User login
For MD-IQ use only
Autism spectrum disorder in children and adolescents: Treatment options
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
SECOND OF 2 PARTS
Evidence supports the crucial role of early intervention and nonpharmacologic approaches
A large percentage of individuals with autism spectrum disorder (ASD) experience persisting significant social deficits in adulthood,1 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.2,3 Childhood is a vital time for making the most significant and lasting changes that can improve functioning of individuals with ASD. Psychiatrists and other physicians who treat children are in a key role to influence outcomes of children at risk for or diagnosed with ASD.
This article provides updates on various aspects of ASD diagnosis and treatment (based on available evidence up to March 2020). Part 1 (
A comprehensive approach is essential
Multiple treatment modalities have been recommended for ASD.5 It is essential to address all aspects of ASD through cognitive, developmental, social-communication, sensory-motor, and behavioral interventions. Nonpharmacologic interventions are crucial in improving long-term outcomes of children with ASD.6
Nonpharmacologic treatments
Nonpharmacologic interventions commonly utilized for children with ASD include behavioral therapies, other psychological therapies, speech-language therapy, occupational therapy, educational interventions, parent coaching/training, developmental social interventions, and other modalities of therapy that are delivered in school, home, and clinic settings.5,7
A recent study examining ASD treatment trends via caregivers’ reports (N = 5,122) from the SPARK (Simons Foundation Powering Autism Research for Knowledge) cohort in the United States reported that 80% of children received speech-language therapy or occupational therapy; 52% got both.5 The study revealed that approximately one-quarter utilized 3 therapies simultaneously; two-thirds had utilized 3 or more therapies in the previous year.5
Interventions for children with ASD need to be individualized.1,8 Evidence-based behavioral interventions for ASD fall into 2 broad categories: Applied Behavior Analysis (ABA), and Naturalistic Developmental Behavioral Interventions (NDBI). Traditionally, ABA has been a key model, guiding treatment for enhancing social-communicating skills and lowering maladaptive behaviors in ASD.9 ABA follows a structured and prescribed format,10,11 and has been shown to be efficacious.1,7 More recently, NDBI, in which interventions are “embedded” in the natural environment of the young child and more actively incorporate a developmental perspective, has been shown to be beneficial in improving and generalizing social-communication skills in young children with ASD.7,11
Early Start Denver Model (ESDM) is an intensive, naturalistic behavioral intervention4 that has been shown to be efficacious for enhancing communication and adaptive behavior in children with ASD.7,8,12 A multisite randomized controlled trial (RCT) by Rogers et al12 that examined the efficacy of ESDM in 118 children (age 14 to 24 months) with ASD found the treatment was beneficial and superior compared with a “community intervention” group, in regards to language ability measured in time by group analyses.The ESDM intervention in this study involved weekly parent coaching for 3 months, along with 24 months of 15 hours/week of one-on-one treatment provided by therapy professionals.12
Reciprocal imitation training (RIT) is another naturalistic intervention that has shown benefit in training children with ASD in imitation skills during play.13 Studies have found that both RIT and ESDM can be parent-implemented, after parents receive training.13,14
Parent-mediated, parent-implemented interventions may have a role in improving outcomes in childhood ASD,7,15 particularly “better generalization and maintenance of skills than therapist-implemented intervention” for lowering challenging behaviors and enhancing verbal and nonverbal communication.16
Various social skills interventions have also been found effective for children with ASD.1 Such interventions are often provided in the school setting.7 Coordination with the child’s school to discuss and advocating for adequate and suitable interventions, educational services, and placement is an essential aspect of ASD treatment.7
Two other school-based, comprehensive treatment model interventions—Learning Experiences and Alternative Programs for Preschoolers and their Parents (LEAP), and TEACCH—have some evidence of leading to improvement in children with ASD.7,17
Some studies have found that music therapy may have high efficacy for children with ASD, even with smaller length and intensity of treatment, particularly in improving social interaction, engagement with parents, joint attention, and communication.3,18 Further research is needed to conclusively establish the efficacy of music therapy for ASD in children and adolescents.
A few studies have assessed the long-term outcomes of interventions for ASD; however, more research is needed.19 Pickles et al19 conducted a follow-up to determine the long-term effects of the Preschool Autism Communication Trial (PACT), an RCT of parent-mediated social communication therapy for children age 2 to 4 with ASD. The children’s average age at follow-up was 10 years. The authors found a significant long-term decrease in ASD symptoms and enhancement of social communication with parents (N = 152).
Technology-based interventions, including games and robotics, have been investigated in recent years, for treatment of children with ASD (eg, for improving social skills).20
Research suggests that the intensity (number of hours) and duration of nonpharmacologic treatments for ASD is critical to improving outcomes (Box1,3,5,7,10,16).
Box
A higher intensity of nonpharmacologic intervention (greater number of hours) has been associated with greater benefit for children with autism spectrum disorder (ASD), in the form of enhancements in IQ and adaptive behavior.1,10,16 In the United States, the intensity of interventions commonly ranges from 30 to 200 or more minutes per week.3 This may mean that a child with ASD who is receiving 30 minutes of speech therapy at school and continues to exhibit significant deficits in speech-language or social-communication may likely benefit from additional hours of speech therapy and/or social-communication skill training, and should be referred accordingly, even for private therapy services if needed and feasible.7 Guidelines created through a systematic review of evidence recommend at least 25 hours per week of comprehensive treatment interventions for children with ASD to address language, social deficits, and behavioral difficulties.1 The duration of intervention has also been shown to play a role in outcomes.1,3,10 Given the complexity and extent of impairment often associated with ASD, it is not surprising that in recent research examining trends in ASD treatment in the United States, most caregivers reported therapy as ongoing.5 The exact intensity and duration of nonpharmacologic interventions may depend on several factors, such as severity of ASD and of the specific deficit being targeted, type of intervention, and therapist skill. The quality of skills of the care provider has also been shown to affect the benefits gained from the intervention.3
Continue to: Pharmacotherapy...
Pharmacotherapy
Medications cannot resolve core features of ASD.21 However, certain medications may help address associated comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), depression, or others, when these conditions have not responded to nonpharmacologic interventions.7,22 Common symptoms that are often treated with pharmacotherapy include aggression, irritability, hyperactivity, attentional difficulties, tics, self-injurious behavior, obsessive-compulsive symptoms, and mood dysregulation/lability.23 Generally speaking, medications might be considered if symptoms are severe and markedly impair functioning. For mild to moderate conditions, psychotherapy and other nonpharmacologic interventions are generally considered first-line. Since none of the medications described below are specific to ASD and psychiatrists generally receive training in prescribing them for other indications, a comprehensive review of their risks and benefits is beyond the scope of this article. No psychotropic medications are known to have robust evidence for safety in preschool children with ASD, and thus are best avoided.
Antipsychotics. Risperidone (for age 5 and older) and aripiprazole (age 6 to 17) are the only medications FDA-approved for use in children and adolescents with ASD, specifically for irritability associated with ASD.21,24 These 2 second-generation antipsychotics may also assist in lowering aggression in patients with ASD.24 First-generation antipsychotics such as haloperidol have been shown to be effective for irritability and aggression in ASD, but the risk of significant adverse effects such as dyskinesias and extrapyramidal symptoms limit their use.24 Two studies (a double-blind study and an open-label extension of that study) in children and adolescents with ASD found that risperidone was more effective and better tolerated than haloperidol in behavioral measures, impulsivity, and even in the social domain.25,26 In addition to other adverse effects and risks, increased prolactin secondary to risperidone use requires close monitoring and caution.24-26 As is the case with the use of other psychotropic medications in children and adolescents, those with ASD who receive antipsychotics should also be periodically reassessed to determine the need for continued use of these medications.27 A multicenter relapse prevention RCT found no statistically significant difference in the time to relapse between aripiprazole and placebo.27 Metabolic syndrome, cardiac risks, and other risks need to be considered before prescribing an antipsychotic.28 Given their serious adverse effects profile, use should be considered only when there is severe impairment or risk of injury, after carefully weighing risks/benefits.
Medications for attentional difficulties. A multisite, randomized, placebo-controlled trial evaluating the use of extended-release guanfacine in children with ASD (N = 62) found the rate of positive response on the Clinical Global Impressions–Improvement scale was 50% for guanfacine vs 9.4% for placebo.29 Clinicians need to monitor for adverse effects of guanfacine, such as fatigue, drowsiness, lightheadedness, lowering of blood pressure and heart rate, and other effects.29 A randomized, double-blind trial of 97 children and adolescents with ASD and ADHD found that atomoxetine had moderate benefit for ADHD symptoms.30 The study reported no serious adverse effects.30 However, it is especially important to monitor for hepatic and cardiac adverse effects (in addition to monitoring for risk of increase in suicidal thoughts/behavior, as in the case of antidepressants) when using atomoxetine, in addition to other side effects and risks. Some evidence suggests that methylphenidate may be effective for attentional difficulties in children and adolescents with ASD21 but may pose a higher risk of adverse effects in this population compared with neurotypical patients.31
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) are sometimes used to reduce obsessive-compulsive symptoms, repetitive behavior, or depressive symptoms in children with ASD, but are not FDA-approved for children or adolescents with ASD. In general, there is inadequate evidence to support the use of SSRIs for ASD in children.31-34 In addition, children with ASD may be at a greater risk of adverse effects from SSRIs.32,34 Despite this, SSRIs are the most commonly prescribed psychotropic medications in children with ASD.32
An RCT examining the efficacy of fluoxetine in 158 children and adolescents with ASD found no significant difference in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) score after 14 weeks of treatment; activation was a common adverse effect.35 A 2005 randomized, double-blind, placebo-controlled trial of 45 children/adolescents with ASD found that low-dose liquid fluoxetine was more effective than placebo for reducing repetitive behaviors in this population.36 Larger studies are warranted to further evaluate the efficacy and safety of fluoxetine (and of SSRIs in general, particularly in the long term) for children and adolescents with ASD.36 A 2009 randomized, placebo-controlled trial of 149 children with ASD revealed no significant difference between citalopram and placebo as measured by Clinical Global Impressions scale or CY-BOCS scores, and noted a significantly elevated likelihood of adverse effects.37
Other antidepressants. There is insufficient evidence to support the use of any other antidepressants in children and adolescents with ASD. A few studies38,39 have examined the use of venlafaxine in children with ASD; however, further research and controlled studies with large sample sizes are required to conclusively establish its benefits. There is a dearth of evidence examining the use of the tetracyclic antidepressant mirtazapine, or other classes of medications such as tricyclic antidepressants or mood stabilizers, in children with ASD; only a few small studies have assessed the efficacy and adverse effects of these medications for such patients.31
Polypharmacy. Although there is no evidence to support polypharmacy in children and adolescents with ASD, the practice appears to be rampant in these patients.28,40 A 2013 retrospective, observational study of psychotropic medication use in children with ASD (N = 33,565) found that 64% were prescribed psychotropic medications, and 35% exhibited evidence of polypharmacy.40 In this study, the total duration of polypharmacy averaged 525 days.40 When addressing polypharmacy, systematic deprescribing or simplification of the psychotropic medication regimen may be needed,28 while taking into account the patient’s complete clinical situation, including (but not limited to) tolerability of the medication regimen, presence or absence of current stressors, presence or absence of adequate supports, use of nonpharmacologic treatments where appropriate, and other factors.
More studies assessing the efficacy and safety of psychotropic medications for children and adolescents with ASD are needed,32 especially studies that evaluate the effects of long-term use, because evidence for pharmacologic treatments for children with ASD is mixed and insufficient.33 There is also a need for evidence-based standards for prescribing psychotropic medications in children and adolescents with ASD.
Psychotropic medications, if used in ASD, should be used only in conjunction with other evidence-based treatment modalities, and not as monotherapy.21 Children and adolescents with ASD may be particularly susceptible to side effects or adverse effects of certain psychotropic medications.31 When considering medications, carefully weigh the risks and benefits.7,21,24,28 Starting low and going slow is generally the preferred strategy.31,32 As always, when recommending medications, discuss in detail with parents the potential side effects, benefits, risks, interactions, and alternatives.
Other agents. Several double-blind, placebo-controlled trials have evaluated using melatonin for sleep difficulties in children and adolescents with ASD.41 A randomized, placebo-controlled, 12-week trial that assessed 160 children with ASD and insomnia found that melatonin plus cognitive-behavioral therapy (CBT) was superior in efficacy to melatonin alone, CBT alone, or placebo.41
The evidence regarding oxytocin use for children with ASD is mixed.31 Some small studies have associated improvement in the social domain with its use. Guastella et al42 conducted a randomized, double-blind, placebo-controlled trial of oxytocin nasal spray for 16 participants (age 12 to 19) with ASD, and found oxytocin enhanced emotional identification. Gordon et al43 conducted a functional MRI study of brain activity with oxytocin use in children with high-functioning ASD (N = 17). They found that oxytocin may augment “salience and hedonic evaluations of socially meaningful stimuli in children with ASD” and thus help social attunement. Further research is needed to evaluate the impact of oxytocin on social behavior.
Complementary and alternative medicine. Although there is limited and inconclusive evidence about the use of complementary and alternative medicine in children and adolescents with ASD, these therapies continue to be commonly used.44-46 A recent survey of parents (N = 211) of children with ASD from academic ASD outpatient clinics in Germany found that 46% reported their child was using or had used some type of complementary and alternative medicine.44 There is inadequate evidence to support the use of a gluten-free, casein-free diet for children/adolescents with ASD.46 A recent cross-sectional study assessing supplement use in 210 children with ASD in Canada found that 75% used supplements, such as multivitamins (77.8%), vitamin D (44.9%), omega 3 (42.5%), probiotics (36.5%), and magnesium (28.1%), despite insufficient evidence to support their safety or efficacy for children with ASD.47 Importantly, 33.5% of parents in this study reported that they did not inform the physician about all their child’s supplements.47 Some of the reasons the parents in this study provided for not disclosing information about supplements to their physicians were “physician lack of knowledge,” “no benefit,” “too time-consuming,” and “scared of judgment.”47 Semi-structured interviews of parents of 21 children with ASD in Australia revealed that parents found information on complementary and alternative medicine and therapies complex and often conflicting.45 In addition to recommendations from health care professionals, evidence suggests that parents often consider the opinions of media, friends, and family when making a decision on using complementary and alternative medicine modalities for children/adolescents with ASD.46 Such findings can inform physician practices regarding supplement use, and highlight the need to educate parents about the evidence regarding these therapies and potential adverse effects and interactions of such therapies,46 along with the need to develop a centralized, evidence-based resource for parents regarding their use.45
Omega 3 supplementation has in general shown few adverse effects47; still, risks/benefits need to be weighed before use. Some evidence suggests that it may decrease hyperactivity in children with ASD.31,48 However, further research, particularly controlled trials with large sample sizes, are needed for a definitive determination of efficacy.31,48 A meta-analysis that included 27 RCTs assessing the efficacy of dietary interventions for various ASD symptoms found that omega 3 supplementation was more effective than placebo, but compared with placebo, the effect size was small.49 A RCT of 73 children with ASD in New Zealand found that omega 3 long chain polyunsaturated fatty acids may benefit some core symptoms of ASD; the authors suggested that further research is needed to conclusively establish efficacy.50
Continue to: A need for advocacy and research..
A need for advocacy and research
Physicians who treat children with ASD can not only make appropriate referrals and educate parents, but also educate their patients’ schools and advocate for their patients to get the level of services they need.23,28
A recent study in the United States found that behavior therapy and speech-language therapy were used less often in the treatment of children with ASD in rural areas compared with those in metro areas.5 This suggests that in addition to increasing parents’ awareness and use of ASD services and providing referrals where appropriate, physicians are in a unique position to advocate for public health policies to improve access, coverage, and training for the provision of such services in rural areas.
There is need for ongoing research to further examine the efficacy and nuances of effects of various treatment interventions for ASD, especially long-term studies with larger sample sizes.11,51 Additionally, research is warranted to better understand the underlying genetic and neurobiological mechanisms of ASD, which would help guide the development of biomarkers,52 innovative treatments, and disease-modifying agents for ASD.7,22 Exploring the effects of potential alliances or joint action between biological and psychosocial interventions for ASD is also an area that needs further research.51
Bottom Line
A combination of treatment modalities (such as speech-language therapy, social skills training, behavior therapy/other psychotherapy, and occupational therapy for sensory sensitivities) is generally needed to improve the long-term outcomes of children and adolescents with autism spectrum disorder (ASD). In addition to the importance of early intervention, the intensity and duration of nonpharmacologic treatments are vital to improving outcomes in ASD.
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53
1. Maglione MA, Gans D, Das L, et al. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2):S169-S178.
2. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
3. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
4. Charman T. Editorial: trials and tribulations in early autism intervention research. J Am Acad Child Adolesc Psychiatry. 2019;58(9):846-848. doi:10.1016/j.jaac.2019.03.004
5. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):517-526. doi:10.1002/aur.2070
6. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi:10.3389/fnins.2016.00393
7. Hyman SL, Levy SE, Myers SM, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
8. Contaldo A, Colombi C, Pierotti C, et al. Outcomes and moderators of Early Start Denver Model intervention in young children with autism spectrum disorder delivered in a mixed individual and group setting. Autism. 2020;24(3):718-729. doi:10.1177/1362361319888344
9. Lei J, Ventola P. Pivotal response treatment for autism spectrum disorder: current perspectives. Neuropsychiatr Dis Treat. 2017;13:1613-1626. doi:10.2147/NDT.S120710
10. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
11. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
12. Rogers SJ, Estes A, Lord C, et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J Am Acad Child Adolesc Psychiatry. 2019;58(9):853-865. doi:10.1016/j.jaac.2019.01.004
13. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. 2007;28(2):163-175.
14. Waddington H, van der Meer L, Sigafoos J, et al. Examining parent use of specific intervention techniques during a 12-week training program based on the Early Start Denver Model. Autism. 2020;24(2):484-498. doi:10.1177/1362361319876495
15. Trembath D, Gurm M, Scheerer NE, et al. Systematic review of factors that may influence the outcomes and generalizability of parent‐mediated interventions for young children with autism spectrum disorder. Autism Res. 2019;12(9):1304-1321.
16. Rogers SJ, Estes A, Lord C, et al. Effects of a brief Early Start Denver Model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
17. Boyd BA, Hume K, McBee MT, et al. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J Autism Dev Disord. 2014;44(2):366-380. doi:10.1007/s10803-013-1877-9
18. Thompson GA, McFerran KS, Gold C. Family-centred music therapy to promote social engagement in young children with severe autism spectrum disorder: a randomized controlled study. Child Care Health Dev. 2014;40(6):840-852. doi:10.1111/cch.12121
19. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
20. Grossard C, Palestra G, Xavier J, et al. ICT and autism care: state of the art. Curr Opin Psychiatry. 2018;31(6):474-483. doi:10.1097/YCO.0000000000000455
21. Cukier S, Barrios N. Pharmacological interventions for intellectual disability and autism. Vertex. 2019;XXX(143)52-63.
22. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104.
23. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257.
24. LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397.
25. Gencer O, Emiroglu FN, Miral S, et al. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217-225.
26. Miral S, Gencer O, Inal-Emiroglu FN, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1-8.
27. Findling RL, Mankoski R, Timko K, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22-30. doi:10.4088/jcp.13m08500
28. McLennan JD. Deprescribing in a youth with an intellectual disability, autism, behavioural problems, and medication-related obesity: a case study. J Can Acad Child Adolesc Psychiatry. 2019;28(3):141-146.
29. Scahill L, McCracken JT, King B, et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197-1206. doi:10.1176/appi.ajp.2015.15010055
30. Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733-741. doi:10.1016/j.jaac.2012.04.011
31. DeFilippis M, Wagner KD. Treatment of autism spectrum disorder in children and adolescents. Psychopharmacol Bull. 2016;46(2):18-41.
32. DeFilippis M. Depression in children and adolescents with autism spectrum disorder. Children (Basel). 2018;5(9):112. doi:10.3390/children5090112
33. Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry. 2018;30(1):78-95. doi:10.1080/09540261.2018.1458706
34. Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. doi:10.1002/14651858.CD004677.pub3
35. Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233-3244. doi:10.1007/s10803-019-04120-y
36. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582-589.
37. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590. doi:10.1001/archgenpsychiatry.2009.30
38. Hollander E, Kaplan A, Cartwright C, et al. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15(2):132-135.
39. Carminati GG, Deriaz N, Bertschy G. Low-dose venlafaxine in three adolescents and young adults with autistic disorder improves self-injurious behavior and attention deficit/hyperactivity disorders (ADHD)-like symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):312-315.
40. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833-840. doi:10.1542/peds.2012-3774
41. Cortesi F, Giannotti F, Sebastiani T, et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res. 2012;21(6):700-709. doi:10.1111/j.1365-2869.2012.01021.x
42. Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692-694. doi:10.1016/j.biopsych.2009.09.020
43. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
44. Höfer J, Bachmann C, Kamp-Becker I, et al. Willingness to try and lifetime use of complementary and alternative medicine in children and adolescents with autism spectrum disorder in Germany: a survey of parents. Autism. 2019;23(7):1865-1870. doi:10.1177/1362361318823545
45. Smith CA, Parton C, King M, et al. Parents’ experiences of information-seeking and decision-making regarding complementary medicine for children with autism spectrum disorder: a qualitative study. BMC Complement Med Ther. 2020;20(1):4. doi:10.1186/s12906-019-2805-0
46. Marsden REF, Francis J, Garner I. Use of GFCF diets in children with ASD. An investigation into parents’ beliefs using the theory of planned behaviour. J Autism Dev Disord. 2019;49(9):3716-3731. doi:10.1007/s10803-019-04035-8
47. Trudeau MS, Madden RF, Parnell JA, et al. Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder. Nutrients. 2019;11(8):1783. doi:10.3390/nu11081783
48. Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658-666. doi:10.1016/j.jaac.2014.01.018
49. Fraguas D, Díaz-Caneja C, Pina-Camacho L, et al. Dietary interventions for autism spectrum disorder: a meta-analysis. Pediatrics. 144(5):e20183218.
50. Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord. 2019;49(5):1778-1794. doi:10.1007/s10803-018-3860-y
51. Green J, Garg S. Annual research review: the state of autism intervention science: progress, target psychological and biological mechanisms and future prospects. J Child Psychol Psychiatry. 2018;59(4):424-443. doi:10.1111/jcpp.1289
52. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.53
Sexual activity alters the microbiome, with potential psychiatric implications
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
Evidence is strong that sexual partners transmit microbiota (bacteria, viruses, fungi, protozoa, and archaea) to each other. While microbial flora are abundant in the gastrointestinal tract, they are also present in the vagina, penis, urethra, mouth, and skin.1 For better or worse, sexual contact of all types means that participants will acquire each other’s microbiota.
The 39 trillion microbiota in the body (which exceed the 30 trillion cells in the body) are commensal and influence both the larger brain in the skull and the smaller enteric brain in the gut. The microbiota and their microbiome genes (1,000 times larger than the human genome) have been linked to depression, anxiety, psychosis, and autism.2-4 They produce 90% of the body’s serotonin, as well as catecholamines (norepinephrine, epinephrine, dopamine), make hormones (eg, cortisol), and modulate the immune system. Microbiota have several important functions, including food digestion, synthesis of vitamins, autoimmunity, hypothalamic-pituitary-adrenal axis regulation, and CNS modulation.
Consequences of dysbiosis
Everyone should be concerned about maintaining a healthy diversity of microbiota in their body, with a predominance of beneficial bacteria such as Lactobacillus and Bacteroides, and avoiding acquiring pathogenic bacteria such as Gardnerella, Prevotella, and Atopobium. Sexual activity involving a partner with unhealthy microbiota may increase the risk of dysbiosis, defined as a reduction in microbiota diversity, including a loss of beneficial bacteria and a rise in harmful bacteria.
Dysbiosis is associated with multiple symptoms, including5:
- brain “fog,” irritability, mood changes, and anxiety
- bloating, loss of intestinal permeability, and insufficient reclamation of nutrients
- congestion of certain organs, such as the liver, gallbladder, and pancreas
- production of antigen-antibody complexes in response to chemicals in partially digested food
- aggravation of inflammatory disorders such as migraine, arthritis, and autoimmune disorders.
Apart from intimate sexual contact, simply sharing a household with someone leads to sharing of gut microflora. Persons who live together, whether genetically related or not, have similar microbiota. Compared with people living in separate households, cohabiting human pairs, dog pairs, and human-dog pairs share most of their microbiota (especially in the skin).
A consequence of acquiring pathogenic microbiota in the vagina is bacterial vaginosis (BV), which is not an infection but an ecologic imbalance in the composition of the vaginal microbiota. BV is caused by a significant decline in the beneficial vaginal Lactobacillus and a marked increase in the non-Lactobacillus taxa (especially Gardnerella and Atopobium).6 It can last for a least 1 week after sexual intercourse. BV is rare or absent among virgins. For a male partner, penile microbiota changes significantly after unprotected sex.6
Pathogenic bacteria can be cultivated from the glans, the coronal sulcus, and the prepuce, as well as from the penile skin, semen, urethra, and urine.6 Diverse bacteria exist in human semen, regardless if the male is fertile or infertile.7Anaerococcus is a biomarker for low sperm quality. Many of the semen bacteria are also found in the vagina of women with BV.7 Semen is a medium for the transmission of bacteria and viruses between men and women, and can contribute to sexually transmitted diseases.8
There are approximately 21 million cases of BV in the United States each year, and BV can also increase the risk of HIV and poor obstetric outcomes.9 The microbiota in the penile skin and urethra in males who have monogamous relationships with females are very similar to the vaginal microbiota of their female partner.
Consequences of BV include:
- decrease in hydrogen peroxide–producing bacilli
- prevalence of anaerobic bacteria (Prevotella, Gardnerella, and Atopobium)
- alkalinization, fishy odor, and gray-white vaginal discharge
- increase in the rate of pelvic inflammatory disease, ectopic pregnancy, endometriosis, preterm birth, and tubal factor infertility.9
Circumcision decreases the risk of BV. There is an increased rate of BV bacterial taxa in men with extramarital affairs and in women with multiple partners. Both oral and vaginal sex increase the abundance of Lactobacillus in the male oral and penile microbiota. Gingivitis has also been reported after oral sex.10
A link to psychiatric disorders
Given that all forms of sexual contact (vaginal, oral, anal, or skin) can transmit microbiota bidirectionally between partners, it is vital to practice safe sex and consider a monogamous relationship rather than indiscriminate promiscuity. Unfortunately, certain psychiatric disorders, such as bipolar disorder, are associated with hypersexuality and multiple partners, which may disrupt the microbiota. This can further disrupt the diversity of an individual’s microbiome and may put them at risk for mood, anxiety, and other psychiatric disorders. Another problem is sexually transmitted infections such as gonorrhea or syphilis require antibiotic therapy. It is well established that antibiotics kill both the bad pathogenic and the good nonpathogenic microbiota, further exacerbating dysbiosis and leading to disruptions in the microbiota-gut-brain (MGB) axis, which then results in psychiatric disorders.
The MGB axis modulates neurological processes via the vagus nerve, the major “highway” connecting the gut and brain for bidirectional traffic. The MGB axis produces microbial metabolites and immune factors that can lead to changes in brain neurotransmitters as well as neuroinflammation and psychiatric symptoms such as depression and anxiety.5
Many researchers are focusing on how to exploit the microbiome to develop novel therapeutic strategies, and encouraging advances are emerging.5 But the exact mechanisms by which the gut microbiome can impact mental health is still a work in progress. It is highly likely that dysbiosis is associated with mood and anxiety symptoms.
The bottom line: Sexual activity—whether it is heavy kissing, vaginal intercourse, oral sex, anal sex, or extensive skin contact—can lead to the exchange of microbiota. If an individual has dysbiosis, that could impact the mental health of their sexual partner(s). This raises the question of whether counseling patients about avoiding indiscriminate sex and practicing safe sex is as important for mental health as diet and exercise counseling is for physical health.
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
1. Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9(1):27-38.
2. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
3. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223-1241.
4. Yolken R, Prandovszky E, Severance EG, et al. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51-57.
5. Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328-1346.
6. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vagina of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi:10.1186/s40168-016-0161-6
7. Hou D, Zhou X, Zhong X, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100(5):1261-1269.
8. Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652.
9. Liu CM, Hungate BA, Tobian AA, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio. 2015;6(3):e00589. doi:10.1128/mBio.00589-15
10. Carda-Diéguez M, Cárdenas N, Aparicio M, et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front Med. 2019;6:178. doi:10.3389/fmed.2019.00178
Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
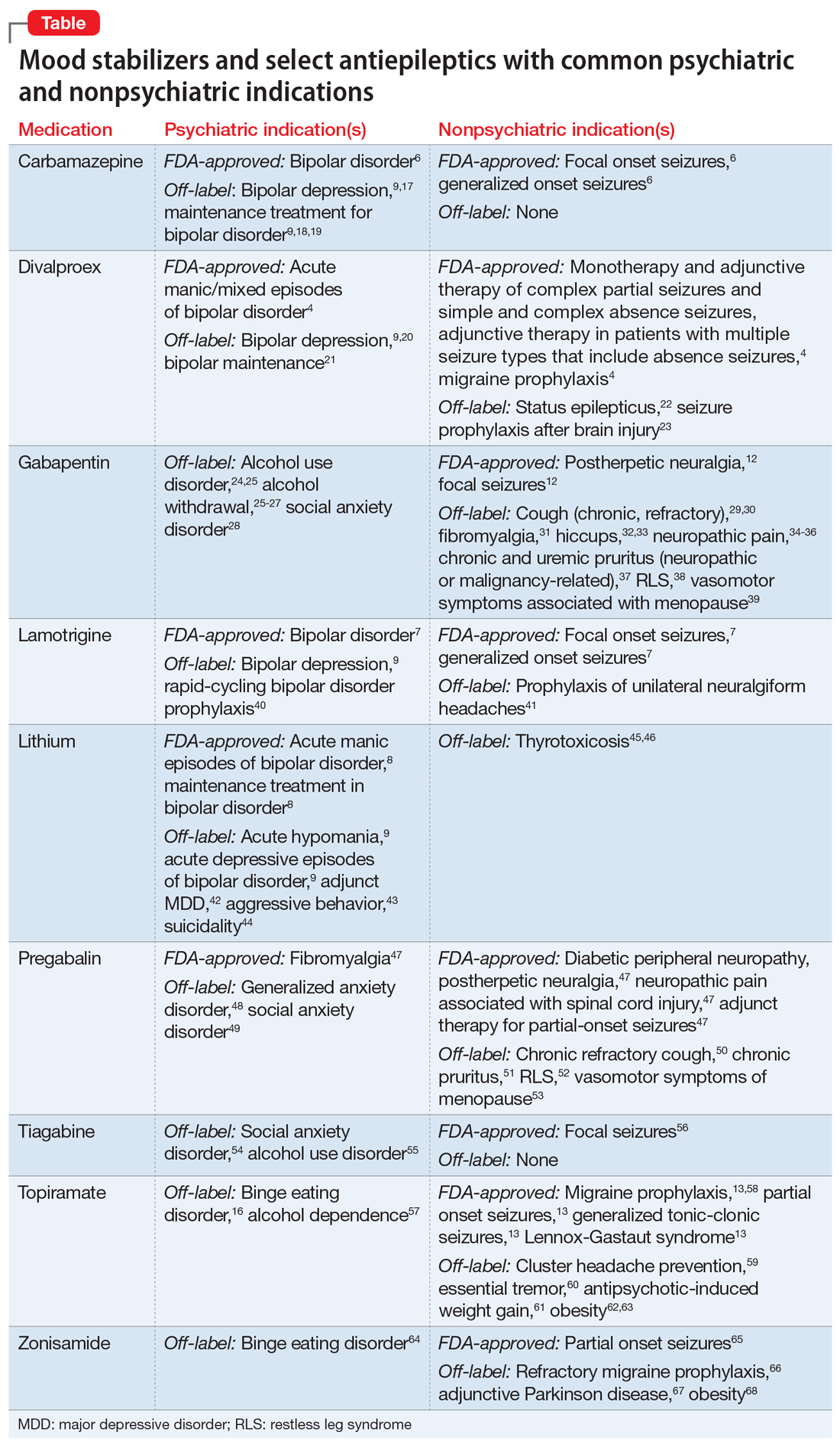
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.

CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.

CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Treatment augmentation strategies for OCD: A review of 8 studies
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.
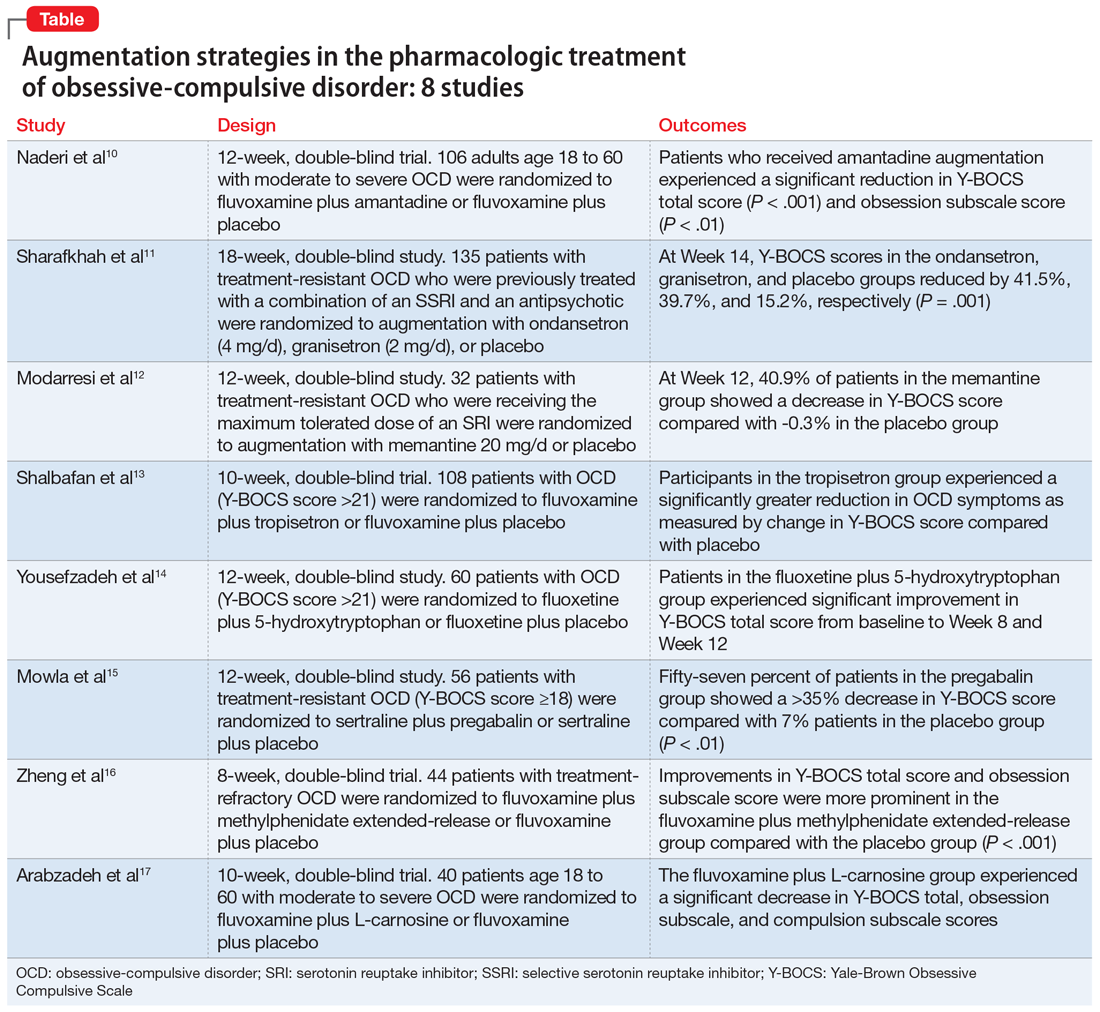
Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
Obsessive-compulsive disorder (OCD) is a chronic, debilitating neuropsychiatric disorder that affects 1% to 3% of the population worldwide.1,2 Together, serotonin reuptake inhibitors (SRIs) and cognitive-behavior therapy (CBT) are considered the first-line treatment for OCD.3 In children and adults, CBT is considered at least as effective as pharmacotherapy.4 Despite being an effective treatment, CBT continues to have barriers to its widespread use, including limited availability of trained CBT therapists, delayed clinical response, and high costs.5
Only approximately one-half of patients with OCD respond to SRI therapy, and a considerable percentage (30% to 40%) show significant residual symptoms even after multiple trials of SRIs.6-8 In addition, SRIs may have adverse effects (eg, sexual dysfunction, gastrointestinal symptoms) that impair patient adherence to these medications.9 Therefore, finding better treatment options is important for managing patients with OCD.
Augmentation strategies are recommended for patients who show partial response to SRI treatment or poor response to multiple SRIs. Augmentation typically includes incorporating additional medications with the primary drug with the goal of boosting the therapeutic efficacy of the primary drug. Typically, these additional medications have different mechanisms of action. However, there are no large-scale randomized controlled trials (RCTs) to inform treatment augmentation after first-line treatments for OCD produce suboptimal outcomes. The available evidence is predominantly based on small-scale RCTs, open-label trials, and case series.
In this article, we review the evidence for treatment augmentation strategies for OCD and summarize 8 studies that show promising results (Table10-17). We focus only on pharmacologic agents and do not include other biological interventions, such as repetitive transcranial magnetic stimulation over supplementary motor area, ablative neurosurgery, or deep brain stimulation.

Continue to: Reference 1...
1. Naderi S, Faghih H , Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessivecompulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/ pcn.12803
Numerous studies support the role of glutamate dysregulation in the pathophysiology of OCD. Cortico-striato-thalamo-cortical (CSTC) abnormalities play a major role in the pathophysiology of OCD as suggested by neuroimaging research studies that indicate glutamate is the fundamental neurotransmitter of the CSTC circuit. Dysregulation of glutamatergic signaling within this circuit has been linked to OCD. Patients with OCD have been found to have an increase of glutamate in the CSF. As a result, medications that affect glutamate levels can be used to treat patients with OCD who do not respond to first-line agents. In patients already taking SRIs, augmentation of glutamate-modulating medications can reduce OCD symptoms. As an uncompetitive antagonist of the N-methyl-
Naderi et al10 evaluated amantadine as augmentative therapy to fluvoxamine for treating patients with moderate to severe OCD.
Study design
- This 12-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of amantadine as an augmentative agent to fluvoxamine in 106 patients age 18 to 60 with moderate to severe OCD.
- Participants met DSM-5 criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score >21. Participants were excluded if they had any substance dependence; an IQ <70; any other Axis I mental disorder; any serious cardiac, renal, or hepatic disease; had received psychotropic medications during the last 6 weeks, were pregnant or breastfeeding, or had rising liver transaminases to 3 times the upper limit of normal or higher.
- Participants received fluvoxamine 100 mg twice daily plus amantadine 100 mg/d, or fluvoxamine 100 mg twice daily plus placebo. All patients received fluvoxamine 100 mg/d for 28 days followed by 200 mg/d for the remainder of the trial.
- The primary outcome measure was difference in Y-BOCS total scores between the amantadine and placebo groups. The secondary outcome was the difference in Y-BOCS obsession and compulsion subscale scores.
Outcomes
- Patients who received amantadine augmentation experienced a significant reduction in Y-BOCS total score (P < .001) and obsession subscale score (P < .01).
- The amantadine group showed good tolerability and safety. There were no clinically significant adverse effects.
- Amantadine is an effective adjuvant to fluvoxamine for reducing OCD symptoms.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
2. Sharafkhah M, Aghakarim Alamdar M, Massoudifar A, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222- 233. doi:10.1097/YIC.0000000000000267
Although selective serotonin reuptake inhibitors (SSRIs) are considered a first-line treatment when teamed with CBT and antipsychotic augmentation, symptom resolution is not always achieved, and treatment resistance is a common problem. Sharafkhah et al11 compared the efficacy of ondansetron and granisetron augmentation specifically for patients with treatment-resistant OCD.
Study Design
- In this 18-week, randomized, double-blind, placebo-controlled study, 135 patients with treatment-resistant OCD who were previously treated with a combination of an SSRI and an antipsychotic received augmentation with ondansetron (n = 45, 4 mg/d), granisetron (n = 45, 2 mg/d), or placebo.
- Patients were rated using Y-BOCS every 2 weeks during phase I (intervention period), which lasted 14 weeks. After completing the intervention, patients were followed for 4 more weeks during phase II (discontinuation period).
- The aim of this study was to determine the safety, efficacy, and tolerability of ondansetron vs granisetron as augmentation for patients with treatment-resistant OCD. A secondary aim was to determine the rate of relapse of OCD symptoms after discontinuing ondansetron as compared with granisetron at 4 weeks after intervention.
Outcomes
- At Week 14, the reductions in Y-BOCS scores in the ondansetron, granisetron, and placebo groups were 41.5%, 39.7%, and 15.2%, respectively (P = .001). The reduction in Y-BOCS score in the ondansetron and granisetron groups was significantly greater than placebo at all phase I visits.
- Complete response was higher in the ondansetron group compared with the granisetron group (P = .041).
- Y-BOCS scores increased in both the ondansetron and granisetron groups during the discontinuation phase, but OCD symptoms were not significantly exacerbated.
Conclusion
- Ondansetron and granisetron can be beneficial as an augmentation strategy for patients with treatment-resistant OCD.
3. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitorrefractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-120268
Increased glutamate levels in CSF, glutamatergic overactivity, and polymorphisms of genes coding the NMDA receptor have been shown to contribute to the occurrence of OCD. Memantine is a noncompetitive antagonist of the NMDA receptor. Various control trials have shown augmentation with memantine 5 mg/d to 20 mg/d significantly reduced symptom severity in patients with moderate to severe OCD. Modarresi et al12 evaluated memantine as a treatment option for patients with severe OCD who did not respond to SRI monotherapy.
Study design
- This 12-week, double-blind, randomized, placebo-controlled trial evaluated the efficacy of memantine augmentation in 32 patients age 18 to 40 who met DSM-5 criteria for OCD, had a Y-BOCS score ≥24, and no psychiatric comorbidity. Participants had not responded to ≥3 adequate trials (minimum 3 months) of SRI therapy, 1 of which was clomipramine.
- Individuals were excluded if they were undergoing CBT; had an additional anxiety disorder, mood disorder, or current drug or alcohol use disorder, or any systemic disorder; had a history of seizures; were pregnant or breastfeeding; or had a history of memantine use.
- Participants already receiving the maximum tolerated dose of an SRI received augmentation with memantine 20 mg/d or placebo.
- The primary outcome measure was change in Y-BOCS score from baseline. The secondary outcome was the number of individuals who achieved treatment response (defined as ≥35% reduction in Y-BOCS score).
Continue to: Outcomes...
Outcomes
- There was a statistically significant difference in Y-BOCS score in patients treated with memantine at Week 8 and Week 12 vs those who received placebo. By Week 8, 17.2% of patients in the memantine group showed a decrease in Y-BOCS score, compared with -0.8% patients in the placebo group. The difference became more significant by Week 12, with 40.9% in the memantine group showing a decrease in Y-BOCS score vs -0.3% in the placebo group. This resulted in 73.3% of patients achieving treatment response.
- Eight weeks of memantine augmentation was necessary to observe a significant improvement in OCD symptoms, and 12 weeks was needed for treatment response.
- The mean Y-BOCS total score decreased significantly in the memantine group from Week 4 to Week 8 (16.8%) and again from Week 8 to Week 12 (28.5%).
- The memantine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- Memantine augmentation in patients with severe OCD who do not respond to an SRI is effective and well-tolerated.
4. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407- 1414. doi:10.1177/0269881119878177
Studies have demonstrated the involvement of the amygdala, medial and lateral orbitofrontal cortex, and dorsal anterior cingulate cortex in OCD. Additionally, studies have also investigated the role of serotonin, dopamine, and glutamate system dysregulation in the pathology of OCD.
The 5-HT3 receptors are ligand-gated ion channels found in the prefrontal cortex, amygdala, and hippocampus. Studies of 5-HT3 receptor antagonists such as ondansetron and granisetron have shown beneficial results in augmentation with SSRIs for patients with OCD.11 Tropisetron, a 5-HT3 receptor antagonist, is highly lipophilic and able to cross the blood brain barrier. It also has dopamine-inhibiting properties that could have benefits in OCD management. Shalbafan et al13 evaluated the efficacy of tropisetron augmentation to fluvoxamine for patients with OCD.
Study design
- In a 10-week, randomized, double-blind, placebo-controlled, parallel-group trial, 108 individuals age 18 to 60 who met DSM-5 criteria for OCD and had a Y-BOCS score >21 received fluvoxamine plus tropisetron or fluvoxamine plus placebo. A total of 48 (44.4%) participants in each group completed the trial. Participants were evaluated using the Y-BOCS scale at baseline and at Week 4 and Week 10.
- The primary outcome was decrease in total Y-BOCS score from baseline to Week 10. The secondary outcome was the difference in change in Y-BOCS obsession and compulsion subscale scores between the groups.
Outcomes
- The Y-BOCS total score was not significantly different between the 2 groups (P = .975). Repeated measures analysis of variance determined a significant effect for time in both tropisetron and placebo groups (Greenhouse-Geisser F [2.72–2303.84] = 152.25, P < .001; and Greenhouse-Geisser F [1.37–1736.81] = 75.57, P < .001, respectively). At Week 10, 35 participants in the tropisetron group and 19 participants in the placebo group were complete responders.
- The baseline Y-BOCS obsession and compulsion subscales did not significantly differ between treatment groups.
Conclusion
- Compared with participants in the placebo group, those in the tropisetron group experienced a significantly greater reduction in OCD symptoms as measured by Y-BOCS score. More participants in the tropisetron group experienced complete response and remission.
- This study demonstrated that compared with placebo, when administered as augmentation with fluvoxamine, tropisetron can have beneficial effects for patients with OCD.
Continue to: Reference 5...
5. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a doubleblind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254- 262. doi:10.1097/YIC.0000000000000321
Nutraceuticals such as glycine, milk thistle, myoinositol, and serotonin (5-hydroxytryptophan) have been proposed as augmentation options for OCD. Yousefzadeh et al14 investigated the effectiveness of using 5-hydroxytryptophan in treating OCD.
Study design
- In a 12-week, randomized, double-blind study, 60 patients who met DSM-5 criteria for moderate to severe OCD (Y-BOCS score >21) were randomly assigned to receive fluoxetine plus 5-hydroxytryptophan 100 mg twice daily or fluoxetine plus placebo.
- All patients were administered fluoxetine 20 mg/d for the first 4 weeks of the study followed by fluoxetine 60 mg/d for the remainder of the trial.
- Symptoms were assessed using the Y-BOCS at baseline, Week 4, Week 8, and Week 12.
- The primary outcome measure was the difference between the 2 groups in change in Y-BOCS total score from baseline to the end of the trial. Secondary outcome measures were the differences in the Y-BOCS obsession and compulsion subscale scores from baseline to Week 12.
Outcomes
- Compared to the placebo group, the 5-hydroxytryptophan group experienced a statistically significant greater improvement in Y-BOCS total score from baseline to Week 8 (P = .002) and Week 12 (P < .001).
- General linear model repeated measure showed significant effects for time × treatment interaction on Y-BOCS total (F = 12.07, df = 2.29, P < .001), obsession subscale (F = 8.25, df = 1.91, P = .001), and compulsion subscale scores (F = 6.64, df = 2.01, P = .002).
- The 5-hydroxytryptophan group demonstrated higher partial and complete treatment response rates (P = .032 and P = .001, respectively) as determined by change in Y-BOCS total score.
- The 5-hydroxytryptophan group showed a significant improvement from baseline to Week 12 in Y-BOCS obsession subscale score (5.23 ± 2.33 vs 3.53 ± 2.13, P = .009).
- There was a significant change from baseline to the end of the trial in the Y-BOCS compulsion subscale score (3.88 ± 2.04 vs 2.30 ± 1.37, P = .002).
Conclusion
- This trial demonstrated the potential benefits of 5-hydroxytryptophan in combination with fluoxetine for patients with OCD.
6. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
Glutamatergic dysfunction has been identified as a potential cause of OCD. Studies have found elevated levels of glutamatergic transmission in the cortical-striatal-thalamic circuit of the brain and elevated glutamate concentration in the CSF in patients with OCD. Pregabalin has multiple mechanisms of action that inhibit the release of glutamate. Mowla et al15 evaluated pregabalin as an augmentation treatment for resistant OCD.
Study design
- This 12-week, double-blind, placebo-controlled clinical trial evaluated the efficacy of adjunctive pregabalin in 56 patients who met DSM-5 criteria for OCD and had not responded to ≥12 weeks of treatment with an adequate and stable dose of sertraline (baseline Y-BOCS score ≥18).
- Individuals who had other major psychiatric disorders, major medical problems, were pregnant, or had past substance or alcohol abuse were excluded.
- Participants were randomly assigned to receive sertraline plus pregabalin (n = 28) or sertraline plus placebo (n = 28). Mean sertraline dosage was 256.5 mg/d; range was 100 mg/d to 300 mg/d. Pregabalin was started at 75 mg/d and increased by 75 mg increments weekly. The mean dosage was 185.9 mg/d; range was 75 mg/d to 225 mg/d.
- The primary outcome measure was change in Y-BOCS score. A decrease >35% in Y-BOCS score was considered a significant response rate.
Outcomes
- There was a statistically significant decrease in Y-BOCS score in patients who received pregabalin. In the pregabalin group, 57.14% of patients (n = 16) showed a >35% decrease in Y-BOCS score compared with 7.14% of patients (n = 2) in the placebo group (P < .01).
- The pregabalin group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- In patients with treatment-resistant OCD who did not respond to sertraline monotherapy, augmentation with pregabalin significantly decreases Y-BOCS scores compared with placebo.
Continue to: Reference 7...
7. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebocontrolled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro. 2018.12.010
Recent evidence suggests dysregulation of serotonin and dopamine in patients with OCD. Methylphenidate is a dopamine and norepinephrine inhibitor and releaser. A limited number of studies have suggested stimulants might be useful for OCD patients. Zheng et al16 conducted a pilot trial to determine whether methylphenidate augmentation may be of benefit in the management of outpatients with OCD.
Study design
- In an 8-week, double-blind, randomized, placebo-controlled trial, 44 patients (29 [66%] men, with a mean [SD] age of 24.7 [6]) with treatment-refractory OCD were randomized to receive fluvoxamine 250 mg/d plus methylphenidate extended-release (MPH-ER) 36 mg/d or fluvoxamine 250 mg/d plus placebo. The MPH-ER dose was 18 mg/d for the first 4 weeks and 36 mg/d for the rest of the trial.
- Biweekly assessments consisted of scores on the Y-BOCS, Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HAM-A).
- The primary outcomes were improvement in Y-BOCS score and the clinical response rate. Secondary outcomes included a change in score on the Y-BOCS subscales, HARS, and HAM-A. Data were analyzed with the intention-to-treat sample.
Outcomes
- Forty-one patients finished the trial. The baseline Y-BOCS total scores and subscale scores did not differ significantly between the 2 groups.
- Improvements in Y-BOCS total score and obsession subscale score were more prominent in the fluvoxamine plus MPH-ER group compared with the placebo group (P < .001).
- HDRS score decreased in both the placebo and MPH-ER groups. HAM-A scores decreased significantly in the MPH-ER plus fluvoxamine group compared with the placebo group.
Conclusion
- This study demonstrated that the combination of fluvoxamine and MPH-ER produces a higher and faster response rate than fluvoxamine plus placebo in patients with OCD.
8. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Glutamate dysregulation is implicated in the pathogenesis of OCD. Glutamate-modulating agents have been used to treat OCD. Studies have shown L-carnosine has a neuroprotective role via its modulatory effect on glutamate. Arabzadeh et al17 evaluated the efficacy of L-carnosine as an adjuvant to fluvoxamine for treating OCD.
Study design
- This 10-week, randomized, double-blind, placebo-controlled trial evaluated the efficacy of adjunctive L-carnosine in 40 patients age 18 to 60 who met DSM-5 criteria for OCD and had moderate to severe OCD (Y-BOCS score ≥21).
- Individuals with any other DSM-5 major psychiatric disorders, serious medical or neurologic illness, substance dependence (other than caffeine or nicotine), mental retardation (based on clinical judgment), were pregnant or breastfeeding, had any contraindication for the use of L‐carnosine or fluvoxamine, or received any psychotropic drugs in the previous 6 weeks were excluded.
- Participants received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the next 6 weeks plus either L-carnosine 500 mg twice daily or placebo. This dosage of L-carnosine was chosen because previously it had been tolerated and effective.
- The primary outcome measure was difference in Y-BOCS total scores. Secondary outcomes were differences in Y-BOCS obsession and compulsion subscale scores and differences in change in score on Y-BOCS total and subscale scores from baseline.
Outcomes
- The L-carnosine group experienced a significant decrease in Y-BOCS total score (P < .001), obsession subscale score (P < .01), and compulsion subscale score (P < .01).
- The group that received fluvoxamine plus L-carnosine also experienced a more complete response (P = .03).
- The L-carnosine group showed good tolerability and safety. There were no clinically significant adverse effects.
Conclusion
- L-carnosine significantly reduces OCD symptoms when used as an adjuvant to fluvoxamine.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
3. Eddy KT, Dutra L, Bradley, R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
4. Franklin ME, Foa EB. Treatment of obsessive compulsive disorder. Annu Rev Clin Psychol. 2011;7:229-243.
5. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5-53.
6. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
7. Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181-191.
8. Atmaca M. Treatment-refractory obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:127-133.
9. Barth M, Kriston L, Klostermann S, et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry. 2016;208(2):114-119.
10. NaderiS, Faghih H, Aqamolaei A, et al. Amantadine as adjuvant therapy in the treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Psychiatry Clin Neurosci. 2019;73(4):169-174. doi:10.1111/pcn.12803
11. SharafkhahM, Aghakarim Alamdar M, MassoudifarA, et al. Comparing the efficacy of ondansetron and granisetron augmentation in treatment-resistant obsessive-compulsive disorder: a randomized double-blind placebo-controlled study. Int Clin Psychopharmacol. 2019;34(5):222-233. doi:10.1097/YIC.0000000000000267
12. ModarresiA, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269. doi:10.1055/s-0043-12026
13. Shalbafan M, Malekpour F, Tadayon Najafabadi B, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: a placebo-controlled, randomized clinical trial. J Psychopharmacol. 2019;33(11):1407-1414. doi:10.1177/0269881119878177
14. Yousefzadeh F, Sahebolzamani E, Sadri A, et al. 5-Hydroxytryptophan as adjuvant therapy in treatment of moderate to severe obsessive-compulsive disorder: a double-blind randomized trial with placebo control. Int Clin Psychopharmacol. 2020;35(5):254-262. doi:10.1097/YIC.0000000000000321
15. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556. doi:10.1017/S1092852919001500
16. Zheng H, Jia F, Han H, et al.Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404. doi:10.1016/j.euroneuro.2018.12.010
17. Arabzadeh S, Shahhossenie M, Mesgarpour B, et al. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: a randomized double-blind study. Hum Psychopharmacol. 2017;32(4). doi:10.1002/hup.2584
Celebrating our colleagues
several of whom I am privileged to work with on a daily basis. We also welcome the newest members of AGA’s Governing Board, Maria T. Abreu, MD, AGAF, who is an outstanding leader and representative of a much larger group of volunteer members who work tirelessly to advance AGA’s initiatives to enhance the clinical practice of gastroenterology and improve patient outcomes. The nominating committee also appointed the following slate of councilors, which is subject to membership vote: Kim Barrett, PhD, AGAF; Lawrence Kosinski, MD, MBA, AGAF; and Sheryl Pfeil, MD, AGAF.
This month’s issue also highlights two newly-developed clinical risk-prediction tools – one designed to assist clinicians in predicting alcoholic hepatitis mortality, and another designed to identify inflammatory bowel disease (IBD) patients at high-risk of developing venous thromboembolism (VTE) post-hospitalization. While no prediction model is perfect, these tools can positively impact clinical decision-making and contribute to improved patient outcomes. We also include recommendations on managing IBD in older patients, and report on a study suggesting an increase in late-stage cancer diagnoses in the wake of the COVID-19 pandemic. AGA’s new clinical guideline on systemic therapy for hepatocellular carcinoma and Clinical Practice Update on non-invasive colorectal cancer screening also are featured. Finally, in this month’s Practice Management Toolbox, Dr. Feuerstein, Dr. Sofia, Dr. Guha, and Dr. Streett offer timely recommendations regarding how to overcome existing barriers to achieve high-value IBD care.
Megan A. Adams, MD, JD, MSc
Editor in Chief
several of whom I am privileged to work with on a daily basis. We also welcome the newest members of AGA’s Governing Board, Maria T. Abreu, MD, AGAF, who is an outstanding leader and representative of a much larger group of volunteer members who work tirelessly to advance AGA’s initiatives to enhance the clinical practice of gastroenterology and improve patient outcomes. The nominating committee also appointed the following slate of councilors, which is subject to membership vote: Kim Barrett, PhD, AGAF; Lawrence Kosinski, MD, MBA, AGAF; and Sheryl Pfeil, MD, AGAF.
This month’s issue also highlights two newly-developed clinical risk-prediction tools – one designed to assist clinicians in predicting alcoholic hepatitis mortality, and another designed to identify inflammatory bowel disease (IBD) patients at high-risk of developing venous thromboembolism (VTE) post-hospitalization. While no prediction model is perfect, these tools can positively impact clinical decision-making and contribute to improved patient outcomes. We also include recommendations on managing IBD in older patients, and report on a study suggesting an increase in late-stage cancer diagnoses in the wake of the COVID-19 pandemic. AGA’s new clinical guideline on systemic therapy for hepatocellular carcinoma and Clinical Practice Update on non-invasive colorectal cancer screening also are featured. Finally, in this month’s Practice Management Toolbox, Dr. Feuerstein, Dr. Sofia, Dr. Guha, and Dr. Streett offer timely recommendations regarding how to overcome existing barriers to achieve high-value IBD care.
Megan A. Adams, MD, JD, MSc
Editor in Chief
several of whom I am privileged to work with on a daily basis. We also welcome the newest members of AGA’s Governing Board, Maria T. Abreu, MD, AGAF, who is an outstanding leader and representative of a much larger group of volunteer members who work tirelessly to advance AGA’s initiatives to enhance the clinical practice of gastroenterology and improve patient outcomes. The nominating committee also appointed the following slate of councilors, which is subject to membership vote: Kim Barrett, PhD, AGAF; Lawrence Kosinski, MD, MBA, AGAF; and Sheryl Pfeil, MD, AGAF.
This month’s issue also highlights two newly-developed clinical risk-prediction tools – one designed to assist clinicians in predicting alcoholic hepatitis mortality, and another designed to identify inflammatory bowel disease (IBD) patients at high-risk of developing venous thromboembolism (VTE) post-hospitalization. While no prediction model is perfect, these tools can positively impact clinical decision-making and contribute to improved patient outcomes. We also include recommendations on managing IBD in older patients, and report on a study suggesting an increase in late-stage cancer diagnoses in the wake of the COVID-19 pandemic. AGA’s new clinical guideline on systemic therapy for hepatocellular carcinoma and Clinical Practice Update on non-invasive colorectal cancer screening also are featured. Finally, in this month’s Practice Management Toolbox, Dr. Feuerstein, Dr. Sofia, Dr. Guha, and Dr. Streett offer timely recommendations regarding how to overcome existing barriers to achieve high-value IBD care.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Question 2
Correct answer: B. Absence of ganglion cells on rectal biopsy.
Rationale
Hirschsprung's disease occurs in approximately 1 out of 5,000 live births and is caused by absence of ganglion cells in the myenteric plexus of the intestine. The condition arises from failure of the neural crest cells to fully migrate caudally along the intestine during early gestation, resulting in a distal portion of the intestine being aganglionic. Rectal and distal sigmoid involvement is seen in around 85% of cases, with the other 15 percent involving more proximal intestine. It can rarely involve the entire colon and small intestine. Ganglion cells inhibit local smooth muscles, resulting in the characteristic inability for aganglionic bowel to relax. This lack of inhibition gives rise to the absence of rectoanal inhibitory reflex (RAIRs) during anorectal manometry. The lack of inhibition also produces a transition zone on contrast enema, with the distal aganglionic bowel being narrow and the more proximal bowel containing ganglia being dilated. Lack of meconium passage in the first 48 hours of life raises concern for Hirschsprung's disease. Other causes for possible failure to pass meconium include cystic fibrosis, anorectal malformation, small left colon syndrome, meconium plug syndrome and megacystis-microcolon-intestinal hypoperistalsis syndrome.
References
Kenny, S et al. Semin Pediatr Surg. 2010 Aug;19(3):194-200.
Wyllie R et al. Pediatric Gastrointestinal and Liver Disease. 4th edition. Elsevier Saunders, Philadelphia, 2011.
Correct answer: B. Absence of ganglion cells on rectal biopsy.
Rationale
Hirschsprung's disease occurs in approximately 1 out of 5,000 live births and is caused by absence of ganglion cells in the myenteric plexus of the intestine. The condition arises from failure of the neural crest cells to fully migrate caudally along the intestine during early gestation, resulting in a distal portion of the intestine being aganglionic. Rectal and distal sigmoid involvement is seen in around 85% of cases, with the other 15 percent involving more proximal intestine. It can rarely involve the entire colon and small intestine. Ganglion cells inhibit local smooth muscles, resulting in the characteristic inability for aganglionic bowel to relax. This lack of inhibition gives rise to the absence of rectoanal inhibitory reflex (RAIRs) during anorectal manometry. The lack of inhibition also produces a transition zone on contrast enema, with the distal aganglionic bowel being narrow and the more proximal bowel containing ganglia being dilated. Lack of meconium passage in the first 48 hours of life raises concern for Hirschsprung's disease. Other causes for possible failure to pass meconium include cystic fibrosis, anorectal malformation, small left colon syndrome, meconium plug syndrome and megacystis-microcolon-intestinal hypoperistalsis syndrome.
References
Kenny, S et al. Semin Pediatr Surg. 2010 Aug;19(3):194-200.
Wyllie R et al. Pediatric Gastrointestinal and Liver Disease. 4th edition. Elsevier Saunders, Philadelphia, 2011.
Correct answer: B. Absence of ganglion cells on rectal biopsy.
Rationale
Hirschsprung's disease occurs in approximately 1 out of 5,000 live births and is caused by absence of ganglion cells in the myenteric plexus of the intestine. The condition arises from failure of the neural crest cells to fully migrate caudally along the intestine during early gestation, resulting in a distal portion of the intestine being aganglionic. Rectal and distal sigmoid involvement is seen in around 85% of cases, with the other 15 percent involving more proximal intestine. It can rarely involve the entire colon and small intestine. Ganglion cells inhibit local smooth muscles, resulting in the characteristic inability for aganglionic bowel to relax. This lack of inhibition gives rise to the absence of rectoanal inhibitory reflex (RAIRs) during anorectal manometry. The lack of inhibition also produces a transition zone on contrast enema, with the distal aganglionic bowel being narrow and the more proximal bowel containing ganglia being dilated. Lack of meconium passage in the first 48 hours of life raises concern for Hirschsprung's disease. Other causes for possible failure to pass meconium include cystic fibrosis, anorectal malformation, small left colon syndrome, meconium plug syndrome and megacystis-microcolon-intestinal hypoperistalsis syndrome.
References
Kenny, S et al. Semin Pediatr Surg. 2010 Aug;19(3):194-200.
Wyllie R et al. Pediatric Gastrointestinal and Liver Disease. 4th edition. Elsevier Saunders, Philadelphia, 2011.
A 2-month-old male presents with abdominal distention and poor appetite. His family notes that the patient has chronic difficulties with constipation, reporting that they have to use a glycerin suppository to help him have a bowel movement every 2-3 days. The family reports that he even needed a suppository in the newborn nursey at day of life 3 due to lack of passage of meconium.
Question 1
Correct answer: A. Amyloidosis involving the small intestine
Rationale
This patient has a protein-losing enteropathy as indicated by his diarrhea, peripheral edema, and positive stool alpha-1 antitrypsin test. Multiple diseases, particularly in their later stages, can be associated with a protein-losing enteropathy including primary intestinal lymphangectasia, Crohn's disease of the small intestine, small intestinal bacterial overgrowth (SIBO), and amyloidosis of the small intestine (A). Celiac disease (B) is not associated with protein-losing enteropathy. While Crohn's disease can be associated with protein-losing enteropathy, ulcerative colitis (C) is not usually associated with it. Small bowel dysmotility (D) does not impact absorption or secretion unless associated with SIBO, making this a wrong answer.
Correct answer: A. Amyloidosis involving the small intestine
Rationale
This patient has a protein-losing enteropathy as indicated by his diarrhea, peripheral edema, and positive stool alpha-1 antitrypsin test. Multiple diseases, particularly in their later stages, can be associated with a protein-losing enteropathy including primary intestinal lymphangectasia, Crohn's disease of the small intestine, small intestinal bacterial overgrowth (SIBO), and amyloidosis of the small intestine (A). Celiac disease (B) is not associated with protein-losing enteropathy. While Crohn's disease can be associated with protein-losing enteropathy, ulcerative colitis (C) is not usually associated with it. Small bowel dysmotility (D) does not impact absorption or secretion unless associated with SIBO, making this a wrong answer.
Correct answer: A. Amyloidosis involving the small intestine
Rationale
This patient has a protein-losing enteropathy as indicated by his diarrhea, peripheral edema, and positive stool alpha-1 antitrypsin test. Multiple diseases, particularly in their later stages, can be associated with a protein-losing enteropathy including primary intestinal lymphangectasia, Crohn's disease of the small intestine, small intestinal bacterial overgrowth (SIBO), and amyloidosis of the small intestine (A). Celiac disease (B) is not associated with protein-losing enteropathy. While Crohn's disease can be associated with protein-losing enteropathy, ulcerative colitis (C) is not usually associated with it. Small bowel dysmotility (D) does not impact absorption or secretion unless associated with SIBO, making this a wrong answer.
A 65-year-old male with no significant past medical history presents with significant diarrhea. He reports that for the past 3 months, he has had four to five bowel movements a day. He characterizes them as greasy and foul smelling, but not entirely watery. He notices no blood or mucous in the stool. Over the same time period, he has also noticed increased swelling in both of his ankles. The physician sends a broad work-up.
Stool testing results include the following:
Clostridioides difficile - Negative.
Stool Ova and Parasite - Negative.
Stool Culture - Negative.
Stool Elastase - within normal limits.
Fecal Fat (spot test) - within normal limits.
Stool Alpha-1 Antitrypsin - Elevated.
A little-known offshoot of hem/onc opens pathway for professional development
Only a small number of pediatric hematologist oncologists and even fewer of our adult counterparts feel comfortable evaluating and treating vascular anomalies.
While admittedly rare, these conditions are still common enough that clinicians in many disciplines encounter them. Hematologist/oncologists are most likely to see vascular malformations, which often present as mass lesions. Complications of these disorders occur across the hematology-oncology spectrum and include clots, pulmonary emboli, cancer predisposition, and an array of functional and psychosocial disorders.
Vascular anomalies are broadly categorized as vascular tumors or malformations. The tumors include hemangiomas, locally aggressive lesions, and true cancers. Malformations can be isolated disorders of one or more blood vessel types (veins, arteries, capillaries or lymphatics), or they can be one part of syndromic disorders. Lymphedema also falls under the heading of vascular anomalies. To make the terminology less confusing, in 2018 the International Society for the Study of Vascular Anomalies refined its classification scheme.
Vascular malformations are thought to be congenital. Although some are obvious at birth, others aren’t apparent until adulthood. In most cases, they grow with a child and may do so disproportionately at puberty and with pregnancies. The fact that vascular malformations persist into adulthood is one reason why their care should be integral to medical hematology-oncology.
Although the cause of a vascular malformation is not always known, a wide range of genetic mutations thought to be pathogenic have been reported. These mutations are usually somatic (only within the involved tissues, not in the blood or germ cells and therefore, not heritable) and tend to cluster in the VEGF-PIK3CA and RAS-MAP signaling pathways.
These genes and pathways will be familiar to any oncologist who cares for patients with solid tumors, notably breast cancer or melanoma. However, unlike the clonal expansion seen in cancers, most vascular malformations will express pathogenic mutations in less than 20% of vascular endothelium within a malformation.
Since 2008, medical management has been limited to sirolimus (rapamycin), a mammalian target of rapamycin inhibitor, which can be effective even when mTOR mutations aren’t apparent. In a seminal phase 2 trial of 57 patients with complex vascular anomalies who were aged 0-29 years, 47 patients had a partial response, 3 patients had stable disease, and 7 patients had progressive disease. None had complete responses. These data highlight the need for more effective treatments.
Recently, vascular anomalists have begun to repurpose drugs from adult oncology that specifically target pathogenic mutations. Some studies underway include Novartis’ international Alpelisib (Piqray) clinical trial for adults and children with PIK3CA-related overgrowth syndromes (NCT04589650) and Merck’s follow-up study of the AKT inhibitor miransertib for PROS and Proteus syndrome. Doses tend to be lower than those used to treat cancers. To date, these have been generally well-tolerated, with sometimes striking but preliminary evidence of efficacy.
During the past 2 years, symposia on vascular anomalies at the annual meeting of the American Society of Hematology have launched what we are hoping is just the start of a broader discussion. In 2020, Fran Blei, MD, chaired Vascular Anomalies 101: Case-Based Discussion on the Diagnosis, Treatment and Lifelong Care of These Patients, and in 2021, Adrienne Hammill, MD, PhD, and Dr. Raj Kasthuri, MBBS, MD, chaired a more specialized symposium: Hereditary Hemorrhagic Telangiectasia (HHT): A Practical Guide to Management.
Dr. Blatt is in the division of pediatric hematology oncology at the University of North Carolina at Chapel Hill. She disclosed no relevant financial relationships.
Only a small number of pediatric hematologist oncologists and even fewer of our adult counterparts feel comfortable evaluating and treating vascular anomalies.
While admittedly rare, these conditions are still common enough that clinicians in many disciplines encounter them. Hematologist/oncologists are most likely to see vascular malformations, which often present as mass lesions. Complications of these disorders occur across the hematology-oncology spectrum and include clots, pulmonary emboli, cancer predisposition, and an array of functional and psychosocial disorders.
Vascular anomalies are broadly categorized as vascular tumors or malformations. The tumors include hemangiomas, locally aggressive lesions, and true cancers. Malformations can be isolated disorders of one or more blood vessel types (veins, arteries, capillaries or lymphatics), or they can be one part of syndromic disorders. Lymphedema also falls under the heading of vascular anomalies. To make the terminology less confusing, in 2018 the International Society for the Study of Vascular Anomalies refined its classification scheme.
Vascular malformations are thought to be congenital. Although some are obvious at birth, others aren’t apparent until adulthood. In most cases, they grow with a child and may do so disproportionately at puberty and with pregnancies. The fact that vascular malformations persist into adulthood is one reason why their care should be integral to medical hematology-oncology.
Although the cause of a vascular malformation is not always known, a wide range of genetic mutations thought to be pathogenic have been reported. These mutations are usually somatic (only within the involved tissues, not in the blood or germ cells and therefore, not heritable) and tend to cluster in the VEGF-PIK3CA and RAS-MAP signaling pathways.
These genes and pathways will be familiar to any oncologist who cares for patients with solid tumors, notably breast cancer or melanoma. However, unlike the clonal expansion seen in cancers, most vascular malformations will express pathogenic mutations in less than 20% of vascular endothelium within a malformation.
Since 2008, medical management has been limited to sirolimus (rapamycin), a mammalian target of rapamycin inhibitor, which can be effective even when mTOR mutations aren’t apparent. In a seminal phase 2 trial of 57 patients with complex vascular anomalies who were aged 0-29 years, 47 patients had a partial response, 3 patients had stable disease, and 7 patients had progressive disease. None had complete responses. These data highlight the need for more effective treatments.
Recently, vascular anomalists have begun to repurpose drugs from adult oncology that specifically target pathogenic mutations. Some studies underway include Novartis’ international Alpelisib (Piqray) clinical trial for adults and children with PIK3CA-related overgrowth syndromes (NCT04589650) and Merck’s follow-up study of the AKT inhibitor miransertib for PROS and Proteus syndrome. Doses tend to be lower than those used to treat cancers. To date, these have been generally well-tolerated, with sometimes striking but preliminary evidence of efficacy.
During the past 2 years, symposia on vascular anomalies at the annual meeting of the American Society of Hematology have launched what we are hoping is just the start of a broader discussion. In 2020, Fran Blei, MD, chaired Vascular Anomalies 101: Case-Based Discussion on the Diagnosis, Treatment and Lifelong Care of These Patients, and in 2021, Adrienne Hammill, MD, PhD, and Dr. Raj Kasthuri, MBBS, MD, chaired a more specialized symposium: Hereditary Hemorrhagic Telangiectasia (HHT): A Practical Guide to Management.
Dr. Blatt is in the division of pediatric hematology oncology at the University of North Carolina at Chapel Hill. She disclosed no relevant financial relationships.
Only a small number of pediatric hematologist oncologists and even fewer of our adult counterparts feel comfortable evaluating and treating vascular anomalies.
While admittedly rare, these conditions are still common enough that clinicians in many disciplines encounter them. Hematologist/oncologists are most likely to see vascular malformations, which often present as mass lesions. Complications of these disorders occur across the hematology-oncology spectrum and include clots, pulmonary emboli, cancer predisposition, and an array of functional and psychosocial disorders.
Vascular anomalies are broadly categorized as vascular tumors or malformations. The tumors include hemangiomas, locally aggressive lesions, and true cancers. Malformations can be isolated disorders of one or more blood vessel types (veins, arteries, capillaries or lymphatics), or they can be one part of syndromic disorders. Lymphedema also falls under the heading of vascular anomalies. To make the terminology less confusing, in 2018 the International Society for the Study of Vascular Anomalies refined its classification scheme.
Vascular malformations are thought to be congenital. Although some are obvious at birth, others aren’t apparent until adulthood. In most cases, they grow with a child and may do so disproportionately at puberty and with pregnancies. The fact that vascular malformations persist into adulthood is one reason why their care should be integral to medical hematology-oncology.
Although the cause of a vascular malformation is not always known, a wide range of genetic mutations thought to be pathogenic have been reported. These mutations are usually somatic (only within the involved tissues, not in the blood or germ cells and therefore, not heritable) and tend to cluster in the VEGF-PIK3CA and RAS-MAP signaling pathways.
These genes and pathways will be familiar to any oncologist who cares for patients with solid tumors, notably breast cancer or melanoma. However, unlike the clonal expansion seen in cancers, most vascular malformations will express pathogenic mutations in less than 20% of vascular endothelium within a malformation.
Since 2008, medical management has been limited to sirolimus (rapamycin), a mammalian target of rapamycin inhibitor, which can be effective even when mTOR mutations aren’t apparent. In a seminal phase 2 trial of 57 patients with complex vascular anomalies who were aged 0-29 years, 47 patients had a partial response, 3 patients had stable disease, and 7 patients had progressive disease. None had complete responses. These data highlight the need for more effective treatments.
Recently, vascular anomalists have begun to repurpose drugs from adult oncology that specifically target pathogenic mutations. Some studies underway include Novartis’ international Alpelisib (Piqray) clinical trial for adults and children with PIK3CA-related overgrowth syndromes (NCT04589650) and Merck’s follow-up study of the AKT inhibitor miransertib for PROS and Proteus syndrome. Doses tend to be lower than those used to treat cancers. To date, these have been generally well-tolerated, with sometimes striking but preliminary evidence of efficacy.
During the past 2 years, symposia on vascular anomalies at the annual meeting of the American Society of Hematology have launched what we are hoping is just the start of a broader discussion. In 2020, Fran Blei, MD, chaired Vascular Anomalies 101: Case-Based Discussion on the Diagnosis, Treatment and Lifelong Care of These Patients, and in 2021, Adrienne Hammill, MD, PhD, and Dr. Raj Kasthuri, MBBS, MD, chaired a more specialized symposium: Hereditary Hemorrhagic Telangiectasia (HHT): A Practical Guide to Management.
Dr. Blatt is in the division of pediatric hematology oncology at the University of North Carolina at Chapel Hill. She disclosed no relevant financial relationships.
Fingers take the fight to COVID-19
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Science says this is the ‘most boring person in the world’
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
FROM PERSONALITY AND SOCIAL PSYCHOLOGY BULLETIN








