User login
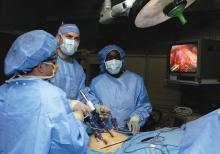
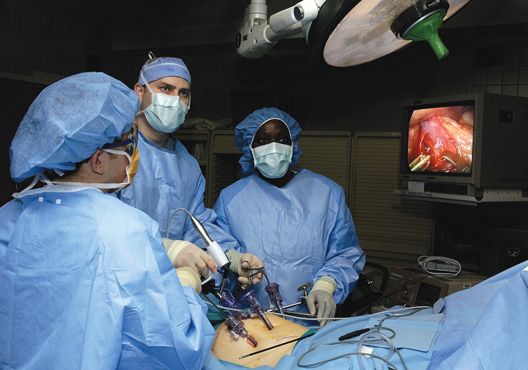
Risk factors identified for urinary retention after lap hernia repair
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Postoperative urinary retention was more likely to occur in patients more than 60 years of age and patients with benign prostatic hyperplasia.
Major finding: POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI.
Study details: A retrospective chart review of 578 patients who had laparoscopic total extraperitoneal inguinal hernia repair between 2009 and 2016.
Disclosures: No disclosures were reported.
Source: Roadman D et al. J Surg Res. 2018;11(231):309-15.
Screening for Prostate Cancer in Black Men
IN THIS ARTICLE
- Prostate cancer screening tools
- Ethic disparities
- Screening guidance
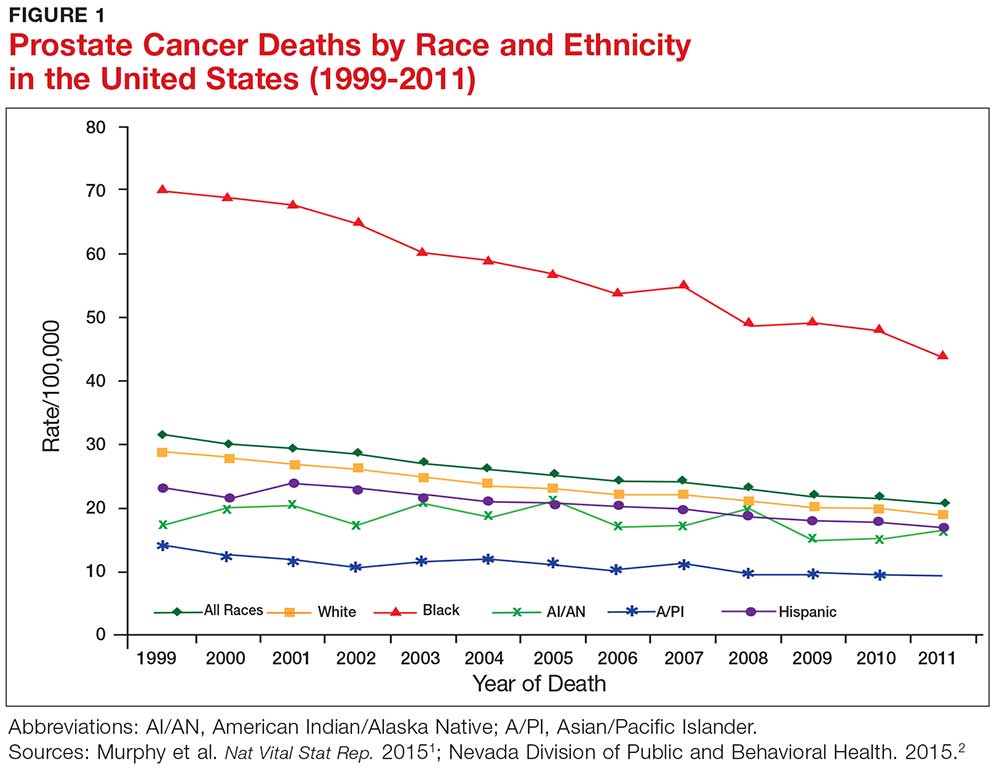
Prostate cancer, the second most common cancer to affect American men, is a slow-growing cancer that is curable when detected early. While the overall incidence has declined in the past 20 years (see Figure 1), prostate cancer remains a major concern among black men due to disproportionate incidence and mortality rates.1-3 A general understanding of the prostate and of prostate cancer lays the groundwork to acknowledge and address this divide.
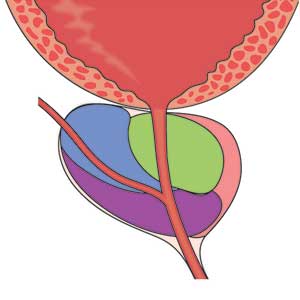
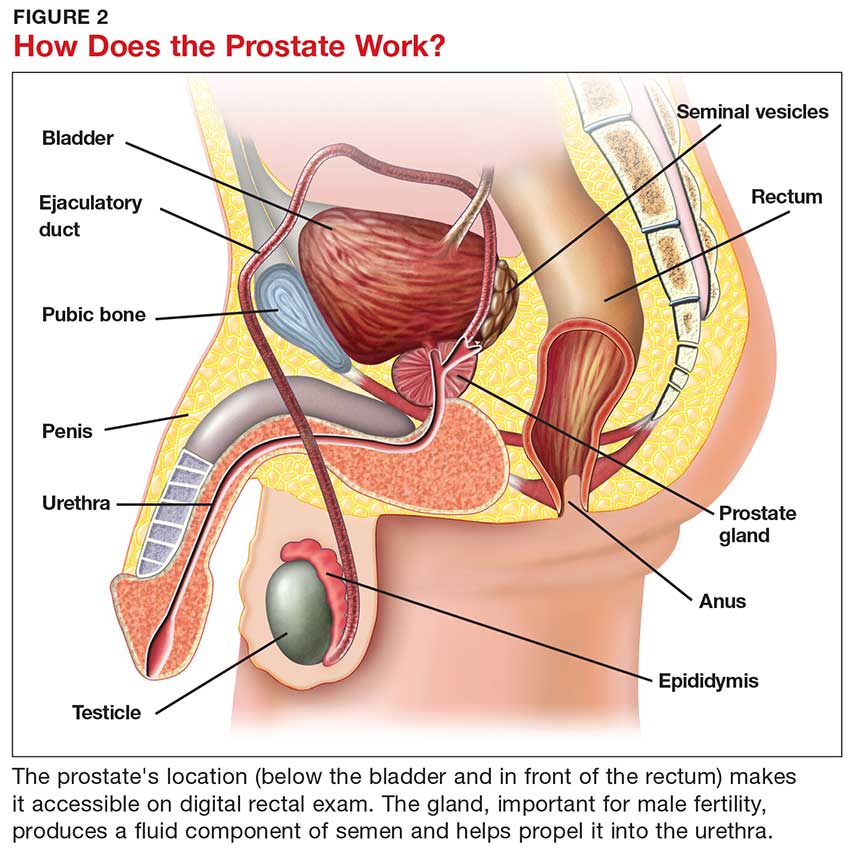
Although most men know where the prostate gland is located, many do not understand how it functions.4 The largest accessory gland of the male reproductive system, the prostate is located below the bladder and in front of the rectum (see Figure 2).5 The urethra passes through this gland; therefore, enlargement of the prostate can cause constriction of the urethra, which can affect the ability to eliminate urine from the body.5
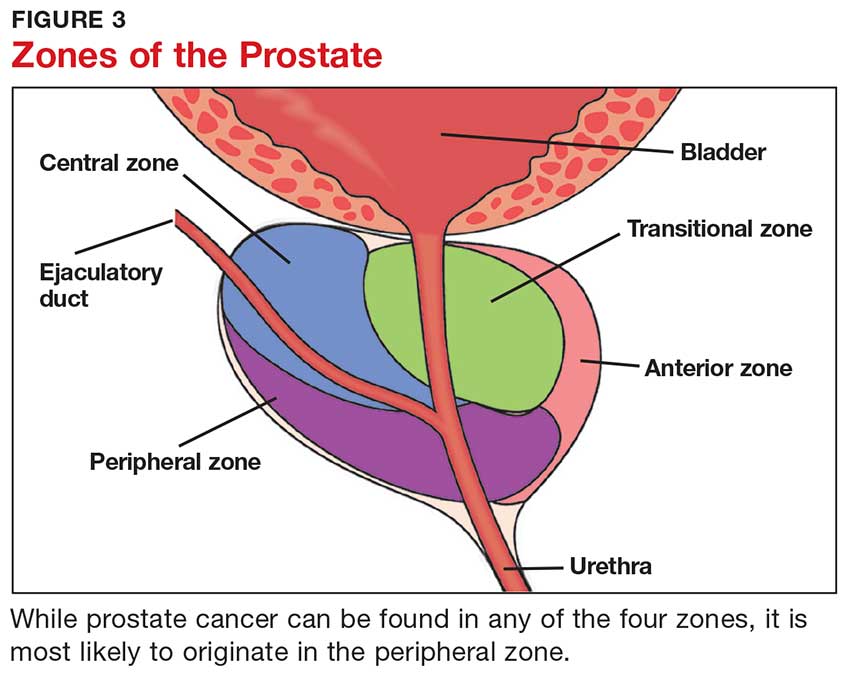
The prostate is broken down into four distinct regions (see Figure 3). Certain types of inflammation may occur more often in some regions of the prostate than others; as such, 75% of prostate cancer occurs in the peripheral zone (the region located closest to the rectal wall).5,6
DIAGNOSING PROSTATE CANCER
Signs and symptoms
According to the CDC, the signs and symptoms of prostate cancer include
- Difficulty starting urination
- Weak or interrupted flow of urine
- Frequent urination (especially at night)
- Difficulty emptying the bladder
- Pain or burning during urination
- Blood in the urine or semen
- Pain in the back, hips, or pelvis
- Painful ejaculation.
However, none of these signs and symptoms are unique to prostate cancer.7 For instance, difficulty starting urination, weak or interrupted flow of urine, and frequent urination can also be attributed to benign prostatic hyperplasia. Further, in its early stages, prostate cancer may not exhibit any signs or symptoms, making accurate screening essential for detection and treatment.7
Screening tools
There are two primary tools for detection of prostate cancer: the prostate-specific antigen (PSA) test and the digital rectal exam (DRE).8 The blood test for PSA is routinely used as a screening tool and is therefore considered a standard test for prostate cancer.9 A PSA level above 4.0 ng/mL is considered abnormal.10 Although measuring the PSA level can improve the odds of early prostate cancer detection, there is considerable debate over its dependability in this regard, as PSA can be elevated for benign reasons.
Sociocultural and genetic risk factors
While both black and white men are at an increased risk for prostate cancer if a first-degree relative (ie, father, brother, son) had the disease, one in five black men will develop prostate cancer in their lifetimes, compared with one in seven white men.3 And despite a five-year survival rate of nearly 100% for regional prostate cancer, black men are more than two times as likely as white men to die of the disease (1 in 23 and 1 in 38, respectively).8,11 From 2011 to 2015, the age-adjusted mortality rate of prostate cancer among black men was 40.8, versus 18.2 for non-Hispanic white men (per 100,000 population).12
Continue to: The disparity in prostate cancer mortality...
The disparity in prostate cancer mortality among black men has been attributed to multiple variables. Cultural differences can play a role in whether patients choose to undergo prostate cancer screening. Black men are, for example, less likely than other men to participate in preventive health care practices.13 Although an in-depth discussion is outside the scope of this article, researchers have identified some plausible factors for this, including economic limitations, lack of access to health care, distrust of the health care system, and an indifference to pain or discomfort.13,14 Decisions surrounding prostate screening can also be affected by a patient’s perceived risk for prostate cancer, the impact of a cancer diagnosis, and the availability of treatment.
Other factors that contribute to the higher incidence and mortality rate among black men include genetic predisposition, health beliefs, and knowledge about the prostate and cancer screenings.15 While most researchers have focused on men ages 40 and older, Ogunsanya et al suggested that educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.15
PRACTICE POINTS
- Prostate cancer remains a major concern among black men due to disproportionate incidence and mortality.
- Developing prostate cancer screening recommendations for black men would help reduce mortality and morbidity in this population.
- Educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.
IMPLICATIONS FOR PRACTICE
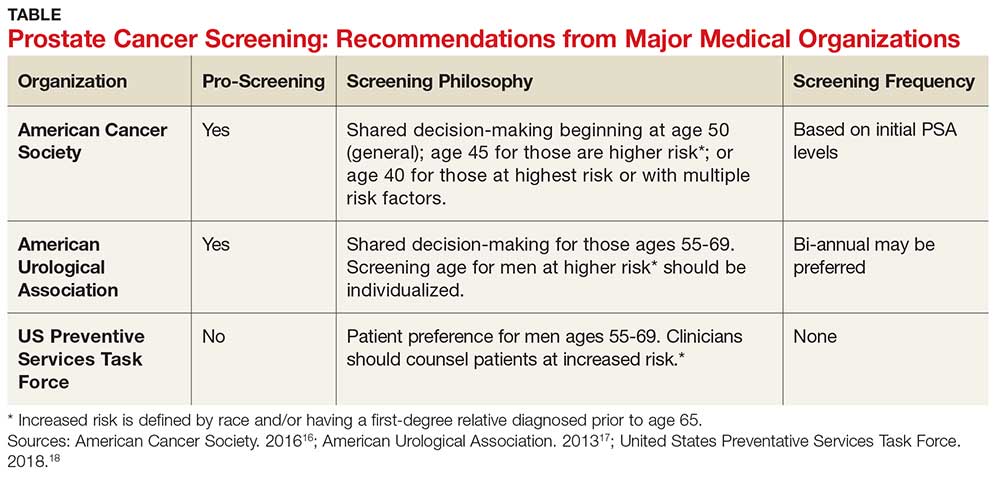
The age at which men should begin screening for prostate cancer has been a source of controversy due to the lack of consensus between the American Cancer Society, the American Urological Association, and the United States Preventive Services Task Force (USPSTF) guidelines (see Table).16-18 The current USPSTF recommendations for prostate cancer screening do not take into account ethnic differences, despite the identified racial disparity.19 Ambiguity in public health policy creates a quandary in the decision-making process regarding testing and treatment.9,19,20
In addition, these guidelines recommend the use of both the DRE and PSA screening tests. Screening should be performed every two years for men who have a PSA level < 2.5 ng/mL, and every year for men who have a level > 2.5 ng/mL.
Continue to: TREATMENT
TREATMENT
Fortunately, there are several treatment options for men who are diagnosed with prostate cancer.22 These include watchful waiting, surgery, radiation, cryotherapy, hormone therapy, and chemotherapy. The type of treatment chosen depends on many factors, such as the tumor grade or cancer stage, the implications for quality of life, and the shared provider/patient decision-making process. Indeed, choosing the right treatment is a specialized approach that varies according to case and circumstance.22
CONCLUSION
There has been an increase in prostate cancer screening in recent years. However, black men still lag behind when it comes to having DRE and PSA tests. Many factors, including cultural perceptions of medical care among black men, often cause delays in seeking evaluation and treatment. Developing consistent and uniform prostate cancer screening recommendations for black men would be an important step in reducing mortality and morbidity in this population.
1. Murphy SL, Kochanek KD, Xu J, Heron M. Deaths: final data for 2012. Nat Vital Stat Rep. 2015;63(9):37-80.
2. Nevada Division of Public and Behavioral Health. Comprehensive report: prostate cancer. September 2015. http://dpbh.nv.gov/Programs/Office_of_Public_Healh_Informatics_and_Epidemiology_(OPHIE)/. Accessed September 19, 2018.
3. Odedina FT, Dagne G, Pressey S, et al. Prostate cancer health and cultural beliefs of black men: the Florida prostate cancer disparity project. Infect Agent Cancer. 2011;6(2):1-7.
4. Winterich JA, Grzywacz JG, Quandt SA, et al. Men’s knowledge and beliefs about prostate cancer: education, race, and screening status. Ethn Dis. 2009;19(2):199-203.
5. Bhavsar A, Verma S. Anatomic imaging of the prostate. Biomed Res Int. 2014,1-9.
6. National Institutes of Health. Zones of the prostate. www.training.seer.cancer.gov/prostate/anatomy/zones.html. Accessed September 7, 2018.
7. CDC. Prostate cancer statistics. June 12, 2017. www.cdc.gov/cancer/prostate/statistics/. Accessed September 7, 2018.
8. American Cancer Society. Prostate cancer risk factors. www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/what-causes.html.
9. Mkanta W, Ndjakani Y, Bandiera F, et al. Prostate cancer screening and mortality in blacks and whites: a hospital-based case-control study. J Nat Med Assoc. 2015;107(2):32-38.
10. Hoffman R. Screening for prostate cancer. N Engl J Med. 2011;365(21):2013-2019.
11. CDC. Who is at risk for prostate cancer? June 7, 2018. www.cdc.gov/cancer/prostate/basic_info/risk_factors.htm. Accessed September 7, 2018.
12. American Cancer Society. Cancer facts and figures 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed September 7, 2018.
13. Woods VD, Montgomery SB, Belliard JC, et al. Culture, black men, and prostate cancer: what is reality? Cancer Control. 2004;11(6):388-396.
14. Braithwaite RL. Health Issues in the Black Community. 2nd ed. San Francisco, Calif: Jossey-Bass Publishers; 2001.
15. Ogunsanya ME, Brown CM, Odedina FT, et al. Beliefs regarding prostate cancer screening among black males aged 18 to 40 years. Am J Mens Health. 2017;11(1):41-53.
16. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. April 14, 2016. www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed September 7, 2018.
17. American Urological Association. Early detection of prostate cancer. 2013. www.auanet.org/guidelines/prostate-cancer-early-detection-(2013-reviewed-for-currency-2018). Accessed September 7, 2018.
18. United States Preventative Services Task Force. Final recommendation statement. Prostate cancer: screening. 2018. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed September 7, 2018.
19. Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. 2016;16(19):1-6.
20. Odedina FT, Campbell E, LaRose-Pierre M, et al. Personal factors affecting African-American men’s prostate cancer screening behavior. J Natl Med Assoc. 2008;100(6):724-733.
IN THIS ARTICLE
- Prostate cancer screening tools
- Ethic disparities
- Screening guidance
Prostate cancer, the second most common cancer to affect American men, is a slow-growing cancer that is curable when detected early. While the overall incidence has declined in the past 20 years (see Figure 1), prostate cancer remains a major concern among black men due to disproportionate incidence and mortality rates.1-3 A general understanding of the prostate and of prostate cancer lays the groundwork to acknowledge and address this divide.

Although most men know where the prostate gland is located, many do not understand how it functions.4 The largest accessory gland of the male reproductive system, the prostate is located below the bladder and in front of the rectum (see Figure 2).5 The urethra passes through this gland; therefore, enlargement of the prostate can cause constriction of the urethra, which can affect the ability to eliminate urine from the body.5

The prostate is broken down into four distinct regions (see Figure 3). Certain types of inflammation may occur more often in some regions of the prostate than others; as such, 75% of prostate cancer occurs in the peripheral zone (the region located closest to the rectal wall).5,6

DIAGNOSING PROSTATE CANCER
Signs and symptoms
According to the CDC, the signs and symptoms of prostate cancer include
- Difficulty starting urination
- Weak or interrupted flow of urine
- Frequent urination (especially at night)
- Difficulty emptying the bladder
- Pain or burning during urination
- Blood in the urine or semen
- Pain in the back, hips, or pelvis
- Painful ejaculation.
However, none of these signs and symptoms are unique to prostate cancer.7 For instance, difficulty starting urination, weak or interrupted flow of urine, and frequent urination can also be attributed to benign prostatic hyperplasia. Further, in its early stages, prostate cancer may not exhibit any signs or symptoms, making accurate screening essential for detection and treatment.7
Screening tools
There are two primary tools for detection of prostate cancer: the prostate-specific antigen (PSA) test and the digital rectal exam (DRE).8 The blood test for PSA is routinely used as a screening tool and is therefore considered a standard test for prostate cancer.9 A PSA level above 4.0 ng/mL is considered abnormal.10 Although measuring the PSA level can improve the odds of early prostate cancer detection, there is considerable debate over its dependability in this regard, as PSA can be elevated for benign reasons.
Sociocultural and genetic risk factors
While both black and white men are at an increased risk for prostate cancer if a first-degree relative (ie, father, brother, son) had the disease, one in five black men will develop prostate cancer in their lifetimes, compared with one in seven white men.3 And despite a five-year survival rate of nearly 100% for regional prostate cancer, black men are more than two times as likely as white men to die of the disease (1 in 23 and 1 in 38, respectively).8,11 From 2011 to 2015, the age-adjusted mortality rate of prostate cancer among black men was 40.8, versus 18.2 for non-Hispanic white men (per 100,000 population).12
Continue to: The disparity in prostate cancer mortality...
The disparity in prostate cancer mortality among black men has been attributed to multiple variables. Cultural differences can play a role in whether patients choose to undergo prostate cancer screening. Black men are, for example, less likely than other men to participate in preventive health care practices.13 Although an in-depth discussion is outside the scope of this article, researchers have identified some plausible factors for this, including economic limitations, lack of access to health care, distrust of the health care system, and an indifference to pain or discomfort.13,14 Decisions surrounding prostate screening can also be affected by a patient’s perceived risk for prostate cancer, the impact of a cancer diagnosis, and the availability of treatment.
Other factors that contribute to the higher incidence and mortality rate among black men include genetic predisposition, health beliefs, and knowledge about the prostate and cancer screenings.15 While most researchers have focused on men ages 40 and older, Ogunsanya et al suggested that educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.15
PRACTICE POINTS
- Prostate cancer remains a major concern among black men due to disproportionate incidence and mortality.
- Developing prostate cancer screening recommendations for black men would help reduce mortality and morbidity in this population.
- Educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.
IMPLICATIONS FOR PRACTICE
The age at which men should begin screening for prostate cancer has been a source of controversy due to the lack of consensus between the American Cancer Society, the American Urological Association, and the United States Preventive Services Task Force (USPSTF) guidelines (see Table).16-18 The current USPSTF recommendations for prostate cancer screening do not take into account ethnic differences, despite the identified racial disparity.19 Ambiguity in public health policy creates a quandary in the decision-making process regarding testing and treatment.9,19,20

In addition, these guidelines recommend the use of both the DRE and PSA screening tests. Screening should be performed every two years for men who have a PSA level < 2.5 ng/mL, and every year for men who have a level > 2.5 ng/mL.
Continue to: TREATMENT
TREATMENT
Fortunately, there are several treatment options for men who are diagnosed with prostate cancer.22 These include watchful waiting, surgery, radiation, cryotherapy, hormone therapy, and chemotherapy. The type of treatment chosen depends on many factors, such as the tumor grade or cancer stage, the implications for quality of life, and the shared provider/patient decision-making process. Indeed, choosing the right treatment is a specialized approach that varies according to case and circumstance.22
CONCLUSION
There has been an increase in prostate cancer screening in recent years. However, black men still lag behind when it comes to having DRE and PSA tests. Many factors, including cultural perceptions of medical care among black men, often cause delays in seeking evaluation and treatment. Developing consistent and uniform prostate cancer screening recommendations for black men would be an important step in reducing mortality and morbidity in this population.
IN THIS ARTICLE
- Prostate cancer screening tools
- Ethic disparities
- Screening guidance
Prostate cancer, the second most common cancer to affect American men, is a slow-growing cancer that is curable when detected early. While the overall incidence has declined in the past 20 years (see Figure 1), prostate cancer remains a major concern among black men due to disproportionate incidence and mortality rates.1-3 A general understanding of the prostate and of prostate cancer lays the groundwork to acknowledge and address this divide.

Although most men know where the prostate gland is located, many do not understand how it functions.4 The largest accessory gland of the male reproductive system, the prostate is located below the bladder and in front of the rectum (see Figure 2).5 The urethra passes through this gland; therefore, enlargement of the prostate can cause constriction of the urethra, which can affect the ability to eliminate urine from the body.5

The prostate is broken down into four distinct regions (see Figure 3). Certain types of inflammation may occur more often in some regions of the prostate than others; as such, 75% of prostate cancer occurs in the peripheral zone (the region located closest to the rectal wall).5,6

DIAGNOSING PROSTATE CANCER
Signs and symptoms
According to the CDC, the signs and symptoms of prostate cancer include
- Difficulty starting urination
- Weak or interrupted flow of urine
- Frequent urination (especially at night)
- Difficulty emptying the bladder
- Pain or burning during urination
- Blood in the urine or semen
- Pain in the back, hips, or pelvis
- Painful ejaculation.
However, none of these signs and symptoms are unique to prostate cancer.7 For instance, difficulty starting urination, weak or interrupted flow of urine, and frequent urination can also be attributed to benign prostatic hyperplasia. Further, in its early stages, prostate cancer may not exhibit any signs or symptoms, making accurate screening essential for detection and treatment.7
Screening tools
There are two primary tools for detection of prostate cancer: the prostate-specific antigen (PSA) test and the digital rectal exam (DRE).8 The blood test for PSA is routinely used as a screening tool and is therefore considered a standard test for prostate cancer.9 A PSA level above 4.0 ng/mL is considered abnormal.10 Although measuring the PSA level can improve the odds of early prostate cancer detection, there is considerable debate over its dependability in this regard, as PSA can be elevated for benign reasons.
Sociocultural and genetic risk factors
While both black and white men are at an increased risk for prostate cancer if a first-degree relative (ie, father, brother, son) had the disease, one in five black men will develop prostate cancer in their lifetimes, compared with one in seven white men.3 And despite a five-year survival rate of nearly 100% for regional prostate cancer, black men are more than two times as likely as white men to die of the disease (1 in 23 and 1 in 38, respectively).8,11 From 2011 to 2015, the age-adjusted mortality rate of prostate cancer among black men was 40.8, versus 18.2 for non-Hispanic white men (per 100,000 population).12
Continue to: The disparity in prostate cancer mortality...
The disparity in prostate cancer mortality among black men has been attributed to multiple variables. Cultural differences can play a role in whether patients choose to undergo prostate cancer screening. Black men are, for example, less likely than other men to participate in preventive health care practices.13 Although an in-depth discussion is outside the scope of this article, researchers have identified some plausible factors for this, including economic limitations, lack of access to health care, distrust of the health care system, and an indifference to pain or discomfort.13,14 Decisions surrounding prostate screening can also be affected by a patient’s perceived risk for prostate cancer, the impact of a cancer diagnosis, and the availability of treatment.
Other factors that contribute to the higher incidence and mortality rate among black men include genetic predisposition, health beliefs, and knowledge about the prostate and cancer screenings.15 While most researchers have focused on men ages 40 and older, Ogunsanya et al suggested that educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.15
PRACTICE POINTS
- Prostate cancer remains a major concern among black men due to disproportionate incidence and mortality.
- Developing prostate cancer screening recommendations for black men would help reduce mortality and morbidity in this population.
- Educating black men about screening for prostate cancer at an earlier age may help them to make informed decisions later in life.
IMPLICATIONS FOR PRACTICE
The age at which men should begin screening for prostate cancer has been a source of controversy due to the lack of consensus between the American Cancer Society, the American Urological Association, and the United States Preventive Services Task Force (USPSTF) guidelines (see Table).16-18 The current USPSTF recommendations for prostate cancer screening do not take into account ethnic differences, despite the identified racial disparity.19 Ambiguity in public health policy creates a quandary in the decision-making process regarding testing and treatment.9,19,20

In addition, these guidelines recommend the use of both the DRE and PSA screening tests. Screening should be performed every two years for men who have a PSA level < 2.5 ng/mL, and every year for men who have a level > 2.5 ng/mL.
Continue to: TREATMENT
TREATMENT
Fortunately, there are several treatment options for men who are diagnosed with prostate cancer.22 These include watchful waiting, surgery, radiation, cryotherapy, hormone therapy, and chemotherapy. The type of treatment chosen depends on many factors, such as the tumor grade or cancer stage, the implications for quality of life, and the shared provider/patient decision-making process. Indeed, choosing the right treatment is a specialized approach that varies according to case and circumstance.22
CONCLUSION
There has been an increase in prostate cancer screening in recent years. However, black men still lag behind when it comes to having DRE and PSA tests. Many factors, including cultural perceptions of medical care among black men, often cause delays in seeking evaluation and treatment. Developing consistent and uniform prostate cancer screening recommendations for black men would be an important step in reducing mortality and morbidity in this population.
1. Murphy SL, Kochanek KD, Xu J, Heron M. Deaths: final data for 2012. Nat Vital Stat Rep. 2015;63(9):37-80.
2. Nevada Division of Public and Behavioral Health. Comprehensive report: prostate cancer. September 2015. http://dpbh.nv.gov/Programs/Office_of_Public_Healh_Informatics_and_Epidemiology_(OPHIE)/. Accessed September 19, 2018.
3. Odedina FT, Dagne G, Pressey S, et al. Prostate cancer health and cultural beliefs of black men: the Florida prostate cancer disparity project. Infect Agent Cancer. 2011;6(2):1-7.
4. Winterich JA, Grzywacz JG, Quandt SA, et al. Men’s knowledge and beliefs about prostate cancer: education, race, and screening status. Ethn Dis. 2009;19(2):199-203.
5. Bhavsar A, Verma S. Anatomic imaging of the prostate. Biomed Res Int. 2014,1-9.
6. National Institutes of Health. Zones of the prostate. www.training.seer.cancer.gov/prostate/anatomy/zones.html. Accessed September 7, 2018.
7. CDC. Prostate cancer statistics. June 12, 2017. www.cdc.gov/cancer/prostate/statistics/. Accessed September 7, 2018.
8. American Cancer Society. Prostate cancer risk factors. www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/what-causes.html.
9. Mkanta W, Ndjakani Y, Bandiera F, et al. Prostate cancer screening and mortality in blacks and whites: a hospital-based case-control study. J Nat Med Assoc. 2015;107(2):32-38.
10. Hoffman R. Screening for prostate cancer. N Engl J Med. 2011;365(21):2013-2019.
11. CDC. Who is at risk for prostate cancer? June 7, 2018. www.cdc.gov/cancer/prostate/basic_info/risk_factors.htm. Accessed September 7, 2018.
12. American Cancer Society. Cancer facts and figures 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed September 7, 2018.
13. Woods VD, Montgomery SB, Belliard JC, et al. Culture, black men, and prostate cancer: what is reality? Cancer Control. 2004;11(6):388-396.
14. Braithwaite RL. Health Issues in the Black Community. 2nd ed. San Francisco, Calif: Jossey-Bass Publishers; 2001.
15. Ogunsanya ME, Brown CM, Odedina FT, et al. Beliefs regarding prostate cancer screening among black males aged 18 to 40 years. Am J Mens Health. 2017;11(1):41-53.
16. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. April 14, 2016. www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed September 7, 2018.
17. American Urological Association. Early detection of prostate cancer. 2013. www.auanet.org/guidelines/prostate-cancer-early-detection-(2013-reviewed-for-currency-2018). Accessed September 7, 2018.
18. United States Preventative Services Task Force. Final recommendation statement. Prostate cancer: screening. 2018. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed September 7, 2018.
19. Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. 2016;16(19):1-6.
20. Odedina FT, Campbell E, LaRose-Pierre M, et al. Personal factors affecting African-American men’s prostate cancer screening behavior. J Natl Med Assoc. 2008;100(6):724-733.
1. Murphy SL, Kochanek KD, Xu J, Heron M. Deaths: final data for 2012. Nat Vital Stat Rep. 2015;63(9):37-80.
2. Nevada Division of Public and Behavioral Health. Comprehensive report: prostate cancer. September 2015. http://dpbh.nv.gov/Programs/Office_of_Public_Healh_Informatics_and_Epidemiology_(OPHIE)/. Accessed September 19, 2018.
3. Odedina FT, Dagne G, Pressey S, et al. Prostate cancer health and cultural beliefs of black men: the Florida prostate cancer disparity project. Infect Agent Cancer. 2011;6(2):1-7.
4. Winterich JA, Grzywacz JG, Quandt SA, et al. Men’s knowledge and beliefs about prostate cancer: education, race, and screening status. Ethn Dis. 2009;19(2):199-203.
5. Bhavsar A, Verma S. Anatomic imaging of the prostate. Biomed Res Int. 2014,1-9.
6. National Institutes of Health. Zones of the prostate. www.training.seer.cancer.gov/prostate/anatomy/zones.html. Accessed September 7, 2018.
7. CDC. Prostate cancer statistics. June 12, 2017. www.cdc.gov/cancer/prostate/statistics/. Accessed September 7, 2018.
8. American Cancer Society. Prostate cancer risk factors. www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/what-causes.html.
9. Mkanta W, Ndjakani Y, Bandiera F, et al. Prostate cancer screening and mortality in blacks and whites: a hospital-based case-control study. J Nat Med Assoc. 2015;107(2):32-38.
10. Hoffman R. Screening for prostate cancer. N Engl J Med. 2011;365(21):2013-2019.
11. CDC. Who is at risk for prostate cancer? June 7, 2018. www.cdc.gov/cancer/prostate/basic_info/risk_factors.htm. Accessed September 7, 2018.
12. American Cancer Society. Cancer facts and figures 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed September 7, 2018.
13. Woods VD, Montgomery SB, Belliard JC, et al. Culture, black men, and prostate cancer: what is reality? Cancer Control. 2004;11(6):388-396.
14. Braithwaite RL. Health Issues in the Black Community. 2nd ed. San Francisco, Calif: Jossey-Bass Publishers; 2001.
15. Ogunsanya ME, Brown CM, Odedina FT, et al. Beliefs regarding prostate cancer screening among black males aged 18 to 40 years. Am J Mens Health. 2017;11(1):41-53.
16. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. April 14, 2016. www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed September 7, 2018.
17. American Urological Association. Early detection of prostate cancer. 2013. www.auanet.org/guidelines/prostate-cancer-early-detection-(2013-reviewed-for-currency-2018). Accessed September 7, 2018.
18. United States Preventative Services Task Force. Final recommendation statement. Prostate cancer: screening. 2018. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed September 7, 2018.
19. Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. 2016;16(19):1-6.
20. Odedina FT, Campbell E, LaRose-Pierre M, et al. Personal factors affecting African-American men’s prostate cancer screening behavior. J Natl Med Assoc. 2008;100(6):724-733.
MRI doubles rate of observation in low-risk prostate cancer
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
FROM UROLOGY
Key clinical point: MRI at screening or diagnosis of low-risk prostate cancer is associated with a higher likelihood of observation versus immediate definitive therapy.
Major finding: MRI was associated with a near doubling of the likelihood of observation.
Study details: Review of SEER Medicare data on 8,144 men diagnosed with low-risk prostate cancers during 2010-2013.
Disclosures: The study was supported by the National Cancer Institute, California Department of Public Health, and Centers for Disease Control and Prevention. The authors reported no relevant conflicts of interest.
Source: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
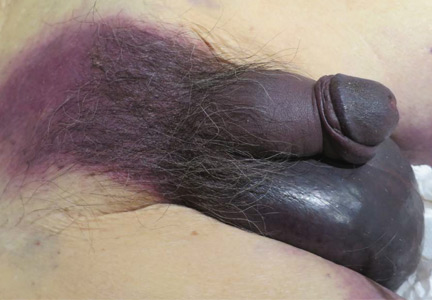
Fournier gangrene
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
In geriatric urinary incontinence, think DIAPERS mnemonic
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Palmoplantar exanthema and liver dysfunction
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
For men with SCD and priapism, hypoxia may prompt RBC adhesion
WASHINGTON – For male patients with sickle cell disease, priapism can be more than just painful and embarrassing. The prolonged erections prompted by vasoocclusive events in the penis may lead to irreversible impotence, but little is known about risk factors for priapism, which remains a difficult-to-treat complication of the disease.
In males with HbSS sickle cell disease (SCD) and priapism, RBC adhesion is increased in hypoxic conditions, according to preliminary findings from work using a newly developed biochip that mimics microvascular conditions in SCD. This significant level of adhesion prompted by hypoxia was not seen in men who did not have priapism, according to study coauthor Erina Quinn, a research assistant in hematology and oncology at Case Western Reserve University, Cleveland, who presented the results at the annual meeting of the Foundation for Sickle Cell Disease Research.
When hemoglobin desaturation occurs, polymerization can be increased, leading to increased end-organ damage, Ms. Quinn said. The biochip is “an effort to measure cellular adhesion in a clinically meaningful way.” The tool can detect hemoglobin phenotype, differentiating among HbSS, HbSbeta+, and HbSC. It can also measure the degree of hemolysis and RBC deformability.
The biochip “mimics postcapillary flow conditions in microchannels,” Ms. Quinn said. The device forces blood samples through microchannels that are at the diameter of smaller venules, approximately 50 mcm, and at a physiological flow rate ranging from 1-13 mm/sec. The microfluidic channels are coated with laminin, a subendothelial matrix protein implicated in RBC adhesion. A second microfluidic biochip mimics hypoxic conditions.
The study enrolled 26 men with the HbSS genotype, 14 of whom reported priapism, and assessed RBC adhesion in blood samples run though both the SCD-modeled biochip and the hypoxia biochip. Investigators also assessed contemporaneous in vivo hemoglobin desaturation, and looked for associations with the in vitro biochip findings.
Of the 26 participants, 16 also had either nocturnal or exertional hemoglobin desaturation. In addition, 10 participants had both priapism and desaturations. These data were collected by retrospective chart review and patient survey.
Patients with priapism were a mean age of 34 years, compared with a mean age of 29 years for the other participants, a nonsignificant difference. There were no significant differences in mean hemoglobin or bilirubin levels, or in reticulocyte counts, between the two groups.
However, white blood count, absolute neutrophil count, and lactate dehydrogenase levels were significantly higher for men with priapism (P = .022, .037, and .008, respectively). Ferritin levels were higher as well, at a mean 2,433 (plus or minus 2,234) mcg/L for those with priapism, compared with a mean 269 (plus or minus 3,015) mcg/L for those without priapism (P = .031).
When absolute reticulocyte count was mapped against lactate dehydrogenase levels to create a measure of degree of hemolysis, “individuals with priapism had a more hemolytic lab profile,” said Ms. Quinn (P = .0186).
Though 10 of 14 men with priapism had hemoglobin desaturation, compared with 5 of 12 who did not have priapism, the difference was not statistically significant.
When the researchers compared microchip analysis of RBC adhesion, though, they found marked differences in RBC adhesion in hypoxic versus nonhypoxic conditions. Significantly more RBCs were adherent under hypoxic conditions – in the hypoxic biochip – for the patients with priapism than for patients without priapism (mean, 529 vs. 3,268 adherent cells; P = .016).
Though numbers were small, RBCs from patients with reported priapism and hemoglobin desaturation in vivo showed increased hypoxia enhanced adhesion in vitro (P = .013), Ms. Quinn said. These was no significant difference between adhesion in normoxic and hypoxic conditions for the patients without priapism.
Future directions of work with the biochip include prospective identification of desaturation events and better characterization of nocturnal symptoms, Ms. Quinn said. The investigators also plan to see whether treatment with supplemental oxygen affects RBC adhesion.
The research was supported by the National Institutes of Health, the Doris Duke Charitable Foundation, and the National Science Foundation. Two coauthors have filed an international patent for the biochip technology.
WASHINGTON – For male patients with sickle cell disease, priapism can be more than just painful and embarrassing. The prolonged erections prompted by vasoocclusive events in the penis may lead to irreversible impotence, but little is known about risk factors for priapism, which remains a difficult-to-treat complication of the disease.
In males with HbSS sickle cell disease (SCD) and priapism, RBC adhesion is increased in hypoxic conditions, according to preliminary findings from work using a newly developed biochip that mimics microvascular conditions in SCD. This significant level of adhesion prompted by hypoxia was not seen in men who did not have priapism, according to study coauthor Erina Quinn, a research assistant in hematology and oncology at Case Western Reserve University, Cleveland, who presented the results at the annual meeting of the Foundation for Sickle Cell Disease Research.
When hemoglobin desaturation occurs, polymerization can be increased, leading to increased end-organ damage, Ms. Quinn said. The biochip is “an effort to measure cellular adhesion in a clinically meaningful way.” The tool can detect hemoglobin phenotype, differentiating among HbSS, HbSbeta+, and HbSC. It can also measure the degree of hemolysis and RBC deformability.
The biochip “mimics postcapillary flow conditions in microchannels,” Ms. Quinn said. The device forces blood samples through microchannels that are at the diameter of smaller venules, approximately 50 mcm, and at a physiological flow rate ranging from 1-13 mm/sec. The microfluidic channels are coated with laminin, a subendothelial matrix protein implicated in RBC adhesion. A second microfluidic biochip mimics hypoxic conditions.
The study enrolled 26 men with the HbSS genotype, 14 of whom reported priapism, and assessed RBC adhesion in blood samples run though both the SCD-modeled biochip and the hypoxia biochip. Investigators also assessed contemporaneous in vivo hemoglobin desaturation, and looked for associations with the in vitro biochip findings.
Of the 26 participants, 16 also had either nocturnal or exertional hemoglobin desaturation. In addition, 10 participants had both priapism and desaturations. These data were collected by retrospective chart review and patient survey.
Patients with priapism were a mean age of 34 years, compared with a mean age of 29 years for the other participants, a nonsignificant difference. There were no significant differences in mean hemoglobin or bilirubin levels, or in reticulocyte counts, between the two groups.
However, white blood count, absolute neutrophil count, and lactate dehydrogenase levels were significantly higher for men with priapism (P = .022, .037, and .008, respectively). Ferritin levels were higher as well, at a mean 2,433 (plus or minus 2,234) mcg/L for those with priapism, compared with a mean 269 (plus or minus 3,015) mcg/L for those without priapism (P = .031).
When absolute reticulocyte count was mapped against lactate dehydrogenase levels to create a measure of degree of hemolysis, “individuals with priapism had a more hemolytic lab profile,” said Ms. Quinn (P = .0186).
Though 10 of 14 men with priapism had hemoglobin desaturation, compared with 5 of 12 who did not have priapism, the difference was not statistically significant.
When the researchers compared microchip analysis of RBC adhesion, though, they found marked differences in RBC adhesion in hypoxic versus nonhypoxic conditions. Significantly more RBCs were adherent under hypoxic conditions – in the hypoxic biochip – for the patients with priapism than for patients without priapism (mean, 529 vs. 3,268 adherent cells; P = .016).
Though numbers were small, RBCs from patients with reported priapism and hemoglobin desaturation in vivo showed increased hypoxia enhanced adhesion in vitro (P = .013), Ms. Quinn said. These was no significant difference between adhesion in normoxic and hypoxic conditions for the patients without priapism.
Future directions of work with the biochip include prospective identification of desaturation events and better characterization of nocturnal symptoms, Ms. Quinn said. The investigators also plan to see whether treatment with supplemental oxygen affects RBC adhesion.
The research was supported by the National Institutes of Health, the Doris Duke Charitable Foundation, and the National Science Foundation. Two coauthors have filed an international patent for the biochip technology.
WASHINGTON – For male patients with sickle cell disease, priapism can be more than just painful and embarrassing. The prolonged erections prompted by vasoocclusive events in the penis may lead to irreversible impotence, but little is known about risk factors for priapism, which remains a difficult-to-treat complication of the disease.
In males with HbSS sickle cell disease (SCD) and priapism, RBC adhesion is increased in hypoxic conditions, according to preliminary findings from work using a newly developed biochip that mimics microvascular conditions in SCD. This significant level of adhesion prompted by hypoxia was not seen in men who did not have priapism, according to study coauthor Erina Quinn, a research assistant in hematology and oncology at Case Western Reserve University, Cleveland, who presented the results at the annual meeting of the Foundation for Sickle Cell Disease Research.
When hemoglobin desaturation occurs, polymerization can be increased, leading to increased end-organ damage, Ms. Quinn said. The biochip is “an effort to measure cellular adhesion in a clinically meaningful way.” The tool can detect hemoglobin phenotype, differentiating among HbSS, HbSbeta+, and HbSC. It can also measure the degree of hemolysis and RBC deformability.
The biochip “mimics postcapillary flow conditions in microchannels,” Ms. Quinn said. The device forces blood samples through microchannels that are at the diameter of smaller venules, approximately 50 mcm, and at a physiological flow rate ranging from 1-13 mm/sec. The microfluidic channels are coated with laminin, a subendothelial matrix protein implicated in RBC adhesion. A second microfluidic biochip mimics hypoxic conditions.
The study enrolled 26 men with the HbSS genotype, 14 of whom reported priapism, and assessed RBC adhesion in blood samples run though both the SCD-modeled biochip and the hypoxia biochip. Investigators also assessed contemporaneous in vivo hemoglobin desaturation, and looked for associations with the in vitro biochip findings.
Of the 26 participants, 16 also had either nocturnal or exertional hemoglobin desaturation. In addition, 10 participants had both priapism and desaturations. These data were collected by retrospective chart review and patient survey.
Patients with priapism were a mean age of 34 years, compared with a mean age of 29 years for the other participants, a nonsignificant difference. There were no significant differences in mean hemoglobin or bilirubin levels, or in reticulocyte counts, between the two groups.
However, white blood count, absolute neutrophil count, and lactate dehydrogenase levels were significantly higher for men with priapism (P = .022, .037, and .008, respectively). Ferritin levels were higher as well, at a mean 2,433 (plus or minus 2,234) mcg/L for those with priapism, compared with a mean 269 (plus or minus 3,015) mcg/L for those without priapism (P = .031).
When absolute reticulocyte count was mapped against lactate dehydrogenase levels to create a measure of degree of hemolysis, “individuals with priapism had a more hemolytic lab profile,” said Ms. Quinn (P = .0186).
Though 10 of 14 men with priapism had hemoglobin desaturation, compared with 5 of 12 who did not have priapism, the difference was not statistically significant.
When the researchers compared microchip analysis of RBC adhesion, though, they found marked differences in RBC adhesion in hypoxic versus nonhypoxic conditions. Significantly more RBCs were adherent under hypoxic conditions – in the hypoxic biochip – for the patients with priapism than for patients without priapism (mean, 529 vs. 3,268 adherent cells; P = .016).
Though numbers were small, RBCs from patients with reported priapism and hemoglobin desaturation in vivo showed increased hypoxia enhanced adhesion in vitro (P = .013), Ms. Quinn said. These was no significant difference between adhesion in normoxic and hypoxic conditions for the patients without priapism.
Future directions of work with the biochip include prospective identification of desaturation events and better characterization of nocturnal symptoms, Ms. Quinn said. The investigators also plan to see whether treatment with supplemental oxygen affects RBC adhesion.
The research was supported by the National Institutes of Health, the Doris Duke Charitable Foundation, and the National Science Foundation. Two coauthors have filed an international patent for the biochip technology.
REPORTING FROM FSCDR 2018
Key clinical point: RBC adhesion was increased, but only in hypoxia, for men with sickle cell disease and priapism.
Major finding: Men who had desaturations and priapism had significantly higher RBC adhesion than those without priapism (P = .013).
Study details: An in vitro and in vivo study of 26 men with HbSS sickle cell disease, with and without priapism.
Disclosures: The study was funded by the National Institutes of Health, the Doris Duke Charitable Foundation, and the National Science Foundation. Two coauthors have filed an international patent for the biochip technology.
Most transgender teens not willing to delay hormone therapy to preserve fertility
TORONTO – The majority of transgender youth attending a pediatric gender clinic were not willing to delay starting hormone therapy in order to pursue fertility preservation, according to a survey study presented during a poster session at the Pediatric Academic Societies annual meeting.
Five percent of 66 young people and 33% of 52 parents surveyed during a visit to a hospital-based gender clinic agreed with the statement: “I would choose to delay hormone therapy to undergo fertility preservation (for my child) if asked today.”
Further, 70% of youth agreed that discomfort with a part of the body they don’t identify with was a factor that influenced their decision or thoughts about fertility preservation. Religious, financial, ethical, and demographic factors were not associated with willingness to delay treatment for fertility concerns.
“While hormone therapy has drastically improved the lives of countless transgender and gender nonconforming youth, its impact on fertility can unfairly force individuals to decide at a very early age whether or not they should preserve the ability to be a biological parent one day,” Rebecca Persky, MD, said in a press release. Dr. Persky, a former Children’s Hospital of Philadelphia (CHOP) resident, is now is a pediatric endocrinology fellow at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A slightly greater proportion of youth (20%) and 12% of parents agreed it was important to have biological children or grandchildren. For those youth who did want to preserve the option of having biological children, that desire was associated with perceiving it as important to their parents (odds ratio, 6.07; P less than .05).
“We didn’t ask any questions about adoption of children or grandchildren, so that might have yielded different results if we had,” Dr. Persky acknowledged in an interview.
A lack of information about whether hormone therapy definitely prevents biologic fertility was associated with parents’ willingness to delay treatment for fertility preservation (OR: 24.57, P less than .05), yet 62% of parents said they felt their children were able to “make a meaningful decision about taking steps to preserve fertility at this point in (his/her/their) life.”
“I thought delaying treatment would be one of the biggest barriers, but even when we asked them if they wanted to preserve their fertility while not delaying or changing their hormone therapy, only the minority [33%] said they would be interested in that,” Dr. Persky said in an interview. “It kind of argues that a lot of these kids just don’t want to have biological children.”
She noted, however, that one limitation of the study was that many of the children surveyed already were receiving hormone therapy such that the questions engaged more on a theoretical level than a practical one.
“Not surprisingly, the strongest factor in the parents’ decisions was whether or not it was important to their child to have biological children,” said Dr. Persky.
The researchers surveyed 66 transgender and gender nonconforming youth who presented for care at the Gender and Sexuality Development Clinic at CHOP. After the findings were released, it was noted by several concerned parties on Twitter that because of the location of the study, the sample was a decidedly selected one.
The mean age was 17 years of patients and 63% of the sample were assigned female sex at birth. The mean age in the 52 parents surveyed was 48 years. The survey included 36 items on knowledge of fertility preservation, the desire to have biological children, and other factors that may affect the decision to pursue fertility preservation.
Gender-specific and age-specific analyses have not been completed, but are in the works, said Dr. Persky, who acknowledged that the area requires more qualitative research.
The authors reported no conflicts of interest.
TORONTO – The majority of transgender youth attending a pediatric gender clinic were not willing to delay starting hormone therapy in order to pursue fertility preservation, according to a survey study presented during a poster session at the Pediatric Academic Societies annual meeting.
Five percent of 66 young people and 33% of 52 parents surveyed during a visit to a hospital-based gender clinic agreed with the statement: “I would choose to delay hormone therapy to undergo fertility preservation (for my child) if asked today.”
Further, 70% of youth agreed that discomfort with a part of the body they don’t identify with was a factor that influenced their decision or thoughts about fertility preservation. Religious, financial, ethical, and demographic factors were not associated with willingness to delay treatment for fertility concerns.
“While hormone therapy has drastically improved the lives of countless transgender and gender nonconforming youth, its impact on fertility can unfairly force individuals to decide at a very early age whether or not they should preserve the ability to be a biological parent one day,” Rebecca Persky, MD, said in a press release. Dr. Persky, a former Children’s Hospital of Philadelphia (CHOP) resident, is now is a pediatric endocrinology fellow at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A slightly greater proportion of youth (20%) and 12% of parents agreed it was important to have biological children or grandchildren. For those youth who did want to preserve the option of having biological children, that desire was associated with perceiving it as important to their parents (odds ratio, 6.07; P less than .05).
“We didn’t ask any questions about adoption of children or grandchildren, so that might have yielded different results if we had,” Dr. Persky acknowledged in an interview.
A lack of information about whether hormone therapy definitely prevents biologic fertility was associated with parents’ willingness to delay treatment for fertility preservation (OR: 24.57, P less than .05), yet 62% of parents said they felt their children were able to “make a meaningful decision about taking steps to preserve fertility at this point in (his/her/their) life.”
“I thought delaying treatment would be one of the biggest barriers, but even when we asked them if they wanted to preserve their fertility while not delaying or changing their hormone therapy, only the minority [33%] said they would be interested in that,” Dr. Persky said in an interview. “It kind of argues that a lot of these kids just don’t want to have biological children.”
She noted, however, that one limitation of the study was that many of the children surveyed already were receiving hormone therapy such that the questions engaged more on a theoretical level than a practical one.
“Not surprisingly, the strongest factor in the parents’ decisions was whether or not it was important to their child to have biological children,” said Dr. Persky.
The researchers surveyed 66 transgender and gender nonconforming youth who presented for care at the Gender and Sexuality Development Clinic at CHOP. After the findings were released, it was noted by several concerned parties on Twitter that because of the location of the study, the sample was a decidedly selected one.
The mean age was 17 years of patients and 63% of the sample were assigned female sex at birth. The mean age in the 52 parents surveyed was 48 years. The survey included 36 items on knowledge of fertility preservation, the desire to have biological children, and other factors that may affect the decision to pursue fertility preservation.
Gender-specific and age-specific analyses have not been completed, but are in the works, said Dr. Persky, who acknowledged that the area requires more qualitative research.
The authors reported no conflicts of interest.
TORONTO – The majority of transgender youth attending a pediatric gender clinic were not willing to delay starting hormone therapy in order to pursue fertility preservation, according to a survey study presented during a poster session at the Pediatric Academic Societies annual meeting.
Five percent of 66 young people and 33% of 52 parents surveyed during a visit to a hospital-based gender clinic agreed with the statement: “I would choose to delay hormone therapy to undergo fertility preservation (for my child) if asked today.”
Further, 70% of youth agreed that discomfort with a part of the body they don’t identify with was a factor that influenced their decision or thoughts about fertility preservation. Religious, financial, ethical, and demographic factors were not associated with willingness to delay treatment for fertility concerns.
“While hormone therapy has drastically improved the lives of countless transgender and gender nonconforming youth, its impact on fertility can unfairly force individuals to decide at a very early age whether or not they should preserve the ability to be a biological parent one day,” Rebecca Persky, MD, said in a press release. Dr. Persky, a former Children’s Hospital of Philadelphia (CHOP) resident, is now is a pediatric endocrinology fellow at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A slightly greater proportion of youth (20%) and 12% of parents agreed it was important to have biological children or grandchildren. For those youth who did want to preserve the option of having biological children, that desire was associated with perceiving it as important to their parents (odds ratio, 6.07; P less than .05).
“We didn’t ask any questions about adoption of children or grandchildren, so that might have yielded different results if we had,” Dr. Persky acknowledged in an interview.
A lack of information about whether hormone therapy definitely prevents biologic fertility was associated with parents’ willingness to delay treatment for fertility preservation (OR: 24.57, P less than .05), yet 62% of parents said they felt their children were able to “make a meaningful decision about taking steps to preserve fertility at this point in (his/her/their) life.”
“I thought delaying treatment would be one of the biggest barriers, but even when we asked them if they wanted to preserve their fertility while not delaying or changing their hormone therapy, only the minority [33%] said they would be interested in that,” Dr. Persky said in an interview. “It kind of argues that a lot of these kids just don’t want to have biological children.”
She noted, however, that one limitation of the study was that many of the children surveyed already were receiving hormone therapy such that the questions engaged more on a theoretical level than a practical one.
“Not surprisingly, the strongest factor in the parents’ decisions was whether or not it was important to their child to have biological children,” said Dr. Persky.
The researchers surveyed 66 transgender and gender nonconforming youth who presented for care at the Gender and Sexuality Development Clinic at CHOP. After the findings were released, it was noted by several concerned parties on Twitter that because of the location of the study, the sample was a decidedly selected one.
The mean age was 17 years of patients and 63% of the sample were assigned female sex at birth. The mean age in the 52 parents surveyed was 48 years. The survey included 36 items on knowledge of fertility preservation, the desire to have biological children, and other factors that may affect the decision to pursue fertility preservation.
Gender-specific and age-specific analyses have not been completed, but are in the works, said Dr. Persky, who acknowledged that the area requires more qualitative research.
The authors reported no conflicts of interest.
REPORTING FROM PAS 18
Key clinical point:
Major finding: Five percent of youth and 33% of parents were willing to delay hormone therapy to undergo fertility preservation.
Study details: Survey study of 66 young people attending a pediatric gender and sexuality clinic and 52 parents of those individuals.
Disclosures: The authors reported no conflicts of interest.
More than 16% of ED sepsis patients not admitted to hospital
SAN DIEGO – More than 16% of emergency department sepsis patients are not admitted to the hospital, preliminary results from a large retrospective cohort study found.
“Nothing is really known about this topic,” lead study author Ithan D. Peltan, MD, said in an interview at an international conference of the American Thoracic Society. “In previous research, we’ve been focused on patients with sepsis who are admitted to the hospital. We have never thoroughly recognized that a fair number of patients who meet clinical criteria for sepsis in the emergency department are actually triaged to outpatient management. We don’t really know anything about these patients. What are their clinical characteristics and what are their outcomes like? And what are the factors that are leading them to be discharged from the ED rather than be admitted to the hospital?”
To find out, he and his associates retrospectively reviewed the medical records of 12,002 adult ED patients who met criteria for sepsis at two tertiary hospitals and two community hospitals in Utah between July 2013 and December 2016. They excluded trauma patients, those who left the ED against medical advice, those who were discharged to hospice or who died in the ED, and eligible patients’ repeat ED encounters. Patients transferred to another acute care facility were considered admitted, while transfers to non-acute care such as skilled nursing or psychiatric facilities were classified as discharges. Next, Dr. Peltan and his associates employed inverse probability weights using a propensity score for ED discharge based on age, sex, Charlson score, ED acuity score, initial ED vital signs, white blood cell count, lactate, sequential organ failure assessment (SOFA)score, busyness of the ED, and study hospital to compare 30-day mortality between patients admitted to the hospital versus those discharged from the ED.
Of the 12,002 patients included in the analysis, 10,032 (83.6%) were admitted, while 1,970 (16.4%) were discharged. Compared with admitted patients, discharged patients were younger (a mean of 53 vs. 60 years, respectively; P less than .001); more likely to be female (65% vs. 55%; P less than .001); more likely to be nonwhite or Hispanic (21% vs 17%; P less than .001), and had fewer comorbidities and physiologic derangements. In addition, crude mortality at 30 days was lower in discharged versus admitted patients (1.0% vs. 6.2%, respectively; P less than .001). After the propensity-adjusted analysis, there was no significant difference in 30-day mortality for discharged vs. admitted sepsis patients (adjusted odds ratio 1.0).
“We were worried that discharged ED sepsis patients were being mismanaged and weren’t going to do well as similar patients who were admitted to the hospital,” Dr. Peltan said. “This analysis is still a work in progress, but with that caveat, our findings so far suggest that physicians are making pretty good decisions overall.”
The researchers also found that, among 89 ED physicians who cared for 20 or more eligible patients, some did not discharge any of their sepsis patients, while others discharged 39% of their sepsis patients. “That was surprising,” Dr. Peltan said. “This could mean that some hospital sepsis admissions depend on physician practice style more than the patient’s condition or treatment needs.”
Researchers emphasized that they do not recommend routine outpatient management for individual sepsis patients. “Almost certainly, some of the discharged patients should have been admitted to the hospital.” Dr. Peltan said. “I think there’s still a lot of opportunity to understand who these patients are, understand why there is so much physician variation, and to develop tools to further optimize triage decisions.”
The study was funded in part by the Intermountain Research and Medical Foundation in Salt Lake City. Dr. Peltan reported having no financial disclosures.
SOURCE: Peltan ID et al. ATS 2018, Abstract A5994/702.
SAN DIEGO – More than 16% of emergency department sepsis patients are not admitted to the hospital, preliminary results from a large retrospective cohort study found.
“Nothing is really known about this topic,” lead study author Ithan D. Peltan, MD, said in an interview at an international conference of the American Thoracic Society. “In previous research, we’ve been focused on patients with sepsis who are admitted to the hospital. We have never thoroughly recognized that a fair number of patients who meet clinical criteria for sepsis in the emergency department are actually triaged to outpatient management. We don’t really know anything about these patients. What are their clinical characteristics and what are their outcomes like? And what are the factors that are leading them to be discharged from the ED rather than be admitted to the hospital?”
To find out, he and his associates retrospectively reviewed the medical records of 12,002 adult ED patients who met criteria for sepsis at two tertiary hospitals and two community hospitals in Utah between July 2013 and December 2016. They excluded trauma patients, those who left the ED against medical advice, those who were discharged to hospice or who died in the ED, and eligible patients’ repeat ED encounters. Patients transferred to another acute care facility were considered admitted, while transfers to non-acute care such as skilled nursing or psychiatric facilities were classified as discharges. Next, Dr. Peltan and his associates employed inverse probability weights using a propensity score for ED discharge based on age, sex, Charlson score, ED acuity score, initial ED vital signs, white blood cell count, lactate, sequential organ failure assessment (SOFA)score, busyness of the ED, and study hospital to compare 30-day mortality between patients admitted to the hospital versus those discharged from the ED.
Of the 12,002 patients included in the analysis, 10,032 (83.6%) were admitted, while 1,970 (16.4%) were discharged. Compared with admitted patients, discharged patients were younger (a mean of 53 vs. 60 years, respectively; P less than .001); more likely to be female (65% vs. 55%; P less than .001); more likely to be nonwhite or Hispanic (21% vs 17%; P less than .001), and had fewer comorbidities and physiologic derangements. In addition, crude mortality at 30 days was lower in discharged versus admitted patients (1.0% vs. 6.2%, respectively; P less than .001). After the propensity-adjusted analysis, there was no significant difference in 30-day mortality for discharged vs. admitted sepsis patients (adjusted odds ratio 1.0).
“We were worried that discharged ED sepsis patients were being mismanaged and weren’t going to do well as similar patients who were admitted to the hospital,” Dr. Peltan said. “This analysis is still a work in progress, but with that caveat, our findings so far suggest that physicians are making pretty good decisions overall.”
The researchers also found that, among 89 ED physicians who cared for 20 or more eligible patients, some did not discharge any of their sepsis patients, while others discharged 39% of their sepsis patients. “That was surprising,” Dr. Peltan said. “This could mean that some hospital sepsis admissions depend on physician practice style more than the patient’s condition or treatment needs.”
Researchers emphasized that they do not recommend routine outpatient management for individual sepsis patients. “Almost certainly, some of the discharged patients should have been admitted to the hospital.” Dr. Peltan said. “I think there’s still a lot of opportunity to understand who these patients are, understand why there is so much physician variation, and to develop tools to further optimize triage decisions.”
The study was funded in part by the Intermountain Research and Medical Foundation in Salt Lake City. Dr. Peltan reported having no financial disclosures.
SOURCE: Peltan ID et al. ATS 2018, Abstract A5994/702.
SAN DIEGO – More than 16% of emergency department sepsis patients are not admitted to the hospital, preliminary results from a large retrospective cohort study found.
“Nothing is really known about this topic,” lead study author Ithan D. Peltan, MD, said in an interview at an international conference of the American Thoracic Society. “In previous research, we’ve been focused on patients with sepsis who are admitted to the hospital. We have never thoroughly recognized that a fair number of patients who meet clinical criteria for sepsis in the emergency department are actually triaged to outpatient management. We don’t really know anything about these patients. What are their clinical characteristics and what are their outcomes like? And what are the factors that are leading them to be discharged from the ED rather than be admitted to the hospital?”
To find out, he and his associates retrospectively reviewed the medical records of 12,002 adult ED patients who met criteria for sepsis at two tertiary hospitals and two community hospitals in Utah between July 2013 and December 2016. They excluded trauma patients, those who left the ED against medical advice, those who were discharged to hospice or who died in the ED, and eligible patients’ repeat ED encounters. Patients transferred to another acute care facility were considered admitted, while transfers to non-acute care such as skilled nursing or psychiatric facilities were classified as discharges. Next, Dr. Peltan and his associates employed inverse probability weights using a propensity score for ED discharge based on age, sex, Charlson score, ED acuity score, initial ED vital signs, white blood cell count, lactate, sequential organ failure assessment (SOFA)score, busyness of the ED, and study hospital to compare 30-day mortality between patients admitted to the hospital versus those discharged from the ED.
Of the 12,002 patients included in the analysis, 10,032 (83.6%) were admitted, while 1,970 (16.4%) were discharged. Compared with admitted patients, discharged patients were younger (a mean of 53 vs. 60 years, respectively; P less than .001); more likely to be female (65% vs. 55%; P less than .001); more likely to be nonwhite or Hispanic (21% vs 17%; P less than .001), and had fewer comorbidities and physiologic derangements. In addition, crude mortality at 30 days was lower in discharged versus admitted patients (1.0% vs. 6.2%, respectively; P less than .001). After the propensity-adjusted analysis, there was no significant difference in 30-day mortality for discharged vs. admitted sepsis patients (adjusted odds ratio 1.0).
“We were worried that discharged ED sepsis patients were being mismanaged and weren’t going to do well as similar patients who were admitted to the hospital,” Dr. Peltan said. “This analysis is still a work in progress, but with that caveat, our findings so far suggest that physicians are making pretty good decisions overall.”
The researchers also found that, among 89 ED physicians who cared for 20 or more eligible patients, some did not discharge any of their sepsis patients, while others discharged 39% of their sepsis patients. “That was surprising,” Dr. Peltan said. “This could mean that some hospital sepsis admissions depend on physician practice style more than the patient’s condition or treatment needs.”
Researchers emphasized that they do not recommend routine outpatient management for individual sepsis patients. “Almost certainly, some of the discharged patients should have been admitted to the hospital.” Dr. Peltan said. “I think there’s still a lot of opportunity to understand who these patients are, understand why there is so much physician variation, and to develop tools to further optimize triage decisions.”
The study was funded in part by the Intermountain Research and Medical Foundation in Salt Lake City. Dr. Peltan reported having no financial disclosures.
SOURCE: Peltan ID et al. ATS 2018, Abstract A5994/702.
AT ATS 2018
Key clinical point: More research is needed to optimize triage decisions for ED sepsis patients and to understand possible disparities in ED disposition.
Major finding: Among adult patients who met clinical criteria for sepsis in the emergency department, 16.4% were not admitted to the hospital.
Study details: A retrospective study of 12,002 adult ED patients who met criteria for sepsis at two tertiary hospitals and two community hospitals in Utah.
Disclosures: The study was funded in part by the Intermountain Research and Medical Foundation in Salt Lake City. Dr. Peltan reported having no financial disclosures.
Source: Peltan ID et al. Abstract 5994/702, ATS 2018.
Diabetes risk may rise with work hours
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
FROM BMJ OPEN DIABETES RESEARCH & CARE