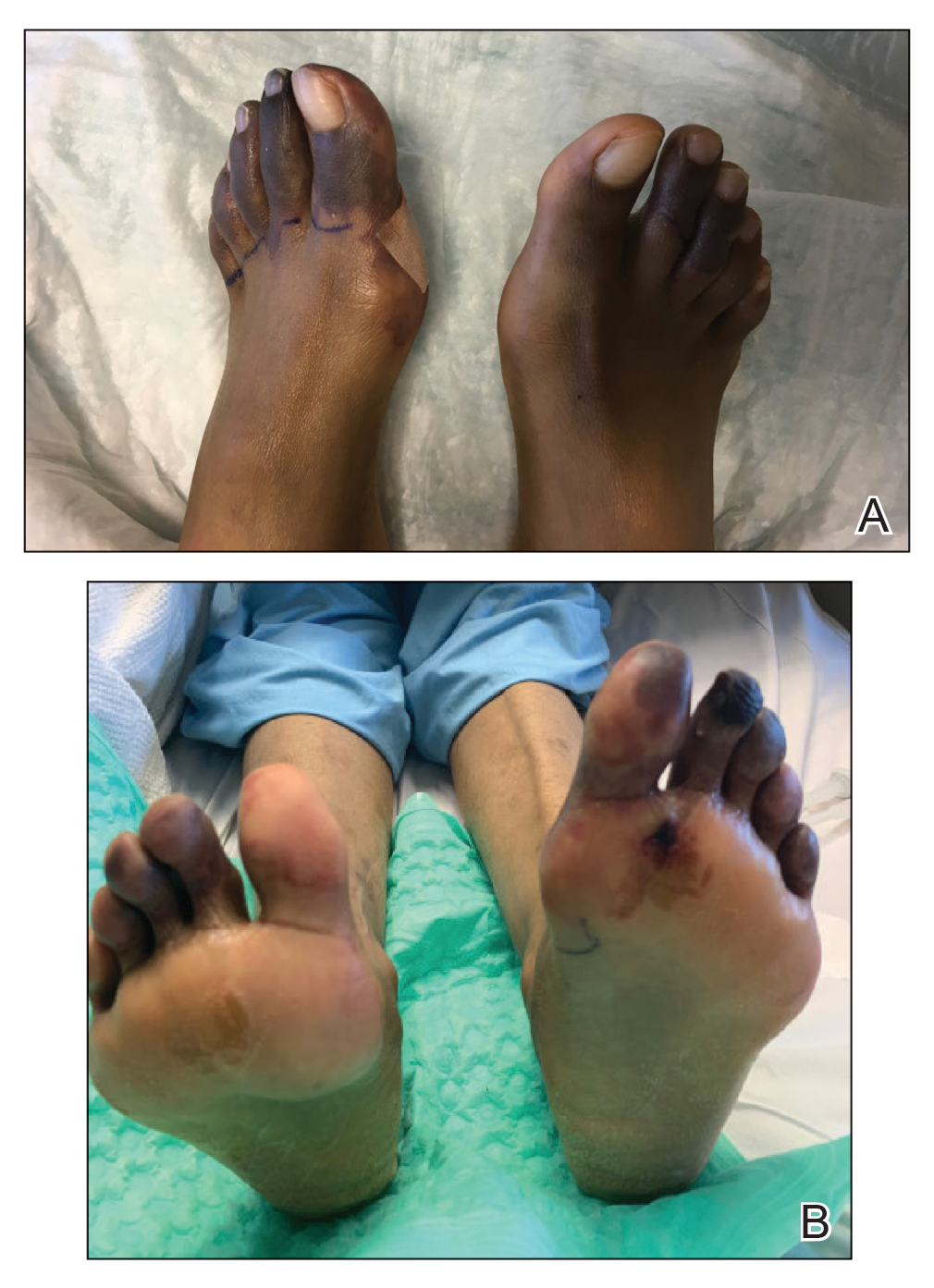
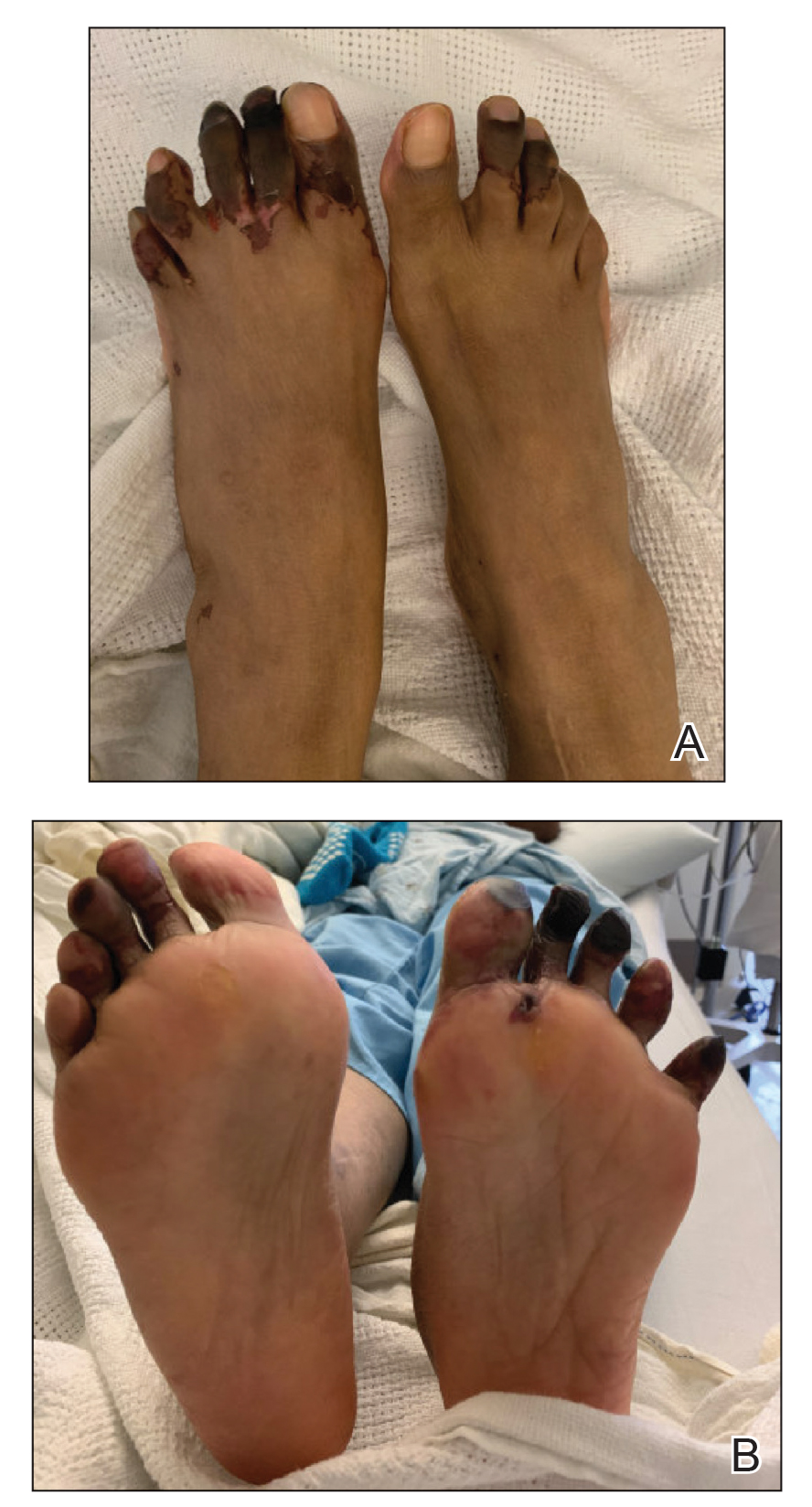
Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).
One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.
Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.

Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).

One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.

Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.

Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).

One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.

Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
Practice Points
- Raynaud phenomenon (RP) is a vascular disorder characterized by episodic vasospasms of the digits often due to cold temperature or stress.
- OnabotulinumtoxinA has been implemented as a treatment of intractable RP after failure with traditional treatments, such as calcium channel blockers, angiotensin receptor blockers, prostaglandins, endothelin receptor blockers, and phosphodiesterase 5 inhibitors.
- A standard technique of delivery of onabotulinumtoxinA involves injection of 5 U/mL into the medial and lateral aspects of each finger at its base (near the metacarpal head) for a total of 50 U per hand or foot.
New finasteride lawsuit brings renewed attention to psychiatric, ED adverse event reports
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ
A pediatrician notices empty fields
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The new transdermal contraceptive patch expands available contraceptive options: Does it offer protection with less VTE risk?
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
The first transdermal contraceptive patch was approved by the US Food and Drug Administration (FDA) in 2001.1 A 2018 survey revealed that 5% of women in the United States between the ages of 15 and 49 years reported the use of a short-acting hormonal contraceptive method (ie, vaginal ring, transdermal patch, injectable) within the past month, with just 0.3% reporting the use of a transdermal patch.2 Transdermal contraceptive patches are an effective form of birth control that may be a convenient option for patients who do not want to take a daily oral contraceptive pill but want similar efficacy and tolerability. Typical failure rates of patches are similar to that of combined oral contraceptives (COCs).1,3
While transdermal hormone delivery results in less peaks and troughs of estrogen compared with COCs, the total estrogen exposure is higher than with COCs; therefore, the risk for venous thromboembolism (VTE) with previously available patches is about twice as high.1 Twirla (Agile), an ethinyl estradiol (EE)/levonorgestrel (LNG) patch, delivers a low and consistent daily dose of hormones over 3 patches replaced once weekly, with no patch on the fourth week.3 Twirla contains 120 μg/day LNG and 30 μg/day EE. OrthoEvra, FDA approved in 2001 as mentioned, contains 150 μg/day norelgestromin and 35 μg/day EE.1 A reduction of the EE dose in COCs has been associated with lower risk for VTE.4
The addition of Twirla to the market offers another contraceptive option for patients who opt for a weekly, self-administered method.
How much lower is the VTE risk?
OBG
Barbara Levy, MD: The reality is we can’t designate a reduction of risk, except, in general, when the dose of ethinyl estradiol is lower, we think that the VTE risk is lower. There has not been a head-to-head comparison in a large enough population to be able to say that the risk is reduced by a certain factor. We just look at the overall exposure to estrogen and say, “In general, for VTE risk, a lower dose is a better thing for women.”
That being said, look at birth control pills, like COCs. We don’t have actual numbers to say that a 30-μg pill is this much less risky than a 35-μg pill. We just put it into a hierarchy, and that’s what we can do with the patch. We can say that, in general, lower is better for VTE risk, but no one can provide absolute numbers.
Continue to: Efficacy...
Efficacy
OBG
Dr. Levy: You have to look at the pivotal trials and look at what the efficacy was in a trial setting. In the real-world setting, the effectiveness is never quite as good as it is in a clinical trial. I think the bottom line for all of us is that combined oral contraception, meaning estrogen with progestin, is equivalently effective across the different options that are available for women. Efficacy really isn’t the factor to use to distinguish which one I’m going to pick. It is about the patient’s convenience and many other factors. But in terms of its clinical effectiveness in preventing pregnancy, from a very practical standpoint, I think we consider them all the same.
Considering route of administration
OBG
Dr. Levy: I think there’s always a benefit in having lots of choices. And for some women, being able to put a patch on once a week is much more convenient, easier to remember, and delivers a very consistent dose of hormone absorbed through the skin, which is different than taking a pill in the morning when your levels go up quickly then diminish over the day. The hormones are higher at a certain time, and then they drop off, so there might be some advantages for people who are very sensitive to swings in hormonal levels. There’s also a convenience factor, where for some people they will choose that. Other people might really dislike having a relatively large patch on their skin somewhere, or they may have skin sensitivity to the adhesive. Overall, I always think that having more options is better and individual girls/women will choose what works best for them.
Counseling tips
OBG
Dr. Levy: Like other patches that are available on the market, these are a once-a-week patch. The patch should be placed on clean, dry skin. No lotions, perfumes, or anything on the skin because you really want them to stick for the whole week, and it’s not going to stick if there’s anything oily on the skin. The first patch is placed on day 1 of a menstrual cycle, the first day of bleeding, and then changed weekly for 3 weeks. Then there’s a 7-day patch-free time in which one would expect to have a period.
In general, breakthrough bleeding was not a significant problem with the patch, but some women will have some irregular spotting and bleeding with any sort of hormonal treatment; some women may have no periods at all. In other words, the estrogen dose and progestin may be of a balance that allows the patient not to have periods. But, in general, most of the women in the trial had regular light menstrual flow during the week when their patch was not on.5
Continue to: Pricing...
Pricing
OBG
Dr. Levy: That’s a tricky question. Insurance plans through Obamacare, the Affordable Care Act, are required to cover every form of contraception. That means they must cover a patch. It doesn’t mean that they have to cover this patch. And because there are generics available of the other patch formulation, it is likely that this would be a higher tier, meaning that there may be a higher copay for someone who wanted to use Twirla versus one of the generic patches.
I can’t say that that’s universally the case, but my experience with most of the health insurance plans is that they tend to put barriers in the way for any of us to prescribe, and for women to use, brand-name products. So Twirla is new on the market; it’s a brand-name product. It may work much better for some people; and in those cases, the health care provider might have to send a letter to the insurance company saying why this one is medically necessary for a patient. There probably will be some hoops to go through for coverage without a copay. I think coverage will be there, but there may be a substantial copay because of the tier level.
OBG
Dr. Levy: I don’t think that the payer is going to buy that argument unless the requirement is to use a patch. If the patient, for example, has some sort of gastrointestinal disease where they don’t absorb things well, so pills don’t work well, we might get to the place where they have to have a patch. If the patient has a lot of breast tenderness or has symptoms on the generic patch that delivers a higher level of estrogen, then we would have to document those symptoms to say, “She’s not tolerating this one and, therefore, we need to go to that one.” So, I think as prescribers we would have to justify not only the lower dose but also the form.
As a clinician, I would always like to put somebody on the lower dose. We do think lower is better, but we have to be sensitive to the costs of all of these things too. I’m very sensitive to my patients’ out-of-pocket costs because, in the end, if the costs are a lot of money or she can only afford one month at a time, then she may miss a window where she may not have the money to buy next month’s supply when it’s due, and get pregnant. We have to balance all of those things as we’re thinking through the best option for an individual.
We have more to learn
OBG
Dr. Levy: I think it’s always exciting when we have new products available, and there’s a lot more we’ll learn as Twirla comes into commercial use and millions instead of thousands of people are using it. Overall, I think it’s fantastic that there’s ongoing research and that there are new products out there. And kudos to the company for doing the research and for getting approval, and I’m looking forward to learning more about it.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
- Galzote RA, Rafie S, Teal R, et al. Transdermal delivery of combined hormonal contraception: a review of the current literature. Int J Womens Health. 2017;9:315-321.
- Contraceptive use in the United States. Guttmacher website. Published May 2021. Accessed August 29, 2021. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states#.
- US National Library of Medicine. Estrogen and progestin (transdermal patch contraceptives). MedlinePlus website. Updated February 15, 2021. Accessed August 29, 2021. https://medlineplus.gov/druginfo/meds/a602006.html.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: ACOG Committee on Practice Bulletins—gynecology. Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:E128-E150.
- Nelson AL, Kaunitz AM, Kroll R, et al. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143. doi: 10.1016/j.contraception.2020.
11.011.
New CMS rule challenges hospitals, but not vendors, to make EHRs safer
In a little-noticed action last month, so as to meet an objective of the Medicare Promoting Interoperability Program, starting next year.
Experts praised the move but said that EHR developers should share the responsibility for ensuring that the use of their products doesn’t harm patients.
A number of safety problems are associated with hospital EHR systems, ranging from insufficient protection against medication errors and inadvertent turnoffs of drug interaction checkers to allowing physicians to use free text instead of coded data for key patient indicators. Although hospitals aren’t required to do anything about safety problems that turn up in their self-audits, practitioners who perform the self-assessment will likely encounter challenges that they were previously unaware of and will fix them, experts say.
Studies over the past decade have shown that improper configuration and use of EHRs, as well as design flaws in the systems, can cause avoidable patient injuries or can fail to prevent them. For example, one large study found that clinical decision support (CDS) features in EHRs prevented adverse drug events (ADEs) in only 61.6% of cases in 2016. That was an improvement over the ADE prevention rate of 54% in 2009. Nevertheless, nearly 40% of ADEs were not averted.
Another study, sponsored by the Leapfrog Group, found that EHRs used in U.S. hospitals failed to detect up to 1 in 3 potentially harmful drug interactions and other medication errors. In this study, about 10% of the detection failures were attributed to design problems in EHRs.
The new CMS measure requires hospitals to evaluate their EHRs using safety guides that were developed in 2014 and were revised in 2016 by the Office of the National Coordinator for Health IT (ONC). Known as the Safety Assurance Factors for Resilience (SAFER) guides, they include a set of recommendations to help health care organizations optimize the safety of EHRs.
Surprises in store for hospitals
Dean Sittig, PhD, a professor at the University of Texas Health Science Center, Houston, told this news organization that a 2018 study he conducted with his colleague Hardeep Singh, MD, MPH, found that eight surveyed health care organizations were following about 75% of the SAFER recommendations.
He said that when hospitals and health care systems start to assess their systems, many will be surprised at what they are not doing or not doing right. Although the new CMS rule doesn’t require them to correct deficiencies, he expects that many will.
For this reason, Dr. Sittig believes the requirement will have a positive effect on patient safety. But the regulation may not go far enough because it doesn’t impose any requirements on EHR vendors, he said.
In a commentary published in JAMA, Dr. Sittig and Dr. Hardeep, a professor at the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, cite a study showing that 40% of “EHR-related products” had “nonconformities” with EHR certification regulations that could potentially harm patients. “Many nonconformities could have been identified by the developer prior to product release,” they say.
Shared responsibility
According to the JAMA commentary, the SAFER guides were developed “to help health care organizations and EHR developers conduct voluntary self-assessments to help eliminate or minimize EHR-related safety risks and hazards.”
In response to a query from this news organization, ONC confirmed that the SAFER guides were intended for use by developers as well as practitioners. ONC said it supports CMS’s approach to incentivize collaborations between EHR vendors and health care organizations. It noted that some entities have already teamed up to the meet the SAFER guides’ recommendations.
Hospitals and EHR developers must share responsibility for safety, Dr. Sittig and Dr. Singh argue, because many SAFER recommendations are based on EHR features that have to be programmed by developers.
For example, one recommendation is that patient identification information be displayed on all portions of the EHR user interface, wristbands, and printouts. Hospitals can’t implement this feature if the developer hasn’t built it into its product.
Dr. Sittig and Dr. Singh suggest three strategies to complement CMS’s new regulation:
- Because in their view, ONC’s EHR certification criteria are insufficient to address many patient safety concerns, CMS should require EHR developers to assess their products annually.
- ONC should conduct annual reviews of the SAFER recommendations with input from EHR developers and safety experts.
- EHR vendors should disseminate guidance to their customers on how to address safety practices, perhaps including EHR configuration guides related to safety.
Safety in EHR certification
At a recent press conference that ONC held to update reporters on its current plans, officials were asked to comment on Dr. Sittig’s and Dr. Singh’s proposition that EHR developers, as well as hospitals, do more to ensure system safety.
Steve Posnack, deputy national coordinator of health IT, noted that the ONC-supervised certification process requires developers to pay attention to how they “implement and integrate safety practices in their software design. We have certification criteria ... around what’s called safety-enhanced design – specific capabilities in the EHR that are sensitive to safety in areas like e-prescribing, medication, and high-risk events, where you want to make sure there’s more attention paid to the safety-related dynamics.”
After the conference, ONC told this news organization that among the safety-related certification criteria is one on user-centered design, which must be used in programming certain EHR features. Another is on the use of a quality management system to guide the creation of each EHR capability.
Nevertheless, there is evidence that not all EHR developers have paid sufficient attention to safety in their products. This is shown in the corporate integrity agreements with the Office of the Inspector General (OIG) of the Department of Health and Human Services (HHS) that developers eClinicalWorks and Greenway agreed to sign because, according to the government, they had not met all of the certification criteria they’d claimed to satisfy.
Under these agreements, the vendors agreed to follow “relevant standards, checklists, self-assessment tools, and other practices identified in the ONC SAFER guides and the ICE Report(s) to optimize the safety and safe use of EHRs” in a number of specific areas.
Even if all EHRs conformed to the certification requirements for safety, they would fall short of the SAFER recommendations, Dr. Sittig says. “Those certification criteria are pretty general and not as comprehensive as the SAFER guides. Some SAFER guide recommendations are in existing certification requirements, like you’re supposed to have drug-drug interaction checking capabilities, and they’re supposed to be on. But it doesn’t say you need to have the patient’s identification on every screen. It’s easy to assume good software design, development, and testing principles are a given, but our experience suggests otherwise.”
Configuration problems
A handful of vendors are working on what the JAMA article suggests, but there are about 1,000 EHR developers, Dr. Sittig notes. Moreover, there are configuration problems in the design of many EHRs, even if the products have the recommended features.
“For example, it’s often possible to meet the SAFER recommendations, but not all the vendors make that the default setting. That’s one of the things our paper says they should do,” Dr. Sittig says.
Conversely, some hospitals turn off certain features because they annoy doctors, he notes. For instance, the SAFER guides recommend that allergies, problem list entries, and diagnostic test results be entered and stored using standard, coded data elements in the EHR, but often the EHR makes it easier to enter free text data.
Default settings can be wiped out during system upgrades, he added. That has happened with drug interaction checkers. “If you don’t test the system after upgrades and reassess it annually, you might go several months without your drug-drug interaction checker on. And your doctors aren’t complaining about not getting alerts. Those kinds of mistakes are hard to catch.”
Some errors in an EHR may be caught fairly quickly, but in a health system that treats thousands of patients at any given time, those mistakes can still cause a lot of potential patient harm, Dr. Sittig points out. Some vendors, he says, are building tools to help health care organizations catch those errors through what is called “anomaly detection.” This is similar to what credit card companies do when they notice you’ve bought a carpet in Saudi Arabia, although you’ve never traveled abroad, he notes.
“You can look at alert firing data and notice that all of a sudden an alert fired 500 times today when it usually fires 10 times, or it stopped firing,” Dr. Sittig observes. “Those kinds of things should be built into all EHRs. That would be an excellent step forward.”
A version of this article first appeared on Medscape.com.
In a little-noticed action last month, so as to meet an objective of the Medicare Promoting Interoperability Program, starting next year.
Experts praised the move but said that EHR developers should share the responsibility for ensuring that the use of their products doesn’t harm patients.
A number of safety problems are associated with hospital EHR systems, ranging from insufficient protection against medication errors and inadvertent turnoffs of drug interaction checkers to allowing physicians to use free text instead of coded data for key patient indicators. Although hospitals aren’t required to do anything about safety problems that turn up in their self-audits, practitioners who perform the self-assessment will likely encounter challenges that they were previously unaware of and will fix them, experts say.
Studies over the past decade have shown that improper configuration and use of EHRs, as well as design flaws in the systems, can cause avoidable patient injuries or can fail to prevent them. For example, one large study found that clinical decision support (CDS) features in EHRs prevented adverse drug events (ADEs) in only 61.6% of cases in 2016. That was an improvement over the ADE prevention rate of 54% in 2009. Nevertheless, nearly 40% of ADEs were not averted.
Another study, sponsored by the Leapfrog Group, found that EHRs used in U.S. hospitals failed to detect up to 1 in 3 potentially harmful drug interactions and other medication errors. In this study, about 10% of the detection failures were attributed to design problems in EHRs.
The new CMS measure requires hospitals to evaluate their EHRs using safety guides that were developed in 2014 and were revised in 2016 by the Office of the National Coordinator for Health IT (ONC). Known as the Safety Assurance Factors for Resilience (SAFER) guides, they include a set of recommendations to help health care organizations optimize the safety of EHRs.
Surprises in store for hospitals
Dean Sittig, PhD, a professor at the University of Texas Health Science Center, Houston, told this news organization that a 2018 study he conducted with his colleague Hardeep Singh, MD, MPH, found that eight surveyed health care organizations were following about 75% of the SAFER recommendations.
He said that when hospitals and health care systems start to assess their systems, many will be surprised at what they are not doing or not doing right. Although the new CMS rule doesn’t require them to correct deficiencies, he expects that many will.
For this reason, Dr. Sittig believes the requirement will have a positive effect on patient safety. But the regulation may not go far enough because it doesn’t impose any requirements on EHR vendors, he said.
In a commentary published in JAMA, Dr. Sittig and Dr. Hardeep, a professor at the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, cite a study showing that 40% of “EHR-related products” had “nonconformities” with EHR certification regulations that could potentially harm patients. “Many nonconformities could have been identified by the developer prior to product release,” they say.
Shared responsibility
According to the JAMA commentary, the SAFER guides were developed “to help health care organizations and EHR developers conduct voluntary self-assessments to help eliminate or minimize EHR-related safety risks and hazards.”
In response to a query from this news organization, ONC confirmed that the SAFER guides were intended for use by developers as well as practitioners. ONC said it supports CMS’s approach to incentivize collaborations between EHR vendors and health care organizations. It noted that some entities have already teamed up to the meet the SAFER guides’ recommendations.
Hospitals and EHR developers must share responsibility for safety, Dr. Sittig and Dr. Singh argue, because many SAFER recommendations are based on EHR features that have to be programmed by developers.
For example, one recommendation is that patient identification information be displayed on all portions of the EHR user interface, wristbands, and printouts. Hospitals can’t implement this feature if the developer hasn’t built it into its product.
Dr. Sittig and Dr. Singh suggest three strategies to complement CMS’s new regulation:
- Because in their view, ONC’s EHR certification criteria are insufficient to address many patient safety concerns, CMS should require EHR developers to assess their products annually.
- ONC should conduct annual reviews of the SAFER recommendations with input from EHR developers and safety experts.
- EHR vendors should disseminate guidance to their customers on how to address safety practices, perhaps including EHR configuration guides related to safety.
Safety in EHR certification
At a recent press conference that ONC held to update reporters on its current plans, officials were asked to comment on Dr. Sittig’s and Dr. Singh’s proposition that EHR developers, as well as hospitals, do more to ensure system safety.
Steve Posnack, deputy national coordinator of health IT, noted that the ONC-supervised certification process requires developers to pay attention to how they “implement and integrate safety practices in their software design. We have certification criteria ... around what’s called safety-enhanced design – specific capabilities in the EHR that are sensitive to safety in areas like e-prescribing, medication, and high-risk events, where you want to make sure there’s more attention paid to the safety-related dynamics.”
After the conference, ONC told this news organization that among the safety-related certification criteria is one on user-centered design, which must be used in programming certain EHR features. Another is on the use of a quality management system to guide the creation of each EHR capability.
Nevertheless, there is evidence that not all EHR developers have paid sufficient attention to safety in their products. This is shown in the corporate integrity agreements with the Office of the Inspector General (OIG) of the Department of Health and Human Services (HHS) that developers eClinicalWorks and Greenway agreed to sign because, according to the government, they had not met all of the certification criteria they’d claimed to satisfy.
Under these agreements, the vendors agreed to follow “relevant standards, checklists, self-assessment tools, and other practices identified in the ONC SAFER guides and the ICE Report(s) to optimize the safety and safe use of EHRs” in a number of specific areas.
Even if all EHRs conformed to the certification requirements for safety, they would fall short of the SAFER recommendations, Dr. Sittig says. “Those certification criteria are pretty general and not as comprehensive as the SAFER guides. Some SAFER guide recommendations are in existing certification requirements, like you’re supposed to have drug-drug interaction checking capabilities, and they’re supposed to be on. But it doesn’t say you need to have the patient’s identification on every screen. It’s easy to assume good software design, development, and testing principles are a given, but our experience suggests otherwise.”
Configuration problems
A handful of vendors are working on what the JAMA article suggests, but there are about 1,000 EHR developers, Dr. Sittig notes. Moreover, there are configuration problems in the design of many EHRs, even if the products have the recommended features.
“For example, it’s often possible to meet the SAFER recommendations, but not all the vendors make that the default setting. That’s one of the things our paper says they should do,” Dr. Sittig says.
Conversely, some hospitals turn off certain features because they annoy doctors, he notes. For instance, the SAFER guides recommend that allergies, problem list entries, and diagnostic test results be entered and stored using standard, coded data elements in the EHR, but often the EHR makes it easier to enter free text data.
Default settings can be wiped out during system upgrades, he added. That has happened with drug interaction checkers. “If you don’t test the system after upgrades and reassess it annually, you might go several months without your drug-drug interaction checker on. And your doctors aren’t complaining about not getting alerts. Those kinds of mistakes are hard to catch.”
Some errors in an EHR may be caught fairly quickly, but in a health system that treats thousands of patients at any given time, those mistakes can still cause a lot of potential patient harm, Dr. Sittig points out. Some vendors, he says, are building tools to help health care organizations catch those errors through what is called “anomaly detection.” This is similar to what credit card companies do when they notice you’ve bought a carpet in Saudi Arabia, although you’ve never traveled abroad, he notes.
“You can look at alert firing data and notice that all of a sudden an alert fired 500 times today when it usually fires 10 times, or it stopped firing,” Dr. Sittig observes. “Those kinds of things should be built into all EHRs. That would be an excellent step forward.”
A version of this article first appeared on Medscape.com.
In a little-noticed action last month, so as to meet an objective of the Medicare Promoting Interoperability Program, starting next year.
Experts praised the move but said that EHR developers should share the responsibility for ensuring that the use of their products doesn’t harm patients.
A number of safety problems are associated with hospital EHR systems, ranging from insufficient protection against medication errors and inadvertent turnoffs of drug interaction checkers to allowing physicians to use free text instead of coded data for key patient indicators. Although hospitals aren’t required to do anything about safety problems that turn up in their self-audits, practitioners who perform the self-assessment will likely encounter challenges that they were previously unaware of and will fix them, experts say.
Studies over the past decade have shown that improper configuration and use of EHRs, as well as design flaws in the systems, can cause avoidable patient injuries or can fail to prevent them. For example, one large study found that clinical decision support (CDS) features in EHRs prevented adverse drug events (ADEs) in only 61.6% of cases in 2016. That was an improvement over the ADE prevention rate of 54% in 2009. Nevertheless, nearly 40% of ADEs were not averted.
Another study, sponsored by the Leapfrog Group, found that EHRs used in U.S. hospitals failed to detect up to 1 in 3 potentially harmful drug interactions and other medication errors. In this study, about 10% of the detection failures were attributed to design problems in EHRs.
The new CMS measure requires hospitals to evaluate their EHRs using safety guides that were developed in 2014 and were revised in 2016 by the Office of the National Coordinator for Health IT (ONC). Known as the Safety Assurance Factors for Resilience (SAFER) guides, they include a set of recommendations to help health care organizations optimize the safety of EHRs.
Surprises in store for hospitals
Dean Sittig, PhD, a professor at the University of Texas Health Science Center, Houston, told this news organization that a 2018 study he conducted with his colleague Hardeep Singh, MD, MPH, found that eight surveyed health care organizations were following about 75% of the SAFER recommendations.
He said that when hospitals and health care systems start to assess their systems, many will be surprised at what they are not doing or not doing right. Although the new CMS rule doesn’t require them to correct deficiencies, he expects that many will.
For this reason, Dr. Sittig believes the requirement will have a positive effect on patient safety. But the regulation may not go far enough because it doesn’t impose any requirements on EHR vendors, he said.
In a commentary published in JAMA, Dr. Sittig and Dr. Hardeep, a professor at the Michael E. DeBakey VA Medical Center and Baylor College of Medicine, cite a study showing that 40% of “EHR-related products” had “nonconformities” with EHR certification regulations that could potentially harm patients. “Many nonconformities could have been identified by the developer prior to product release,” they say.
Shared responsibility
According to the JAMA commentary, the SAFER guides were developed “to help health care organizations and EHR developers conduct voluntary self-assessments to help eliminate or minimize EHR-related safety risks and hazards.”
In response to a query from this news organization, ONC confirmed that the SAFER guides were intended for use by developers as well as practitioners. ONC said it supports CMS’s approach to incentivize collaborations between EHR vendors and health care organizations. It noted that some entities have already teamed up to the meet the SAFER guides’ recommendations.
Hospitals and EHR developers must share responsibility for safety, Dr. Sittig and Dr. Singh argue, because many SAFER recommendations are based on EHR features that have to be programmed by developers.
For example, one recommendation is that patient identification information be displayed on all portions of the EHR user interface, wristbands, and printouts. Hospitals can’t implement this feature if the developer hasn’t built it into its product.
Dr. Sittig and Dr. Singh suggest three strategies to complement CMS’s new regulation:
- Because in their view, ONC’s EHR certification criteria are insufficient to address many patient safety concerns, CMS should require EHR developers to assess their products annually.
- ONC should conduct annual reviews of the SAFER recommendations with input from EHR developers and safety experts.
- EHR vendors should disseminate guidance to their customers on how to address safety practices, perhaps including EHR configuration guides related to safety.
Safety in EHR certification
At a recent press conference that ONC held to update reporters on its current plans, officials were asked to comment on Dr. Sittig’s and Dr. Singh’s proposition that EHR developers, as well as hospitals, do more to ensure system safety.
Steve Posnack, deputy national coordinator of health IT, noted that the ONC-supervised certification process requires developers to pay attention to how they “implement and integrate safety practices in their software design. We have certification criteria ... around what’s called safety-enhanced design – specific capabilities in the EHR that are sensitive to safety in areas like e-prescribing, medication, and high-risk events, where you want to make sure there’s more attention paid to the safety-related dynamics.”
After the conference, ONC told this news organization that among the safety-related certification criteria is one on user-centered design, which must be used in programming certain EHR features. Another is on the use of a quality management system to guide the creation of each EHR capability.
Nevertheless, there is evidence that not all EHR developers have paid sufficient attention to safety in their products. This is shown in the corporate integrity agreements with the Office of the Inspector General (OIG) of the Department of Health and Human Services (HHS) that developers eClinicalWorks and Greenway agreed to sign because, according to the government, they had not met all of the certification criteria they’d claimed to satisfy.
Under these agreements, the vendors agreed to follow “relevant standards, checklists, self-assessment tools, and other practices identified in the ONC SAFER guides and the ICE Report(s) to optimize the safety and safe use of EHRs” in a number of specific areas.
Even if all EHRs conformed to the certification requirements for safety, they would fall short of the SAFER recommendations, Dr. Sittig says. “Those certification criteria are pretty general and not as comprehensive as the SAFER guides. Some SAFER guide recommendations are in existing certification requirements, like you’re supposed to have drug-drug interaction checking capabilities, and they’re supposed to be on. But it doesn’t say you need to have the patient’s identification on every screen. It’s easy to assume good software design, development, and testing principles are a given, but our experience suggests otherwise.”
Configuration problems
A handful of vendors are working on what the JAMA article suggests, but there are about 1,000 EHR developers, Dr. Sittig notes. Moreover, there are configuration problems in the design of many EHRs, even if the products have the recommended features.
“For example, it’s often possible to meet the SAFER recommendations, but not all the vendors make that the default setting. That’s one of the things our paper says they should do,” Dr. Sittig says.
Conversely, some hospitals turn off certain features because they annoy doctors, he notes. For instance, the SAFER guides recommend that allergies, problem list entries, and diagnostic test results be entered and stored using standard, coded data elements in the EHR, but often the EHR makes it easier to enter free text data.
Default settings can be wiped out during system upgrades, he added. That has happened with drug interaction checkers. “If you don’t test the system after upgrades and reassess it annually, you might go several months without your drug-drug interaction checker on. And your doctors aren’t complaining about not getting alerts. Those kinds of mistakes are hard to catch.”
Some errors in an EHR may be caught fairly quickly, but in a health system that treats thousands of patients at any given time, those mistakes can still cause a lot of potential patient harm, Dr. Sittig points out. Some vendors, he says, are building tools to help health care organizations catch those errors through what is called “anomaly detection.” This is similar to what credit card companies do when they notice you’ve bought a carpet in Saudi Arabia, although you’ve never traveled abroad, he notes.
“You can look at alert firing data and notice that all of a sudden an alert fired 500 times today when it usually fires 10 times, or it stopped firing,” Dr. Sittig observes. “Those kinds of things should be built into all EHRs. That would be an excellent step forward.”
A version of this article first appeared on Medscape.com.
Antibiotic use and colon cancer: More evidence of link
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The Implications of Power Mobility on Body Weight in a Veteran Population
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
New guidance on preventing cutaneous SCC in solid organ transplant patients
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
Transgender individuals twice as likely to die as general population
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.
Mortality is consistently twice as high in transgender people receiving hormone treatment, compared with cisgender individuals in the general population and has not decreased over time, results of a 5 decades–long study from the Netherlands indicate.
Particularly concerning is that trans women (male to female) had a mortality risk nearly double that of cis men (born and remain male) in the general Dutch population (standardized mortality ratio, 1.8), while it was nearly triple that of cis women (SMR, 2.8).
Compared with cisgender women, transgender women were more than twice as likely to die from heart disease, three times more likely to die from lung cancer, and almost nine times more likely to die from infection. HIV-related disease mortality risk was nearly 50 times higher for trans women than cis women, and the risk of suicide was almost seven times greater.
Suicide and other nonnatural causes of death were more common in trans men, compared with cis women.
The report, by Christel J.M. de Blok, MD, of Amsterdam University Medical Center and colleagues, was published online Sept. 2 in The Lancet Diabetes & Endocrinology.
The study included trans men who received testosterone to transition from female to male and trans women who received estrogen plus an antiandrogen to transition from male to female.
Is gender-affirming hormone therapy associated with increased mortality?
Senior author Martin den Heijer, MD, also of Amsterdam University Medical Center, said: “The findings of our large, nationwide study highlight a substantially increased mortality risk among transgender people that has persisted for decades.”
But he pointed out that, overall, the data do not appear to suggest the premature deaths were related to gender-affirming hormone treatment.
However, he conceded that more work is needed on this aspect of care. “There is insufficient evidence at present to determine long-term safety of [gender-affirming hormone treatment]. More research is needed to fully establish whether it in any way affects mortality risk for transgender people,” said Dr. den Heijer.
Endocrinologist Will Malone, MD, of Twin Falls, Idaho, told this news organization, “The study confirms, like others before it, that individuals taking cross-sex hormones are more likely to die prematurely from a number of causes.”
“While the authors speculate that this higher mortality rate is not connected to cross-sex hormones, the study was not designed to be able to make such a claim,” he said, pointing to limited follow-up times.
In an accompanying commentary, Vin Tangpricha, MD, PhD, an endocrinologist from Emory University, Atlanta, noted: “Transgender men do not appear to have as significantly increased comorbidity following receipt of gender-affirming hormone therapy when compared with transgender women.”
Dr. Tangpricha added future studies should examine which factors – hormone regimen, hormone concentrations, access to health care, or other biological factors – explain the higher increased risk of morbidity and mortality observed in trans women as opposed to trans men.
However, Dr. de Blok and colleagues note that, as there were relatively few deaths among transgender men in the cohort, analysis on cause of death in this group is limited.
Transgender individuals more likely to die younger
For their study, Dutch researchers retrospectively examined data from 4,568 transgender people attending their clinic (2,927 transgender women and 1,641 transgender men) treated in 1972-2018. People were excluded if they started treatment before the age of 17 or if they had received puberty-blocking drugs.
Data on age at start of hormone treatment, type of treatment, smoking habits, medical history, and last date of follow-up were gathered from medical records. Where possible, SMRs were determined for deaths among trans men and trans women, compared with rates for the adult Dutch general population.
Median age at the start of cross-sex hormone treatment was 30 years in transgender women and 23 years in transgender men. But the median follow-up time was only 11 years in transgender women and 5 years in transgender men.
A total of 317 (10.8%) trans women died, and 44 (2.7%) trans men died. The findings were higher than expected, compared with the general population of cisgender women (SMR, 1.8) but not cisgender men (SMR 1.2).
Mortality risk did increase more in transgender people who started gender-affirming hormone treatment in the past 2 decades compared with earlier, a fact that Dr. de Blok said was surprising.
Trans men, for example, compared with cis women, had an SMR of 2.1-2.4 in 2000-2018 (compared with 1.8 overall).
“This may be due to changes in clinical practice. ... In the past, health care providers were reluctant to provide hormone treatment to people with a history of comorbidities such as cardiovascular disease. However, because of the many benefits of enabling people to access hormone therapy, nowadays this rarely results in treatment being denied,” Dr. de Blok noted.
More research needed, especially in trans-identifying youth
Dr. Malone remarked that previous studies have shown associations between taking cross-sex hormones and elevated mortality, while also “not designed to detect causality,” have “generally accepted that natal males who take estrogen have estrogen-related increases in the rates of heart disease, stroke, and deep venous thrombosis.”
He added that the risks of testosterone use in natal females were less well established, “but testosterone is also felt to increase their risk of heart disease.”
He stressed the limited follow-up times in the study by Dr. de Blok and colleagues.
This “strongly suggests that the rate of elevated mortality far exceeds the doubling measured by the study, especially for natal females.”
Dr. Malone is one of several clinicians and researchers who has formed the Society for Evidence-Based Gender Medicine, a nonprofit organization that now has at least 100 physician members. SEGM is concerned about the lack of quality evidence for the use of hormonal and surgical interventions as first-line treatment, especially for young people with gender dysphoria.
Dr. Tangpricha also highlighted that the findings do not apply to transgender people who began treatment before age 17 years or those who had taken puberty blockers before gender-affirming hormone treatment.
There are no long-term data on transgender individuals who have received gender-affirming hormone therapies close to the time of puberty.
These data, such as those from the Trans Youth Care study, should be available in the future, he added.
The authors have reported no relevant financial relationships. Dr. Tangpricha has reported receiving funding from the National Institutes of Health and served as past president of the World Professional Association for Transgender Health. He is editor-in-chief of Endocrine Practice and has provided expert testimony for Kirkland and Ellis.
A version of this article first appeared on Medscape.com.