User login
Transgender men need counseling on contraceptive and reproductive choices
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
Researchers have highlighted the need for contraception counseling for transgender men, after a study found around half of transgender men had not been asked by their health care providers about their fertility desires.
Writing in Contraception, researchers reported the results of an anonymous, online survey of 197 female-to-male transgender men, 86% of whom were taking masculinizing hormones.
Overall, 17% of respondents had experienced a pregnancy, with 60 pregnancies reported in total. While participants who had never taken hormones had a nearly 200% higher incidence of pregnancy, compared with those who had taken testosterone, one pregnancy occurred while the subject was taking testosterone, and five of seven reported abortions were in participants who had used testosterone prior to conception.
A total of 30 participants (16.4%) believed testosterone was a contraceptive method, and 10 said that a health care provider had advised them to use testosterone for contraception. However, nearly half of all participants did report using condoms for contraception, making it the most common method. IUDs were the second most common method of contraception currently used.
Nearly one-third of participants said they had used some type of contraceptive pill at some point, 17 said they had tried more than one type of pill, and 36 had used combination pills. However, of those who had used combination pills, nearly half stopped using them because of side effects or because of concern about extra feminine hormones.
The authors noted that most study participants expressed a desire to become a parent. Around one-quarter wanted to bear a child while the majority said they would consider adoption.
One-quarter of respondents had fears about not achieving a desired pregnancy, and for some, those fears began after initiating hormone treatments. Just over half the respondents said their health care provider had not asked about their fertility desires.
“Transgender men have unintended pregnancy as well as future fertility desires, and yet, there is a paucity of reproductive health care best practices research for this unique population,” wrote Alexis Light, MD, MPH, of MedStar Washington Hospital Center, and her coauthors. “This survey confirms earlier studies: Transgender men do become pregnant, both intentionally and unintentionally, and some transgender men engage in behaviors that can lead to unintended pregnancy.”
The study authors called for doctors to be more equipped and prepared to counsel transgender men on contraception and discuss other reproductive health concerns.
The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
SOURCE: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
FROM CONTRACEPTION
Key clinical point: Transgender men need advice on reproductive and contraceptive choices.
Major finding: Half of transgender men were not asked about their fertility desires.
Study details: An online survey of 197 female-to-male transgender men.
Disclosures: The study was supported by MedStar Washington Hospital Center. No conflicts of interest were declared.
Source: Light A et al. Contraception. 2018 Jun 23. doi: 10.1016/j.contraception.2018.06.006.
Smoking tied to localized prostate cancer recurrence, metastasis, death
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
While previous studies have linked smoking to adverse outcomes in prostate cancer, including death, this study argues that the higher rate of prostate cancer–related death among smokers is a real effect with a biological cause, Stephen J. Freedland, MD, said in an editorial.
The current study included only men healthy enough to undergo aggressive treatment, which is an “important and necessary step to level the playing field,” Dr. Freedland wrote.
In that context, current smokers in this study had an 89% increased risk of prostate cancer–related death. “This finding shows that when we treat patients equally and minimize deaths from other causes, there is a stronger link between smoking and death from prostate cancer,” Dr. Freedland noted.
The finding also supports the notion that many smokers won’t live long enough to die from prostate cancer, given its slow-growing nature and the effects of smoking on competing causes of death, he added.
A scenario in which smokers did not live long enough to die of prostate cancer would predict a lower risk of prostate cancer–related death among smokers, he explained.
Because smoking has such clear adverse effects, physicians of all specialties should be hypervigilant about urging patients to quit smoking, Dr. Freedland concluded.
“If all of us remembered we are physicians first and oncologists and/or prostate cancer experts second, we will realize we are uniquely poised to help our patients, as the time of a new cancer diagnosis is a teachable moment when patients are very receptive to overall health advice,” he wrote.
Dr. Freedland is with the Center for Integrated Research on Cancer and Lifestyle, Cedars-Sinai Medical Center, Los Angeles. These comments are derived from his editorial in JAMA Oncology. Dr. Freeland had no reported conflict of interest disclosures.
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
Patients with localized prostate cancer who were smokers at the time of local therapy had a higher risk of experiencing adverse outcomes and death related to the disease, a systematic review and meta-analysis has shown.
Current smokers in the study had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality after undergoing primary radical prostatectomy or radiotherapy.
“Our findings encourage radiation oncologists and urologists to counsel patients to stop smoking, using primary prostate cancer treatment as a teachable moment,” wrote Dr. Foerster and coauthors. The report was published in JAMA Oncology.
The investigators performed a database search of studies published from January 2000 to March 2017 and selected 11 articles for quantitative analysis. Those studies, which were all observational and not randomized, comprised 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy. Of those patients, 4,202 (18.6%) were current smokers.
Current smokers had a higher risk of cancer-specific mortality, the investigators found based on analysis of five studies (hazard ratio, 1.89; 95% confidence interval, 1.37-2.69; P less than .001).
They also had a significantly higher risk of biochemical recurrence, based on 10 studies (HR, 1.40; 95% CI, 1.18-1.66; P less than .001), and high risk of metastasis based on 3 studies (HR, 2.51; 95% CI, 1.80-3.51; P less than .001), the report shows.
Future studies need to more closely examine the link between smoking cessation and longer-term oncologic outcomes, as well as a more precise assessment of smoking exposure, the researchers concluded.
In the current study, results were inconclusive as to whether former smoking and time to cessation had any relationship to outcomes after radical prostatectomy or radiotherapy.
“Available data were sparse and heterogenous,” they noted.
Dr. Foerster is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
SOURCE: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
FROM JAMA ONCOLOGY
Key clinical point: Patients with localized prostate cancer should be encouraged to quit smoking because of the risk of potentially worse oncologic outcomes.
Major finding: Current smokers had a higher risk of biochemical recurrence, metastasis, and cancer-specific mortality, with hazard ratios of 1.40, 2.51, and 1.89, respectively.
Study details: A systematic review and meta-analysis of 11 studies involving 22,549 patients with prostate cancer undergoing radical prostatectomy or radiotherapy.
Disclosures: The first author is supported by the Scholarship Foundation of Swiss Urology. One coauthor reported disclosures related to Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, and Wolff.
Source: Foerster B et al. JAMA Oncol. 2018 May 24. doi: 10.1001/jamaoncol.2018.1071.
Statin effect in prostate cancer may be caused by reduced inflammation
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
SAN FRANCISCO – according to the results of a new genetic analysis of prostate cancers in men in the Health Professionals Follow Up Study.
A previous analysis from the Health Professionals Follow Up Study published in 2015 showed no difference in the risk of lethal prostate cancer for those who began using statins after diagnosis of their tumors.
A genetic analysis of these lethal cases revealed that patients taking long-term statins had a lower incidence of phosphatase and tensin homolog (PTEN)–null cancers, which are associated with worse outcomes. Integrating molecular and epidemiologic data, PTEN and PI3K (phosphatidylinositol) signaling and inflammation and immune activation appear to be two potential mechanisms contributing to this association.
Genetic analysis of normal prostate tissue also showed unique traits among statin users. “We found that the top ten pathways that came up were almost all involved in inflammation or immune activation, and we found those differences only in tumor and adjacent normal prostate tissue. We didn’t see any pathways that were differentially expressed by statin use within the tumor tissue itself,” said Emma Allott, PhD, who presented the research at a poster session at the annual meeting of the American Urological Association.
An association between statin use and improved survival was first described in a 2006 study based on results from the Health Professionals Follow Up Study. Since then, “we’ve been generating molecular data on cancers that developed in statin users and nonusers,” said Dr. Allott of the University of North Carolina, Chapel Hill.
Of the 5,792 prostate cancer diagnoses, 17% were advanced cases, defined as stage T3b or more, having spread to lymph nodes or metastasized, or lethal; 13% were lethal, 46% were positive for the ERG oncogene, and 14% were PTEN-null. “Statin use was associated with a lower risk of PTEN-null prostate cancer, so that seems to drive some of the reduced association with lethal disease,” said Dr. Allott.
There was no association between lethality and ERG-positive or ERG-negative status. Those who used statins for more than 5 years were less likely to have a PTEN-null tumor (hazard ratio, 0.42; 95% confidence interval, 0.20-0.90) but not more likely to have a PTEN-positive tumor (HR, 1.18; 95% CI, 0.95-1.46).
Compared with never users, long-term statin users also were less likely to have advanced prostate cancer (multivariate analysis, HR, 0.62; 95% CI, 0.45-0.85) as well as lethal prostate cancer (HR, 0.52; 95% CI, 0.35-0.78).
The researchers conducted a gene set enrichment analysis in statin users and found an enrichment of T-cell, B-cell, and PI3K signaling in tumor-adjacent normal prostate tissue, as well as other changes. “We think maybe there’s a microenvironment inflammation component to the mechanism through which statins are associated with lower risk of lethal prostate cancer,” said Dr. Allott.
The molecular data could identify patient subgroups that could benefit from statins. Dr. Allott said that is the goal, but it will take time. “That’s more obviously translatable to the clinic, but we don’t yet have enough data in this cohort to look at that.”
SOURCE: Allott E et al. AUA 2018, Abstract MP21-01.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Researchers hope that genetic analyses could eventually point to prostate cancer patients who might benefit from statin use.
Major finding: Long-term statin users had lower odds of having a pTEN-null tumor (hazard ratio, 0.42), which is associated with worse outcomes.
Study details: Retrospective analysis of 5,792 diagnoses of prostate cancer among 44,076 men.
Disclosures: The study was funded by the Irish Cancer Society, the John Fitzpatrick Fellowship, and the National Cancer Institute. Dr. Allott reported no relevant financial relationships.
Source: Allott E et al. AUA 2018, Abstract MP21-01.
Prostate cancer risk before age 55 higher for black men
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
according to a new study that drew subjects aged 40-54 years from three public and two private hospitals in the Chicago area.
Black race, rather than socioeconomic or clinical factors, appeared to be the strongest nonmodifiable predictor of prostate cancer risk in that age group, the researchers concluded, based on multivariate analyses that examined the association between prostate cancer risk and clinical setting, race, genetically determined West African ancestry, and clinical and socioeconomic risk factors.
The results suggest that screening practices should be altered, said study investigator Oluwarotimi S. Nettey, MD, of Northwestern University, Chicago. “You might want to think about screening black men who are younger than 55.”
“In the prebiopsy space, most studies have looked at race, age, PSA [level], and prostate volume, and they’ve said that the reason we see that black men have disparate prostate cancer risk on diagnosis is probably because of access to care issues, so that’s been the confounder. We tried to control for this by looking at socioeconomic status through income, marriage, and education, as well as hospital setting,” said Dr. Nettey, who presented the study at a poster session at the annual meeting of the American Urological Association.
Previous studies have examined populations and then conducted a secondary analysis on outcomes in black men. The current study has greater power and is more convincing because outcomes in black men was the primary outcome of the study, according to Robert L. Waterhouse Jr., MD, who is the public policy liaison for the R. Frank Jones Urological Society of the National Medical Association. Dr. Waterhouse, a urologist in Charlotte, N.C., attended the poster session and was not involved in the research.
“This study helps to provide some evidence that black heritage is indeed a significant risk factor in men who develop prostate cancer at an earlier age, and efforts at identifying prostate cancer at an earlier age [should consider] black race as a high-risk group,” said Dr. Waterhouse.
For patients of all ages, biopsies were positive in 63.1% of black men, compared with 41.5% of nonblack men (P less than .001). Cancers were also more advanced in black men: 47.5% were Gleason 3+4 in black men, compared with 40% in nonblack men (P less than .001), and 14.4% were Gleason 4+4 in black men, compared with 9.6% in nonblack men (P = .02).
After researchers controlled for other risk factors, black race was associated with heightened risk of prostate cancer diagnosis (OR, 5.66; P = .02), as was family history (OR, 4.98; P = .01).
There was no association between West African ancestry and prostate cancer risk either as a continuous variable or in quartiles.
Limitations of the study include the fact that race was self-reported and that this was a referred population.
The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
SOURCE: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Black race appears to be a key risk factor for prostate cancer in younger men.
Major finding: Black men younger than age 55 years undergoing prostate biopsies were 5.6 times more likely than other men to have a positive biopsy result.
Study details: Retrospective analysis of 564 men.
Disclosures: The study received funding from the National Institutes of Health and the U.S. Department of Veterans Affairs. Dr. Nettey reported having no financial disclosures.
Source: Nettey OS et al. AUA Annual Meeting. Abstract MP 21-17.
Primary hPTH often goes unnoticed
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Calcium levels in the high normal range may be confounding diagnoses.
Major finding: About 20% of primary hyperparathyroidism cases could have been spotted by the primary care physician based on tests that had been ordered.
Study details: A retrospective analysis of 742 patients at a tertiary care kidney stone clinic.
Disclosures: The study received no funding. Mr. Boyd declared no relevant financial relationships.
Source: Boyd C et al. AUA 2018, Abstract MP13-03.
Phototherapy has lasting benefit in low-risk prostate cancer
SAN FRANCISCO – In men with low-risk prostate cancer, vascular-targeted phototherapy (VTP) led to a significant reduction in subsequent conversions to radiation therapy or prostatectomy, compared with patients who underwent active surveillance. The latest results come at 4 years of follow-up, and confirm a reduction of risk seen in the original study at 2 years post treatment.
The new analysis was presented by Inderbir Gill, MD, in a late-breaking abstract session at the annual meeting of the American Urological Association.
About half of men with low-risk prostate cancer start out with active surveillance, but 25%-60% of them will go on to radical therapy (RT) within the next 5-10 years. Put another way, about 70% of men with low-risk prostate cancer will undergo RT within a decade. This protocol is generally effective, but it comes at a cost: Sixty-six percent of men undergoing radiotherapy and 82% of men undergoing prostatectomy experience incontinence, and 4% and 20%, respectively, experience erectile dysfunction at 2 years.
That adds up to an unmet clinical need: Patients with low-risk prostate cancer would be well served by an alternative therapy that cuts the risk of RT. VTP, along with alternatives cryoablation and high-intensity ultrasound, were developed to meet that need.
The original, phase 3 trial enrolled 413 men at 47 centers in Europe. Participants could have one cancer core that was free of Gleason patterns 4 or 5 as long as its length was between 3 and 5 mm. The study also included men with two or three positive cores, as long as the length was less than 5 mm, but they were excluded if they had Gleason patterns of 4 or 5.The participants were randomized to active surveillance or VTP, which consisted of 4 mg/kg padeliporfin delivered intravenously. Optical fibers inserted into the prostate to target the treatment zone were activated by laser light.
In the VTP group, 185 men completed 24 months of follow-up, as did 174 in the active surveillance group. Overall, 69% of the participants achieved follow-up at 3 years, and 64% at 4 years. At 2 years, 6% of men in the VTP group went on to undergo RT, compared with 29% in the surveillance group (P less than .0001). There were no significant differences between the two groups with respect to incontinence or erectile dysfunction.
In the extension study, similar patterns were seen at 3 and 4 years. At 4 years, 53% of men in the active surveillance group had converted to RT, compared with 24% in the treatment group (hazard ratio, 0.31; 95% confidence interval, 0.21-0.45). The absolute difference in risk for conversion to RT between the two groups was 3% at year 1, 26% at year 2, 30% at year 3, and 29% at year 4.
“It’s more of the same. Essentially there was about a 25% reduction in risk of conversion to radical treatment, and that benefit is maintained,” said Dr. Gill, professor and chair of urology at the University of Southern California, Los Angeles.
As expected, survival rates were similar at 4 years. In both groups, 99% were metastasis free, and cancer-specific survival was 100% in both. Overall survival was 98% in the VTP group, and 99% in the active surveillance group.
Currently, in very-low-risk patients, active surveillance is recommended and generally accepted. But many low-risk patients choose RT, putting them at risk for impotency and incontinence. “With this treatment, if you can maintain a 25% reduction in crossover treatment, then more men with low-risk prostate cancer are going to be spared radical treatment than if you were just doing active surveillance. And if you don’t get radical therapy, then your long-term quality of life is better,” said Dr. Gill.
VTP does lead to a small increase in risk of impotency and in incontinence in the short term, but patients tend to recover, and by 2 years, there is no significant difference between the groups.
Cryoablation and high-intensity focused ultrasound are also available. Those are effective, but they seem to have a higher profile of incontinence, impotency, and urethral injury, although they haven’t been directly compared to VTP, so comparing their side effect profiles “is literally comparing apples to oranges,” Dr. Gill said.
SAN FRANCISCO – In men with low-risk prostate cancer, vascular-targeted phototherapy (VTP) led to a significant reduction in subsequent conversions to radiation therapy or prostatectomy, compared with patients who underwent active surveillance. The latest results come at 4 years of follow-up, and confirm a reduction of risk seen in the original study at 2 years post treatment.
The new analysis was presented by Inderbir Gill, MD, in a late-breaking abstract session at the annual meeting of the American Urological Association.
About half of men with low-risk prostate cancer start out with active surveillance, but 25%-60% of them will go on to radical therapy (RT) within the next 5-10 years. Put another way, about 70% of men with low-risk prostate cancer will undergo RT within a decade. This protocol is generally effective, but it comes at a cost: Sixty-six percent of men undergoing radiotherapy and 82% of men undergoing prostatectomy experience incontinence, and 4% and 20%, respectively, experience erectile dysfunction at 2 years.
That adds up to an unmet clinical need: Patients with low-risk prostate cancer would be well served by an alternative therapy that cuts the risk of RT. VTP, along with alternatives cryoablation and high-intensity ultrasound, were developed to meet that need.
The original, phase 3 trial enrolled 413 men at 47 centers in Europe. Participants could have one cancer core that was free of Gleason patterns 4 or 5 as long as its length was between 3 and 5 mm. The study also included men with two or three positive cores, as long as the length was less than 5 mm, but they were excluded if they had Gleason patterns of 4 or 5.The participants were randomized to active surveillance or VTP, which consisted of 4 mg/kg padeliporfin delivered intravenously. Optical fibers inserted into the prostate to target the treatment zone were activated by laser light.
In the VTP group, 185 men completed 24 months of follow-up, as did 174 in the active surveillance group. Overall, 69% of the participants achieved follow-up at 3 years, and 64% at 4 years. At 2 years, 6% of men in the VTP group went on to undergo RT, compared with 29% in the surveillance group (P less than .0001). There were no significant differences between the two groups with respect to incontinence or erectile dysfunction.
In the extension study, similar patterns were seen at 3 and 4 years. At 4 years, 53% of men in the active surveillance group had converted to RT, compared with 24% in the treatment group (hazard ratio, 0.31; 95% confidence interval, 0.21-0.45). The absolute difference in risk for conversion to RT between the two groups was 3% at year 1, 26% at year 2, 30% at year 3, and 29% at year 4.
“It’s more of the same. Essentially there was about a 25% reduction in risk of conversion to radical treatment, and that benefit is maintained,” said Dr. Gill, professor and chair of urology at the University of Southern California, Los Angeles.
As expected, survival rates were similar at 4 years. In both groups, 99% were metastasis free, and cancer-specific survival was 100% in both. Overall survival was 98% in the VTP group, and 99% in the active surveillance group.
Currently, in very-low-risk patients, active surveillance is recommended and generally accepted. But many low-risk patients choose RT, putting them at risk for impotency and incontinence. “With this treatment, if you can maintain a 25% reduction in crossover treatment, then more men with low-risk prostate cancer are going to be spared radical treatment than if you were just doing active surveillance. And if you don’t get radical therapy, then your long-term quality of life is better,” said Dr. Gill.
VTP does lead to a small increase in risk of impotency and in incontinence in the short term, but patients tend to recover, and by 2 years, there is no significant difference between the groups.
Cryoablation and high-intensity focused ultrasound are also available. Those are effective, but they seem to have a higher profile of incontinence, impotency, and urethral injury, although they haven’t been directly compared to VTP, so comparing their side effect profiles “is literally comparing apples to oranges,” Dr. Gill said.
SAN FRANCISCO – In men with low-risk prostate cancer, vascular-targeted phototherapy (VTP) led to a significant reduction in subsequent conversions to radiation therapy or prostatectomy, compared with patients who underwent active surveillance. The latest results come at 4 years of follow-up, and confirm a reduction of risk seen in the original study at 2 years post treatment.
The new analysis was presented by Inderbir Gill, MD, in a late-breaking abstract session at the annual meeting of the American Urological Association.
About half of men with low-risk prostate cancer start out with active surveillance, but 25%-60% of them will go on to radical therapy (RT) within the next 5-10 years. Put another way, about 70% of men with low-risk prostate cancer will undergo RT within a decade. This protocol is generally effective, but it comes at a cost: Sixty-six percent of men undergoing radiotherapy and 82% of men undergoing prostatectomy experience incontinence, and 4% and 20%, respectively, experience erectile dysfunction at 2 years.
That adds up to an unmet clinical need: Patients with low-risk prostate cancer would be well served by an alternative therapy that cuts the risk of RT. VTP, along with alternatives cryoablation and high-intensity ultrasound, were developed to meet that need.
The original, phase 3 trial enrolled 413 men at 47 centers in Europe. Participants could have one cancer core that was free of Gleason patterns 4 or 5 as long as its length was between 3 and 5 mm. The study also included men with two or three positive cores, as long as the length was less than 5 mm, but they were excluded if they had Gleason patterns of 4 or 5.The participants were randomized to active surveillance or VTP, which consisted of 4 mg/kg padeliporfin delivered intravenously. Optical fibers inserted into the prostate to target the treatment zone were activated by laser light.
In the VTP group, 185 men completed 24 months of follow-up, as did 174 in the active surveillance group. Overall, 69% of the participants achieved follow-up at 3 years, and 64% at 4 years. At 2 years, 6% of men in the VTP group went on to undergo RT, compared with 29% in the surveillance group (P less than .0001). There were no significant differences between the two groups with respect to incontinence or erectile dysfunction.
In the extension study, similar patterns were seen at 3 and 4 years. At 4 years, 53% of men in the active surveillance group had converted to RT, compared with 24% in the treatment group (hazard ratio, 0.31; 95% confidence interval, 0.21-0.45). The absolute difference in risk for conversion to RT between the two groups was 3% at year 1, 26% at year 2, 30% at year 3, and 29% at year 4.
“It’s more of the same. Essentially there was about a 25% reduction in risk of conversion to radical treatment, and that benefit is maintained,” said Dr. Gill, professor and chair of urology at the University of Southern California, Los Angeles.
As expected, survival rates were similar at 4 years. In both groups, 99% were metastasis free, and cancer-specific survival was 100% in both. Overall survival was 98% in the VTP group, and 99% in the active surveillance group.
Currently, in very-low-risk patients, active surveillance is recommended and generally accepted. But many low-risk patients choose RT, putting them at risk for impotency and incontinence. “With this treatment, if you can maintain a 25% reduction in crossover treatment, then more men with low-risk prostate cancer are going to be spared radical treatment than if you were just doing active surveillance. And if you don’t get radical therapy, then your long-term quality of life is better,” said Dr. Gill.
VTP does lead to a small increase in risk of impotency and in incontinence in the short term, but patients tend to recover, and by 2 years, there is no significant difference between the groups.
Cryoablation and high-intensity focused ultrasound are also available. Those are effective, but they seem to have a higher profile of incontinence, impotency, and urethral injury, although they haven’t been directly compared to VTP, so comparing their side effect profiles “is literally comparing apples to oranges,” Dr. Gill said.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Vascular-targeted phototherapy may improve quality of life by reducing the need for radical therapy.
Major finding: VTP-treated patients had lower risk of conversion to radical therapy (HR, 0.31).
Study details: Extension study of a phase III trial (n = 413).
Disclosures: The study was funded by Steba Biotech and various government and foundation grants. Dr. Gill reported no relevant financial relationships.
USPSTF takes another stab at PSA screening recs
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
Painless penile ulcer and tender inguinal lymphadenopathy
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
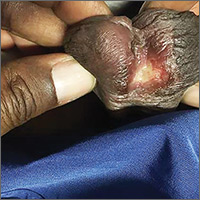
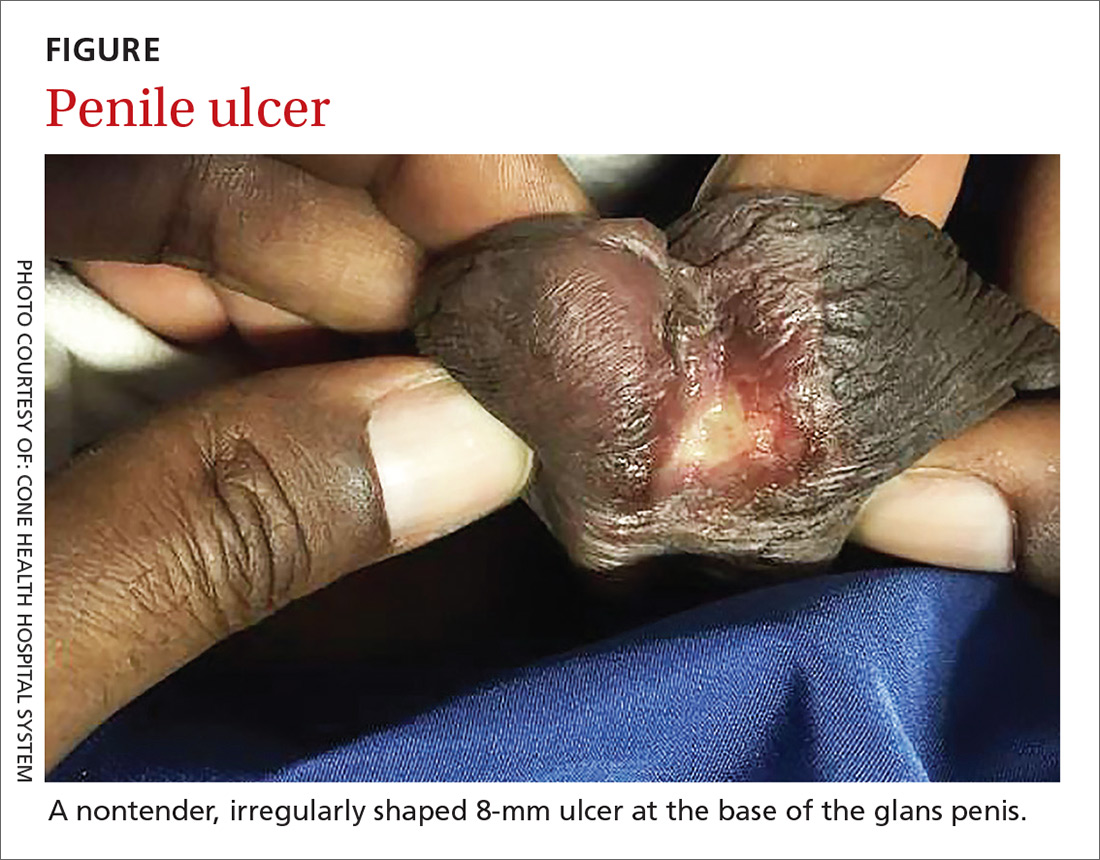
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.
A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.

A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.

A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
History of posttraumatic stress disorder • priapism • Dx?
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
Testosterone therapy tied to kidney stone risk
SAN FRANCISCO – , according to an analysis of more than 50,000 men with low testosterone.
When researchers compared hypogonadal men to age- and comorbidity-matched controls, they found a statistically significantly higher number of clinical diagnoses of a kidney stone, or of patients undergoing a kidney stone–related procedure.
The new study is the first large-scale analysis of the question in humans, according to Tyler McClintock, MD, who presented the findings at a poster session at the annual meeting of the American Urological Association. Dr. McClintock is a urology resident at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Dr. McClintock and his colleagues analyzed data from the Military Health System Data Repository (MDR). The MDR includes beneficiaries of the TRICARE program for service members, retirees, and their families. They looked at 26,586 men aged 40-64 years who had been diagnosed with low testosterone and who had received continuous testosterone replacement therapy between April 2006 and March 2014. The researchers compared them to 26,586 controls with low testosterone who did not receive testosterone replacement therapy.
Stone events were significantly higher in the treatment group. There were 67 extracorporeal shock wave lithotripsy procedures in the treatment group, compared with 51 among controls. Similar trends were seen with ureteroscopy with lithotripsy (75 vs. 46) and clinical diagnoses of kidney stone (1,059 vs. 794).
The researchers also broke down stone events by type of testosterone replacement therapy. A total of 5.4% of patients who received pellets (9 of 167) experienced an event (P = .27), compared with 5.1% of those who received injections (218 of 4,259; P = .004) and 3.5% of those who received it topically (655 of 18,895; P less than .0001).
At 2 years, Dr. McClintock reported that there were significantly more kidney stone events in the testosterone-treated group than in the untreated group (659 and 482, respectively; P less than .001). Two years after starting testosterone replacement therapy, significantly more of the treatment group had experienced a stone episode, compared with the matched controls during the same time period (3.9% and 3%, respectively; P less than .001).
Dr. McClintock said the study is convincing in part because it used data from TRICARE, which sets a lower testosterone level even than AUA guidelines for determining if a patient is eligible for testosterone therapy.
“It would suggest that those are the real low testosterone patients, not necessarily men who heard an ad or went to a test center,” noted Patrick Shepherd Lowry, MD, associate professor of urology at Scott & White Medical Center, Temple, Texas, who attended the presentation but was not involved in the study. “It’s preliminary, but it’s very interesting. It hasn’t been shown before.”
The Department of Defense funded the study. Dr. McClintock reported having no relevant financial disclosures.
SOURCE: McClintock T. AUA Annual Meeting. Abstract MP13-19.
SAN FRANCISCO – , according to an analysis of more than 50,000 men with low testosterone.
When researchers compared hypogonadal men to age- and comorbidity-matched controls, they found a statistically significantly higher number of clinical diagnoses of a kidney stone, or of patients undergoing a kidney stone–related procedure.
The new study is the first large-scale analysis of the question in humans, according to Tyler McClintock, MD, who presented the findings at a poster session at the annual meeting of the American Urological Association. Dr. McClintock is a urology resident at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Dr. McClintock and his colleagues analyzed data from the Military Health System Data Repository (MDR). The MDR includes beneficiaries of the TRICARE program for service members, retirees, and their families. They looked at 26,586 men aged 40-64 years who had been diagnosed with low testosterone and who had received continuous testosterone replacement therapy between April 2006 and March 2014. The researchers compared them to 26,586 controls with low testosterone who did not receive testosterone replacement therapy.
Stone events were significantly higher in the treatment group. There were 67 extracorporeal shock wave lithotripsy procedures in the treatment group, compared with 51 among controls. Similar trends were seen with ureteroscopy with lithotripsy (75 vs. 46) and clinical diagnoses of kidney stone (1,059 vs. 794).
The researchers also broke down stone events by type of testosterone replacement therapy. A total of 5.4% of patients who received pellets (9 of 167) experienced an event (P = .27), compared with 5.1% of those who received injections (218 of 4,259; P = .004) and 3.5% of those who received it topically (655 of 18,895; P less than .0001).
At 2 years, Dr. McClintock reported that there were significantly more kidney stone events in the testosterone-treated group than in the untreated group (659 and 482, respectively; P less than .001). Two years after starting testosterone replacement therapy, significantly more of the treatment group had experienced a stone episode, compared with the matched controls during the same time period (3.9% and 3%, respectively; P less than .001).
Dr. McClintock said the study is convincing in part because it used data from TRICARE, which sets a lower testosterone level even than AUA guidelines for determining if a patient is eligible for testosterone therapy.
“It would suggest that those are the real low testosterone patients, not necessarily men who heard an ad or went to a test center,” noted Patrick Shepherd Lowry, MD, associate professor of urology at Scott & White Medical Center, Temple, Texas, who attended the presentation but was not involved in the study. “It’s preliminary, but it’s very interesting. It hasn’t been shown before.”
The Department of Defense funded the study. Dr. McClintock reported having no relevant financial disclosures.
SOURCE: McClintock T. AUA Annual Meeting. Abstract MP13-19.
SAN FRANCISCO – , according to an analysis of more than 50,000 men with low testosterone.
When researchers compared hypogonadal men to age- and comorbidity-matched controls, they found a statistically significantly higher number of clinical diagnoses of a kidney stone, or of patients undergoing a kidney stone–related procedure.
The new study is the first large-scale analysis of the question in humans, according to Tyler McClintock, MD, who presented the findings at a poster session at the annual meeting of the American Urological Association. Dr. McClintock is a urology resident at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Dr. McClintock and his colleagues analyzed data from the Military Health System Data Repository (MDR). The MDR includes beneficiaries of the TRICARE program for service members, retirees, and their families. They looked at 26,586 men aged 40-64 years who had been diagnosed with low testosterone and who had received continuous testosterone replacement therapy between April 2006 and March 2014. The researchers compared them to 26,586 controls with low testosterone who did not receive testosterone replacement therapy.
Stone events were significantly higher in the treatment group. There were 67 extracorporeal shock wave lithotripsy procedures in the treatment group, compared with 51 among controls. Similar trends were seen with ureteroscopy with lithotripsy (75 vs. 46) and clinical diagnoses of kidney stone (1,059 vs. 794).
The researchers also broke down stone events by type of testosterone replacement therapy. A total of 5.4% of patients who received pellets (9 of 167) experienced an event (P = .27), compared with 5.1% of those who received injections (218 of 4,259; P = .004) and 3.5% of those who received it topically (655 of 18,895; P less than .0001).
At 2 years, Dr. McClintock reported that there were significantly more kidney stone events in the testosterone-treated group than in the untreated group (659 and 482, respectively; P less than .001). Two years after starting testosterone replacement therapy, significantly more of the treatment group had experienced a stone episode, compared with the matched controls during the same time period (3.9% and 3%, respectively; P less than .001).
Dr. McClintock said the study is convincing in part because it used data from TRICARE, which sets a lower testosterone level even than AUA guidelines for determining if a patient is eligible for testosterone therapy.
“It would suggest that those are the real low testosterone patients, not necessarily men who heard an ad or went to a test center,” noted Patrick Shepherd Lowry, MD, associate professor of urology at Scott & White Medical Center, Temple, Texas, who attended the presentation but was not involved in the study. “It’s preliminary, but it’s very interesting. It hasn’t been shown before.”
The Department of Defense funded the study. Dr. McClintock reported having no relevant financial disclosures.
SOURCE: McClintock T. AUA Annual Meeting. Abstract MP13-19.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Kidney stone risk may be a factor when considering testosterone replacement therapy.
Major finding: In untreated men, 482 kidney stone events occurred, compared with 659 in those receiving testosterone.
Study details: A case-control analysis of 26,586 treated men and 26,586 matched controls.
Disclosures: The Department of Defense funded the study. Dr. McClintock reported having no relevant financial disclosures.
Source: McClintock T. AUA Annual Meeting. Abstract MP13-19.