User login
Carol Bernstein Part II
Parental leave for residents
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Prostate cancer screening
To the Editor: In their article on men’s health,1 Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
To the Editor: In their article on men’s health,1 Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
To the Editor: In their article on men’s health,1 Chaitoff and colleagues present the scenario of a 60-year-old patient, with no other history given, whose recent screening prostate-specific antigen (PSA) level was 5.1 ng/mL, and who asks his doctor:
- Should I have agreed to the screening?
- How effective is the screening?
- What are the next steps?
These questions are consistent with the patient having read the latest US Preventive Services Task Force (USPSTF) report on PSA screening, which states: “Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results…”2
I would tell the patient that he can expect greater benefit from PSA screening than reported by the USPSTF simply by adhering to the screening protocol. Intention-to-treat analysis applied to the trial results diminished the apparent benefits of PSA screening by counting fatal prostate cancers experienced by nonadherent study participants as screening failures.3 In other words, screening works better in those who actually get screened!
The authors state1 that “in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it.” Nevertheless, prostate cancer remains the second most common cause of cancer deaths in American men, after lung cancer.4 In addition to the reduction in prostate cancer-specific mortality with screening, patients should consider the reduction in morbidity from painful bone metastases and pathologic fractures, which are common in advanced prostate cancer.
A false-positive elevated PSA can be caused by reversible benign conditions, such as prostate infection or trauma, which can resolve over time, returning the PSA to its baseline level. Studies have demonstrated that simply repeating the PSA test a few weeks later will significantly reduce the number of false-positive PSA screening tests.5
Also, it is not optimal to screen for prostate cancer using a single PSA measurement. This patient’s PSA of 5.1 ng/mL cannot distinguish between chronic benign prostatic hyperplasia and a fast-growing but still curable malignancy. If the patient’s PSA had been tested annually and was known to be stable at its current level, a benign or indolent condition would be most likely, allowing for the possibility of continuing noninvasive observation. If his PSA was 1.1 ng/mL a year ago, and his PSA remains elevated when retested in a few weeks, the likelihood of malignancy would increase, increasing the yield of biopsy.
Lastly, consider false-negatives. A man with a PSA of 2.0 ng/mL would not have undergone biopsy in any of the trials, but if he had a history of several consecutive annual PSA levels less than 1.0 ng/mL, the doubling of his PSA during an interval less than or equal to 1 year could signal an early aggressive prostate cancer. Increases in PSA velocity can reveal the rapid proliferation of malignant prostate cells before the tumor is large enough to cross a static threshold PSA. We have zero data indicating how much benefit can be derived from the use of PSA velocity in this fashion. However, clinicians who carefully track serial PSA changes in each patient have anecdotes of success in early detection and cure of aggressive prostate cancers that would not have been detected by the trial protocols using fixed PSA thresholds. Until such trials are done, we can only tell patients that the ability to compute PSA velocity may be another source of benefit of annual screening of PSA.
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
- Chaitoff A, Killeen TC, Nielsen C. Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880. doi:10.3949/ccjm.85a.18011
- US Preventive Services Task Force. Prostate cancer: screening. May 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1?ds=1&s=PSA. Accessed November 6, 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2(3):109–112. doi:10.4103/2229-3485.83221
- Cancer.Net. Prostate cancer: statistics. www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed November 6, 2018.
- Lavallée LT, Binette A, Witiuk K, et al. Reducing the harm of prostate cancer screening: repeated prostate-specific antigen testing. Mayo Clin Proc 2016; 91(1):17–22. doi:10.1016/j.mayocp.2015.07.030
Correction: Men’s health 2018
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
In the article by Chaitoff et al (Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements. Cleve Clin J Med 2018; 85(11):871–880, doi:10.3949/ccjm.85a.18011), the prostate-specific antigen level of a 60-year-old man was given as 5.1 mg/dL. The unit of measure should have been 5.1 ng/mL. This has been corrected online.
Adjunctive testosterone may reduce depressive symptoms in men
Testosterone treatment has potential as an adjunct therapy for men with depressive disorders, a meta-analysis has suggested. However, more research is needed.
“This meta-analysis provides important new evidence that testosterone treatment may also be effective and efficacious for eugonadal and older men when higher testosterone dosages are administered,” Andreas Walther, PhD, and his coauthors wrote Nov. 14 in JAMA Psychiatry. However, they noted that safety monitoring in testosterone treatment trials remained important because of an absence of sufficiently powered, long-term studies to assess the increased risk of adverse events with treatment.
The link between testosterone and depression has been debated extensively because testosterone is a neuroactive steroid hormone known to influence mood and appetitive behavior, Dr. Walther and his coauthors wrote. Although testosterone treatment for various disorders in hypogonadal men has been backed by evidence, the results of randomized, placebo-controlled clinical trials for its use in depression have been inconsistent. Indeed, testosterone treatment was currently not recommended in national or international guidelines because of an “prevailing uncertainty about its efficacy, age criteria, dosage, ideal duration and method of application,” wrote Dr. Walther, of the department of biological psychology at Technische Universität Dresden (Germany).
For the current review, the researchers identified 27 randomized, controlled trials altogether including 1,890 men who were receiving testosterone treatment and had reported depressive symptoms on validated depression scales.
Results showed evidence for a moderate antidepressant association of testosterone treatment, compared with placebo (Hedges g, 0.21; 95% confidence interval, 0.10-0.32), and an efficacy odds ratio of 2.30 (95% CI, 1.30-4.06). According to the researchers, based on reference ranges for depressive symptoms, “The National Institute for Health and Care Excellence guidelines on depression suggest a reduction of 3.0 and 2.0 points on BDI scores to be clinically significant for normal depression and treatment-resistant depression, respectively,” they wrote.
Testosterone treatment also showed an efficacy OR of 2.30, a finding that the authors said suggested the “potential of testosterone treatment as adjunct therapy for men with depressive disorders.”
They said the results suggested that better treatment response might require higher doses but acknowledged that the finding required replication.
In addition, Dr. Walther and his coauthors found acceptability of testosterone treatment was high, with an OR of 0.79 for testosterone treatment–related loss to follow-up, compared with placebo. Remarkably, they added, initial testosterone status did not moderate the effects of testosterone treatment on depressive symptoms.
“Large, preregistered RCTs of good quality investigating testosterone treatment’s effect in men on depression as the primary outcome” are needed, they concluded.
Dr. Walther and his coauthors cited a few limitations, including the low number of randomized, controlled trials addressing the effects of testosterone treatment in men who were depressed but otherwise healthy.
No conflicts of interest were reported.
SOURCE: Walther A et al. JAMA Psychiatry. 2018 14 Nov. doi: 10.1001/jamapsychiatry.2018.2734.
The role of testosterone in the pathophysiology and treatment of depressive disorders in men is controversial. The meta-analysis by Walther et al. is well performed and adds to the body of evidence suggesting that testosterone treatment can lead to small improvements in men with depressive symptoms.
However, it is uncertain whether these improvements are clinically meaningful. The data should not be interpreted as testosterone treatment leads to remission or enhances response to antidepressant treatment in this population. In short, the current meta-analysis suggests testosterone replacement may enhance mood among nondepressed hypogonadal men. It is worth noting that the long-term safety of testosterone treatment remains unknown. Until more research is available, clinicians should continue to follow the Endocrine Society guideline for testosterone replacement therapy of androgen-deficient men. The data do not currently support the use of testosterone therapy, particularly in supraphysiologic doses, for the treatment of depressive disorders in men.
Shalender Bhasin, MD, is affiliated with the Brigham and Women’s Hospital in Boston, and Stuart Seidman, MD, is affiliated with Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 14. doi: 10.1001/jamapsychiatry.2018.2661). Dr. Bhasin reported receiving research grants from several sources, and Dr. Seidman reported no disclosures.
The role of testosterone in the pathophysiology and treatment of depressive disorders in men is controversial. The meta-analysis by Walther et al. is well performed and adds to the body of evidence suggesting that testosterone treatment can lead to small improvements in men with depressive symptoms.
However, it is uncertain whether these improvements are clinically meaningful. The data should not be interpreted as testosterone treatment leads to remission or enhances response to antidepressant treatment in this population. In short, the current meta-analysis suggests testosterone replacement may enhance mood among nondepressed hypogonadal men. It is worth noting that the long-term safety of testosterone treatment remains unknown. Until more research is available, clinicians should continue to follow the Endocrine Society guideline for testosterone replacement therapy of androgen-deficient men. The data do not currently support the use of testosterone therapy, particularly in supraphysiologic doses, for the treatment of depressive disorders in men.
Shalender Bhasin, MD, is affiliated with the Brigham and Women’s Hospital in Boston, and Stuart Seidman, MD, is affiliated with Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 14. doi: 10.1001/jamapsychiatry.2018.2661). Dr. Bhasin reported receiving research grants from several sources, and Dr. Seidman reported no disclosures.
The role of testosterone in the pathophysiology and treatment of depressive disorders in men is controversial. The meta-analysis by Walther et al. is well performed and adds to the body of evidence suggesting that testosterone treatment can lead to small improvements in men with depressive symptoms.
However, it is uncertain whether these improvements are clinically meaningful. The data should not be interpreted as testosterone treatment leads to remission or enhances response to antidepressant treatment in this population. In short, the current meta-analysis suggests testosterone replacement may enhance mood among nondepressed hypogonadal men. It is worth noting that the long-term safety of testosterone treatment remains unknown. Until more research is available, clinicians should continue to follow the Endocrine Society guideline for testosterone replacement therapy of androgen-deficient men. The data do not currently support the use of testosterone therapy, particularly in supraphysiologic doses, for the treatment of depressive disorders in men.
Shalender Bhasin, MD, is affiliated with the Brigham and Women’s Hospital in Boston, and Stuart Seidman, MD, is affiliated with Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Nov 14. doi: 10.1001/jamapsychiatry.2018.2661). Dr. Bhasin reported receiving research grants from several sources, and Dr. Seidman reported no disclosures.
Testosterone treatment has potential as an adjunct therapy for men with depressive disorders, a meta-analysis has suggested. However, more research is needed.
“This meta-analysis provides important new evidence that testosterone treatment may also be effective and efficacious for eugonadal and older men when higher testosterone dosages are administered,” Andreas Walther, PhD, and his coauthors wrote Nov. 14 in JAMA Psychiatry. However, they noted that safety monitoring in testosterone treatment trials remained important because of an absence of sufficiently powered, long-term studies to assess the increased risk of adverse events with treatment.
The link between testosterone and depression has been debated extensively because testosterone is a neuroactive steroid hormone known to influence mood and appetitive behavior, Dr. Walther and his coauthors wrote. Although testosterone treatment for various disorders in hypogonadal men has been backed by evidence, the results of randomized, placebo-controlled clinical trials for its use in depression have been inconsistent. Indeed, testosterone treatment was currently not recommended in national or international guidelines because of an “prevailing uncertainty about its efficacy, age criteria, dosage, ideal duration and method of application,” wrote Dr. Walther, of the department of biological psychology at Technische Universität Dresden (Germany).
For the current review, the researchers identified 27 randomized, controlled trials altogether including 1,890 men who were receiving testosterone treatment and had reported depressive symptoms on validated depression scales.
Results showed evidence for a moderate antidepressant association of testosterone treatment, compared with placebo (Hedges g, 0.21; 95% confidence interval, 0.10-0.32), and an efficacy odds ratio of 2.30 (95% CI, 1.30-4.06). According to the researchers, based on reference ranges for depressive symptoms, “The National Institute for Health and Care Excellence guidelines on depression suggest a reduction of 3.0 and 2.0 points on BDI scores to be clinically significant for normal depression and treatment-resistant depression, respectively,” they wrote.
Testosterone treatment also showed an efficacy OR of 2.30, a finding that the authors said suggested the “potential of testosterone treatment as adjunct therapy for men with depressive disorders.”
They said the results suggested that better treatment response might require higher doses but acknowledged that the finding required replication.
In addition, Dr. Walther and his coauthors found acceptability of testosterone treatment was high, with an OR of 0.79 for testosterone treatment–related loss to follow-up, compared with placebo. Remarkably, they added, initial testosterone status did not moderate the effects of testosterone treatment on depressive symptoms.
“Large, preregistered RCTs of good quality investigating testosterone treatment’s effect in men on depression as the primary outcome” are needed, they concluded.
Dr. Walther and his coauthors cited a few limitations, including the low number of randomized, controlled trials addressing the effects of testosterone treatment in men who were depressed but otherwise healthy.
No conflicts of interest were reported.
SOURCE: Walther A et al. JAMA Psychiatry. 2018 14 Nov. doi: 10.1001/jamapsychiatry.2018.2734.
Testosterone treatment has potential as an adjunct therapy for men with depressive disorders, a meta-analysis has suggested. However, more research is needed.
“This meta-analysis provides important new evidence that testosterone treatment may also be effective and efficacious for eugonadal and older men when higher testosterone dosages are administered,” Andreas Walther, PhD, and his coauthors wrote Nov. 14 in JAMA Psychiatry. However, they noted that safety monitoring in testosterone treatment trials remained important because of an absence of sufficiently powered, long-term studies to assess the increased risk of adverse events with treatment.
The link between testosterone and depression has been debated extensively because testosterone is a neuroactive steroid hormone known to influence mood and appetitive behavior, Dr. Walther and his coauthors wrote. Although testosterone treatment for various disorders in hypogonadal men has been backed by evidence, the results of randomized, placebo-controlled clinical trials for its use in depression have been inconsistent. Indeed, testosterone treatment was currently not recommended in national or international guidelines because of an “prevailing uncertainty about its efficacy, age criteria, dosage, ideal duration and method of application,” wrote Dr. Walther, of the department of biological psychology at Technische Universität Dresden (Germany).
For the current review, the researchers identified 27 randomized, controlled trials altogether including 1,890 men who were receiving testosterone treatment and had reported depressive symptoms on validated depression scales.
Results showed evidence for a moderate antidepressant association of testosterone treatment, compared with placebo (Hedges g, 0.21; 95% confidence interval, 0.10-0.32), and an efficacy odds ratio of 2.30 (95% CI, 1.30-4.06). According to the researchers, based on reference ranges for depressive symptoms, “The National Institute for Health and Care Excellence guidelines on depression suggest a reduction of 3.0 and 2.0 points on BDI scores to be clinically significant for normal depression and treatment-resistant depression, respectively,” they wrote.
Testosterone treatment also showed an efficacy OR of 2.30, a finding that the authors said suggested the “potential of testosterone treatment as adjunct therapy for men with depressive disorders.”
They said the results suggested that better treatment response might require higher doses but acknowledged that the finding required replication.
In addition, Dr. Walther and his coauthors found acceptability of testosterone treatment was high, with an OR of 0.79 for testosterone treatment–related loss to follow-up, compared with placebo. Remarkably, they added, initial testosterone status did not moderate the effects of testosterone treatment on depressive symptoms.
“Large, preregistered RCTs of good quality investigating testosterone treatment’s effect in men on depression as the primary outcome” are needed, they concluded.
Dr. Walther and his coauthors cited a few limitations, including the low number of randomized, controlled trials addressing the effects of testosterone treatment in men who were depressed but otherwise healthy.
No conflicts of interest were reported.
SOURCE: Walther A et al. JAMA Psychiatry. 2018 14 Nov. doi: 10.1001/jamapsychiatry.2018.2734.
FROM JAMA PSYCHIATRY
Key clinical point: Testosterone appears to be moderately effective in reducing depressive symptoms in men.
Major finding: Testosterone treatment was associated with a significant reduction in depressive symptoms, compared with placebo, with an efficacy of odds ratio of 2.30 (95% confidence interval, 1.30-4.06).
Study details: A systematic review and meta-analysis involving 27 randomized, placebo-controlled trials involving a broad range of men who were treated with testosterone and reported depressive symptoms on validated depression scales.
Disclosures: No conflicts of interest were reported.
Source: Walther A et al. JAMA Psychiatry. 2018 Nov 14. doi: 10.1001/jamapsychiatry.2018.2734.
Influenza update 2018–2019: 100 years after the great pandemic
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
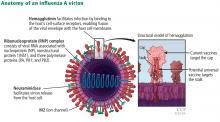
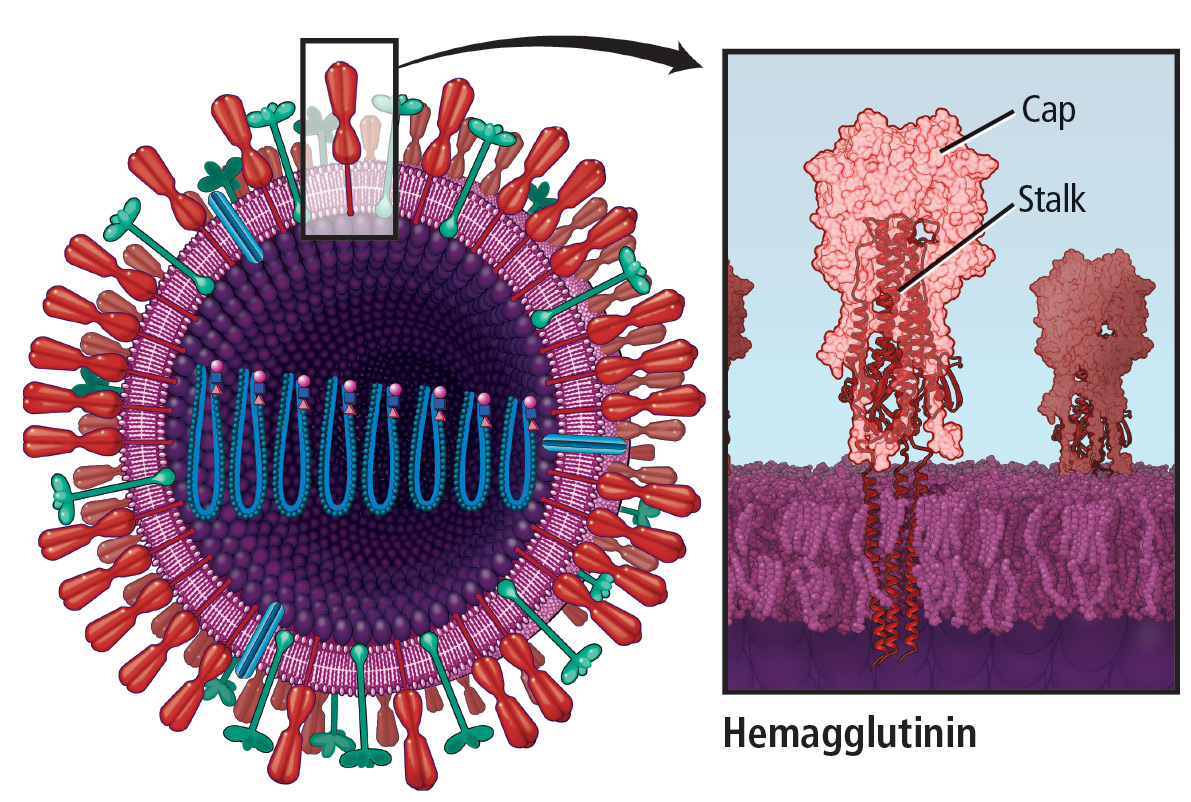
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
Men’s health 2018: BPH, prostate cancer, erectile dysfunction, supplements
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
Primary care physicians are tasked with a wide variety of issues affecting men. This article reviews the latest research in 4 areas of men’s health commonly addressed in primary care:
- Medical management of benign prostatic hyperplasia (BPH)
- Prostate cancer screening and treatment
- Medical management of erectile dysfunction
- Use of supplements.
MEDICAL MANAGEMENT OF BPH
An 84-year-old man with a history of hypertension, type 2 diabetes, hyperlipidemia, BPH, mild cognitive impairment, and osteoarthritis presents for a 6-month follow-up, accompanied by his son.
Two years ago he was started on a 5-alpha reductase inhibitor and an alpha-blocker for worsening BPH symptoms. His BPH symptoms are currently under control, with an American Urological Association (AUA) symptom index score of 7 of a possible 35 (higher scores being worse).
However, both the patient and son are concerned about the number of medications he is on and wonder if some could be eliminated.
Assessment tools
BPH is a common cause of lower urinary tract symptoms in older men. Evidence-based tools to help the clinician and patient decide on when to consider treatment for symptoms are:
- The AUA symptom index1
- The International Prostate Symptom Score (IPSS).2
An AUA symptom index score or IPSS score of 8 through 19 of a possible 35 is consistent with moderate symptoms, while a score of 20 or higher indicates severe symptoms.
Combination therapy or monotherapy?
Monotherapy with an alpha-blocker or a 5-alpha reductase inhibitor is often the first-line treatment for BPH-related lower urinary tract symptoms.3 However, combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor is another evidence-based option.
The Medical Therapy of Prostatic Symptoms study,4 a randomized controlled trial, reported that long-term combination therapy reduced the risk of BPH clinical progression better than monotherapy. The same trial also found that either combination therapy or finasteride alone (a 5-alpha reductase inhibitor) reduced the risk of acute urinary retention and the future need for invasive therapy.
Monotherapy after a period of combination therapy?
There is also evidence to support switching from combination to monotherapy after an initial treatment period.
Matsukawa et al5 examined the effects of withdrawing the alpha-blocker from BPH combination therapy in a study in 140 patients. For 12 months, all patients received the alpha-blocker silodosin and the 5-alpha reductase inhibitor dutasteride. At 12 months, the remaining 132 patients (8 patients had been lost to follow-up) were randomized to continue combination therapy or to take dutasteride alone for another 12 months. They were evaluated at 0, 12, and 24 months by questionnaires (the IPSS and Overactive Bladder Symptom Score) and urodynamic testing (uroflowmetry, cystometrography, and pressure-flow studies).
There were no significant differences in subjective symptoms and bladder outlet obstruction between patients who continued combination therapy and those who switched to dutasteride monotherapy. In the monotherapy group, those whose symptoms worsened weighed more (68.8 kg vs 62.6 kg, P =.002) and had a higher body mass index (BMI) (26.2 kg/m2 vs 22.8 kg/m2, P < .001) than those whose symptoms stayed the same or got better.
These findings of successful alpha-blocker withdrawal were consistent with those of other studies.
The Symptom Management After Reducing Therapy study6 showed that 80% of men with an IPSS score less than 20 who changed to dutasteride monotherapy did not have a noticeable worsening of their symptoms.
Baldwin et al7 noted similar success after withdrawing the alpha-blocker doxazosin in patients on finasteride.
Review all medications
The National Health and Nutrition Examination Survey noted that the estimated prevalence of polypharmacy increased from 8% in 1999 to 15% in 2011.8 Many commonly used medications, such as decongestants, antihistamines, and anticholinergic agents, can worsen BPH symptoms,9 so it is reasonable to consistently review the patient’s medications to weigh the risks and benefits and determine which ones align with the patient’s personal care goals.
BPH: Take-home points
- Combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH.
- Polypharmacy is a significant problem in the elderly.
- Withdrawing the alpha-blocker component from BPH combination therapy can be considered after 1 year of combination therapy in patients whose symptoms have been well controlled.
PROSTATE CANCER SCREENING AND TREATMENT
A 60-year-old patient calls you after receiving his laboratory testing report from his insurance physical. His prostate-specific antigen (PSA) level is 5.1 ng/mL, and he has several questions:
- Should he have agreed to the screening?
- How effective is the screening?
- What are the next steps?
Is PSA screening useful?
Over the last few years, there has been great debate as to the utility of screening for prostate cancer.
The US Centers for Disease Control and Prevention10 reported that in 2014, an estimated 172,258 men in the United States were diagnosed with prostate cancer, but only 28,343 men died of it. These statistics support the notion that screening programs may be detecting what might otherwise be a silent disease.
The US Preventive Services Task Force (USPSTF)11 recommends against blanket PSA screening, in view of the low probability that it reduces the risk of death from prostate cancer. For men ages 55 through 69, current guidelines give a grade C recommendation to PSA screening, meaning there is moderate agreement that the benefit is likely small, and screening should be selectively offered based on professional judgment and patient preference. In men ages 70 and older who are not at high risk, the guideline gives screening a grade D recommendation, meaning there is moderate evidence that there is no benefit from the practice. This is a change from the 2012 USPSTF guidelines,12 which gave a grade D recommendation to PSA screening for all ages.
The American Urological Association13 recommends against PSA screening in men under age 40 or ages 70 and older. It does not recommend routine screening in those ages 40 to 54 at average risk, but it says the decision should be individualized in this age group in those at higher risk (eg, with a positive family history, African American). At ages 55 through 69, it recommends shared decision-making, taking into account cancer risk and life expectancy. In those who opt for screening, an interval of 2 years or more may be preferred over annual screening to reduce the risk of overdiagnosis.
The USPSTF recommendations rely heavily on data from 2 trials: the European Randomized Study of Screening for Prostate Cancer (ERSPC)14 and the Prostate, Lung, Colorectal, and Ovarian Screening (PLCO) trial.15
The ERSPC14 demonstrated that screening for prostate cancer reduced deaths from prostate cancer by 20%, with an absolute risk difference of 0.71 deaths per 1,000 men; 1,410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer. Screening also decreased the risk of developing metastatic disease by 30%.16 On the negative side, screening increased the risk of overdiagnosis and other harms such as bleeding, sepsis, and incontinence.
The PLCO trial,15 in contrast, found no difference in death rates between men randomly assigned to annual screening and those assigned to usual care. Differences between the trial results were thought to be due to different practice settings as well as study implementation and compliance.
Tsodikov et al17 reanalyzed data from the ERSPC and the PLCO trial using 3 different mathematical models to estimate the effects of screening in both trials compared with no screening. The analysis found no evidence that the effects of screening vs not screening differed between the 2 trials, ultimately concluding that PSA screening reduced prostate cancer deaths by 25% to 32%, which the authors inferred was primarily a result of earlier detection of cancer.
The Cluster Randomized Trial of PSA Testing for Prostate Cancer,18 published in March 2018, explored the effect of single PSA screening vs no screening on prostate cancer mortality rates in 419,582 men ages 50 through 69. Although screening detected more cases of low-risk prostate cancer, there was no significant difference in prostate cancer mortality rates after a median follow-up of 10 years. However, 10% to 15% of the control group was estimated to have also been screened, and these results do not directly speak to the efficacy of serial PSA screening.
Extended follow-up of this trial is planned to report on long-term survival benefits and whether screening lowers the risk of metastasis.
Imaging-guided prostate biopsy
Once a patient is found to have an elevated PSA level, standard practice has been to perform transrectal ultrasonography to obtain 12 core biopsy samples. The results indicate whether the prostate contains cancer, how aggressive the cancer is (Gleason score), and whether there is extracapsular extension.
In the past, magnetic resonance imaging (MRI) of the prostate before biopsy was thought to be too costly, and many insurance plans do not currently cover it.
Pahwa et al,19 however, in a cost-effectiveness study using a decision-analysis model, found that using MRI to detect lesions and then guide biopsy by triaging patients into proper treatment pathways added health benefits in a cost-effective manner in 94.05% of simulations. These benefits were found across all age groups.
This study demonstrated that doctors could use MRI to better evaluate patients for potentially harmful lesions. If a focus of cancer is found, it can be biopsied; if no cancer is seen on MRI, the patient can avoid biopsy completely. Additionally, though MRI tended to miss low-risk cancers, these cancers are thought to disproportionately lead to higher healthcare costs through unnecessary treatment. Therefore, a negative MRI study was believed to be an excellent sign that the patient does not have aggressive prostate cancer. This approach led to a net gain of 0.251 additional quality-adjusted life years compared with the standard biopsy strategy.
The Prostate MRI Imaging Study20 also found MRI to be effective in the prostate cancer workup. In this trial, 576 men who had never undergone biopsy underwent multiparametric MRI, transrectal ultrasonography-guided biopsy, and the reference standard, ie, transperineal template prostate mapping biopsy. Of those who underwent biopsy, 71% received a diagnosis of prostate cancer, and 40% had clinically significant disease. In patients with clinically significant disease, MRI was more sensitive than ultrasonography-guided biopsy (93% vs 48%, P < .0001) but less specific (41% vs 96%, P < .0001).
Based on these findings, if biopsy were performed only in those who had suspicious lesions on MRI, 27% of men with elevated PSA could avoid biopsy and its potential complications such as bleeding and sepsis, which occurred in 5.9% of the biopsy group.
The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? trial21 more recently studied MRI with or without targeted biopsy vs standard transrectal ultrasonography-guided biopsy in 500 men who had not undergone biopsy before, and reported similar results. MRI with or without biopsy led to fewer biopsies and less overdetection of clinically insignificant prostate cancers compared with the standard approach. Furthermore, those in the MRI-targeted biopsy group were 13% less likely to receive a diagnosis of clinically insignificant cancer than those who received the standard biopsy (adjusted difference −13 percentage points, 95% confidence interval [CI] −19 to −7, P < .001).
Together, these data provide another argument for adding multiparametric MRI to the workup of men with an elevated PSA level.
Surveillance vs treatment for prostate cancer
Once prostate cancer is diagnosed, surveillance is becoming an increasingly common management strategy.
The Prostate Cancer Intervention Versus Observation Trial (PIVOT),22 one of the largest and longest trials involving cancer patients, offered further evidence that active surveillance and less intervention for men with prostate cancer is a better approach in many cases. This trial compared prostatectomy and observation alone in a randomized fashion. Inclusion for the study required men to be medically fit for radical prostatectomy, along with having histologically confirmed localized prostate cancer (stage T1-T2NxM0 in the tumor-node-metastasis classification system) of any grade diagnosed within the last 12 months.
During 19.5 years of follow-up, 223 (61.3%) of the 364 men randomly assigned to radical prostatectomy died, compared with 245 (66.8%) of 367 men in the observation group; the difference was not statistically different (P = .06). Only 9.4% of the deaths were due to prostate cancer, 7.4% in the surgery group and 11.4% in the observation group (P = .06).
Surgery was associated with a lower all-cause mortality rate than observation in the subgroup of patients with intermediate-risk prostate cancer (defined as PSA 10–20 ng/mL and a Gleason score of 7). Surgery was also associated with less disease progression.22
This finding is in line with previous data from the Scandinavian Prostate Cancer Group Study Number 4,23 as well as the much larger Prostate Testing for Cancer and Treatment (ProtecT) trial,24 both of which reported that metastasis was 1.5 and 2.6 times as common, respectively, in participants in the active surveillance groups. However, in the PIVOT trial, those in the surgery group were significantly more likely than those in the observation group to have erectile dysfunction and urinary incontinence at 10 years.
Therefore, in men with localized disease and in those with low-risk PSA levels, both the PIVOT and ProtecT trials suggest that death from prostate cancer is uncommon and that observation may be more appropriate.
Prostate cancer: Take-home points
- A new look at 2 large trials of PSA screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial that found no benefit from 1-time screening may reopen debate on the topic.
- MRI offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Surgery for prostate cancer may not prolong life but could reduce disease progression, at the risk of more adverse effects.
- Shared decision-making should be practiced when deciding whether to use active surveillance or active treatment of diagnosed prostate cancer.
MANAGEMENT OF ERECTILE DYSFUNCTION
A 62-year-old man with hypertension, hyperlipidemia, peripheral artery disease, and type 2 diabetes presents for a 6-month follow-up. His medications include aspirin, metformin, lisinopril, and atorvastatin, all of which he takes without problems. Over the past several months, he has noticed that his erections are not adequate for sexual intercourse. He recently heard that a generic version of sildenafil has just become available, and he wonders if it might benefit him.
Erectile dysfunction is common, associated with chronic diseases
Erectile dysfunction, ie, persistent inability to obtain and maintain an erection sufficient to permit satisfactory sexual intercourse,25,26 is estimated to affect nearly 20% of men over the age of 20 and 75% of men over the age of 75.27
In age-adjusted models, erectile dysfunction has been shown28 to be associated with:
- History of cardiovascular disease (odds ratio [OR] 1.63, 95% CI 1.02–2.63)
- Diabetes (OR 3.90, 95% CI 2.16–7.04)
- Treated hypertension vs no hypertension (OR 2.22, 95% CI 1.30–3.80)
- Current smoking vs never smoking (OR 1.63, 95% CI 1.01–2.62)
- BMI greater than 30 kg/m2 vs less than 25 kg/m2 (OR 1.80, 95% CI 1.03–3.14).
Because of the strong association between cardiovascular disease and erectile dysfunction, the presence of one often suggests the need to screen for the other.29 While tools such as the International Index of Erectile Function (IIEF-5) have been developed to evaluate erectile dysfunction, it is most often diagnosed on the basis of clinical impression, while validated assessment methods are reserved for clinical trials.28
Multiple causes of erectile dysfunction
Erectile dysfunction arises from inadequate penile tissue response to a sexual signal. The response can be disrupted at several points. For example, damage to vascular smooth muscle cells (eg, from age or obesity) and endothelial cells (from smoking or diabetes) and narrowing of the vascular lumen (from atherosclerosis or hypertension) have all been shown to impair engorgement of the corpus cavernosum.30 In addition, denervation from prostate surgery or spinal trauma and psychogenic causes should be recognized in discussions with patients.
Drugs for erectile dysfunction
Pharmacologic management of erectile dysfunction includes oral, sublingual, intracavernosal, and intraurethral therapies.31 Treatment in primary care settings usually includes addressing underlying chronic diseases32 and prescribing phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil). These drugs work by increasing local concentrations of cyclic guanosine monophosphate in the corpus cavernosum to induce vasodilation.33
While these 4 drugs are still patent-protected, a manufacturer has been allowed to introduce a generic version of sildenafil into US markets, and a generic version of tadalafil is expected to be available soon.
Sildenafil, tadalafil, and vardenafil have been studied and found to have some degree of effectiveness in erectile dysfunction caused by damage to the penile vasculature, denervation, and spinal cord injury.34 All drugs of this class have adverse effects including headache, facial flushing, and nasal congestion, but the drugs are generally well tolerated.35
Sildenafil and tadalafil improve IIEF-5 scores by a similar margin, raising scores on the erectile domain subsection from approximately 14 of a possible 30 to approximately 24 of 30 in a trial of both drugs.36 However, multiple crossover studies comparing the 2 drugs have shown that nearly 75% of patients prefer tadalafil to sildenafil,36,37 perhaps because of tadalafil’s longer duration of action.34
There is little evidence to suggest that vardenafil is more effective or more often preferred by patients than tadalafil or sidenafil.34,38 And though data on the newest drug on the market, avanafil, are limited, a meta-analysis concluded that it may be less effective than tadalafil and without significant differences in terms of safety.39
Other treatments
Lifestyle modifications, especially smoking cessation and exercise, have been shown to reduce the risk of erectile dysfunction with varying effect sizes across studies.40–42 Moreover, factors such as obesity, alcohol use, and smoking may cause irreversible harm, and thus a healthy lifestyle should be encouraged.41
While there is only weak evidence for the use of psychological interventions alone for treating most types of erectile dysfunction, one meta-analysis found that the combination of psychological intervention and a phosphodiesterase-5 inhibitor improved sexual satisfaction more than drug therapy alone.43
Erectile dysfunction: Take-home points
- Erectile dysfunction is common, affecting nearly 20% of men over the age of 20 and over 75% of men over the age of 75.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
- Treating underlying chronic diseases may help, and phosphodiesterase-5 inhibitors are effective; tadalafil may be most often preferred.
SUPPLEMENT USE AND MEN’S HEALTH
A 68-year-old man with a history of hypertension, BPH, and erectile dysfunction presents for a 6-month follow-up. His medication use includes lisinopril, which he takes without problems. He denies any new physical symptoms. His physical examination is unremarkable. He says he has heard about supplements that might help with his sexual performance and hopes to discuss recommendations during the visit.
A burgeoning, unregulated industry
Since the passage of the Dietary Supplement and Health Education Act in 1994, a law that decreased oversight of the supplement industry, spending on supplements has skyrocketed to over $41.1 billion each year.44 Advertisements for these products typically claim that they improve general mental and physical health, sexual and romantic performance, leanness, and muscularity.45 A national survey of men ages 57 and older reported that the most popular products were aimed at nutrition (such as multivitamins), cardiovascular health (such as omega-3 fatty acids), and chronic conditions (such as saw palmetto for BPH).46
Little evidence of efficacy
There is little evidence to support the use of most supplements to improve men’s health. For example, a study in 82,405 men found no association between mortality rates and multivitamin use (hazard ratio [HR] 1.07, 95% CI 0.96–1.19).47 Even for specific uses, such as cognitive performance, randomized trials exploring the effects of multivitamins in men have been largely negative.48
The positive trials that have been reported are often of low quality and are funded by supplement manufacturers. For example, one of the few trials that reported a positive association between multivitamin supplementation and cognition in men was underpowered (N = 51) and found improvement in only 1 of 19 cognitive domains.49 Despite the poor design and results to the contrary, this industry-funded study nevertheless concluded that multivitamins may play a role in improving elements of memory.
Evidence of possible harm from antioxidants
While not always specific to men, many meta-analyses have explored the effects of antioxidant supplements on cardiovascular and mortality risk. Most of them concluded that antioxidant supplements have no benefit and that some may actually be harmful.
For example, multiple meta-analyses of vitamin E supplementation found no cardiovascular benefit but possible increases in all-cause mortality rates in those taking high doses (risk ratio 1.04, 95% CI 1.01–1.07).50,51
Another meta-analysis of 180,938 participants in high-quality studies found an increased risk of all-cause mortality associated with independent intake of several antioxidant vitamins, including beta-carotene (risk ratio 1.07, 95% CI 1.02–1.11) and vitamin A (risk ratio 1.16, 95% CI 1.10–1.24), while intake of vitamin C and selenium had no impact on mortality.52
Similarly, although nearly 10% of US adults report taking omega-3 fatty acid supplements, a review of 24 randomized controlled trials and meta-analyses published between 2005 and 2012 concluded that only 2 supported the use of these supplements for any health benefit.53
Can supplements improve sexual function, prostate health?
To improve sexual function. A 2015 narrative review of the ingredients in General Nutrition Center’s top 30 best-selling products targeted at improving men’s sexual performance (including improving libido and erectile dysfunction) found only poor evidence for any efficacy.54 The few studies that did support the use of select supplements, including B vitamins in people with diabetes, L-arginine, and yohimbine, were deemed to be of poor quality or showed a smaller effect size compared with standard medical therapy.
To prevent prostate cancer. Studies of supplement use to improve prostate health have had mixed results. For example, multiple large case-control studies have suggested that taking vitamin D55,56 or vitamin C57 is not associated with prostate cancer risk, while increased vitamin A58,59 and E60,61 intake is associated with inconsistent increases in prostate cancer risk.
In the Selenium and Vitamin E Cancer Prevention Trial,62 a randomized controlled trial in 35,533 men, those assigned to receive vitamin E supplementation were 17% more likely to get prostate cancer than were those assigned to placebo (HR 1.17, 99% CI 1.004–1.36, P = .008).
However, there are plausible biologic links between nutraceuticals and prostate cancer. For example, studies have linked genetic polymorphisms in vitamin D receptors63 as well as intake of natural androgen receptor modulators, such as the most active polyphenol in green tea,64 to prostate cancer risk and aggressiveness in certain populations. This led a recent review to conclude that there is some biologic plausibility, but at present little epidemiologic evidence, to support any dietary supplement’s ability to broadly affect prostate cancer risk.65
Interest continues in exploring the targeted use of nutraceuticals as adjuvant therapy in specific populations at risk of prostate cancer.66,67
To treat BPH. There is a similar dearth of clinical or population-based evidence that supplements can broadly affect BPH symptoms. For example, in a 2012 Cochrane review of Serenoa repens (saw palmetto) utilizing only high-quality evidence, there was no evidence that supplement use significantly reduced lower urinary tract symptoms, nocturia, or peak urine flow in BPH patients, and this was true even when the supplement was taken at triple-strength doses.68
For other diseases. There is also limited evidence that supplements can affect other chronic diseases. For example, a meta-analysis of 3,803 patients found that glucosamine, chondroitin, and their combination had no impact on joint pain or joint space narrowing in patients with osteoarthritis of the knee or hip.69
Even when there is some evidence to suggest benefit from supplementation, study heterogeneity and varying evidence quality limit confidence in the conclusions. For example, meta-analyses suggest garlic may improve blood pressure control in those with hypertension70 and improve lipid and blood glucose control in type 2 diabetes.71 However, most of the trials included in those systematic reviews were underpowered, with samples as low as 10 patients, and many suffered from improper design, such as inadequate blinding of researchers. In addition, these meta-analyses often do not report adverse events, suggesting that higher quality studies would be needed to adequately measure event rates. As such, there is need for caution and a case-by-case review before recommending even a seemingly benign supplement like garlic to patients.
In total, there is only limited evidence to support the efficacy of supplements across many diseases and concerns common to men in primary care. This includes improving general health, cardiovascular health, sexual functioning, or other chronic diseases. While a supplement’s placebo effect may at times provide some benefit, supplements are much less strictly regulated since the passing of the 1994 act, and even vitamin supplementation has been shown to be associated with negative health outcomes. As such, a patient’s use of supplements requires careful consideration and shared decision-making.
Supplements: Take-home points
- Supplements are only loosely regulated by the federal government.
- There is some biologic but limited epidemiologic evidence for the use of multivitamins to improve cognition or mortality rates; for the use of antioxidant vitamins or omega-3 fatty acids to improve cardiovascular health; for the use of any of the top-selling sexual enhancement supplements to improve libido or erectile function; and for the use of vitamins or other supplements for improving BPH or reducing prostate cancer risk. Using supplements may in some cases be harmful.
- Given the heterogeneity of studies of supplements to manage chronic diseases and a lack of reporting of adverse events, careful consideration is needed when recommending supplements to patients.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017; 197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
- Urological Sciences Research Foundation. International Prostate Symptom Score (IPSS). http://www.usrf.org/questionnaires/AUA_SymptomScore.html. Accessed October 16, 2018.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185(5):1793–1803. doi:10.1016/j.juro.2011.01.074
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349(25):2387–2398. doi:10.1056/NEJMoa030656
- Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017; 198(4):905–912. doi:10.1016/j.juro.2017.05.031
- Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5a-reductase inhibitor dutasteride. Eur Urol 2003; 44(4):461–466. pmid:14499682
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha-blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001; 58(2):203–209. pmid:11489700
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314(17):1818–1831. doi:10.1001/jama.2015.13766
- DuBeau CE, Yalla SV, Resnick NM. Improving the utility of urine flow rate to exclude outlet obstruction in men with voiding symptoms. J Am Geriatr Soc 1998; 46(9):1118–1124. pmid:9736105
- US Department of Health and Human Services Health Resources and Services Administration. United States Cancer Statistics: 1999-2014 Incidence and Mortality Web-Based Report. Atlanta; 2017. https://nccd.cdc.gov/uscs/. Accessed October 17, 2018.
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 16, 2018.
- US Preventive Services Task Force. Archived: prostate cancer: screening. Original release date: May 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed October 16, 2018.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol 2013; 190(2):419–426. doi:10.1016/j.juro.2013.04.119
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360(13):1320–1328. doi:10.1056/NEJMoa0810084
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009; 360(13):1310–1319. doi:10.1056/NEJMoa0810696
- Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62(5):745–752. doi:10.1016/j.eururo.2012.05.068
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018; 319(9):883–895. doi:10.1001/jama.2018.0154
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging–guided strategies for detection of prostate cancer in biopsy-naive men. Radiology 2017; 285(1):157–166. doi:10.1148/radiol.2017162181
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017; 377(2):132–142. doi:10.1056/NEJMoa1615869
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370(10):932–942. doi:10.1056/NEJMoa1311593
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375(15):1415–1424. doi:10.1056/NEJMoa1606220
- Morley JE. Impotence. Am J Med 1986; 80(5):897–905. pmid:3518438
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270(1):83–90. pmid:8510302
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007; 120(2):151–157. doi:10.1016/j.amjmed.2006.06.010
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002; 14(4):226–244. doi:10.1038/sj.ijir.3900857
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65(5):968–978. doi:10.1016/j.eururo.2013.08.023
- Heaton JPW, Adams MA. Causes of erectile dysfunction. Endocrine 2004; 23(2-3):119–123. doi:10.1385/ENDO:23:2-3:119
- Montorsi F, Salonia A, Deho F, et al. Pharmacological management of erectile dysfunction. BJU Int 2003; 91(5):446–454. pmid:12603396
- Cai X, Tian Y, Wu T, Cao CX, Bu SY, Wang KJ. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014; 16(3):461–466. doi:10.4103/1008-682X.123678
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure–lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 1999; 83(5):21C–28C. pmid:10078539
- Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6(1):75–84. doi:10.1517/14656566.6.1.75
- Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res 2002; 7(10):435–446. pmid:12435622
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005; 96(9):1323–1332. doi:10.1111/j.1464-410X.2005.05892.x
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol 2004; 45(4):499–509. doi:10.1016/j.eururo.2003.11.030
- Martin-Morales A, Haro JM, Beardsworth A, Bertsch J, Kontodimas S; EDOS Group. Therapeutic effectiveness and patient satisfaction after 6 months of treatment with tadalafil, sildenafil, and vardenafil: results from the erectile dysfunction observational study (EDOS). Eur Urol 2007; 51(2):541–550. doi:10.1016/j.eururo.2006.09.027
- Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63(5):902–912. doi:10.1016/j.eururo.2013.01.012
- Cao S, Yin X, Wang Y, Zhou H, Song F, Lu Z. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013; 8(4):e60443. doi:10.1371/journal.pone.0060443
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000; 56(2):302–306. pmid:10925098
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291(24):2978–2984. doi:10.1001/jama.291.24.2978
- Schmidt HM, Munder T, Gerger H, Frühauf S, Barth J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014; 11(6):1376–1391. doi:10.1111/jsm.12520
- New Hope Network. Supplement Business Report 2017. Boulder; 2017. http://images.info.newhope.com/Web/NewHopeNaturalMedia/%7B3a3f3b03-6130-41d4-9e66-84f29eeebe44%7D_2017_Supplement_Business_Report_-_Extended_TOC.pdf. Accessed October 16, 2018.
- Labre MP. Burn fat, build muscle: a content analysis of men’s health and men’s fitness. Int J Mens Health 2005; 4(2):187–200.
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008; 300(24):2867–2878. doi:10.1001/jama.2008.892
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol 2011; 173(8):906–914. doi:10.1093/aje/kwq447
- McNeill G, Avenell A, Campbell MK, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J 2007; 6(1):10. doi:10.1186/1475-2891-6-10
- Harris E, Macpherson H, Vitetta L, Kirk J, Sali A, Pipingas A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum Psychopharmacol Clin Exp 2012; 27(4):370–377. doi:10.1002/hup.2236
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003; 361(9374):2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142(1):37–46. pmid:15537682
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297(8):842–857. doi:10.1001/jama.297.8.842
- Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med 2014; 174(3):460–462. doi:10.1001/jamainternmed.2013.12765
- Cui T, Kovell RC, Brooks DC, Terlecki RP. A urologist’s guide to ingredients found in top-selling nutraceuticals for men’s sexual health. J Sex Med 2015; 12(11):2105–2117. doi:10.1111/jsm.13013
- Schenk JM, Till CA, Tangen CM, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Prev Biomarkers 2014; 23(8):1484–1493. doi:10.1158/1055-9965.EPI-13-1340
- Albanes D, Mondul AM, Yu K, et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Prev Biomarkers 2011; 20(9):1850–1860. doi:10.1158/1055-9965.EPI-11-0403
- Roswall N, Larsen SB, Friis S, et al. Micronutrient intake and risk of prostate cancer in a cohort of middle-aged, Danish men. Cancer Causes Control 2013; 24(6):1129–1135. doi:10.1007/s10552-013-0190-4
- Mondul AM, Watters JL, Männistö S, et al. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011; 173(7):813-821. doi:10.1093/aje/kwq429
- Schenk JM, Riboli E, Chatterjee N, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Prev Biomarkers 2009; 18(4):1227–1231. doi:10.1158/1055-9965.EPI-08-0984
- Bidoli E, Talamini R, Zucchetto A, et al. Dietary vitamins E and C and prostate cancer risk. Acta Oncol 2009; 48(6):890–894. doi:10.1080/02841860902946546
- Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Prev Biomarkers 2007; 16(6):1128–1135. doi:10.1158/1055-9965.EPI-06-1071
- Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14):1549–1556. doi:10.1001/jama.2011.1437
- Jingwi EY, Abbas M, Ricks-Santi L, et al. Vitamin D receptor genetic polymorphisms are associated with PSA level, Gleason score and prostate cancer risk in African-American men. Anticancer Res 2015; 35(3):1549–1558. pmid:25750310
- Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011; 25(4):1198–1207. doi:10.1096/fj.10-167924
- Yacoubian A, Dargham RA, Khauli RB, Bachir BG. Overview of dietary supplements in prostate cancer. Curr Urol Rep 2016; 17(11):78. doi:10.1007/s11934-016-0637-8
- Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol 2016; 40:160–169. doi:10.1016/j.semcancer.2016.06.003
- Shui IM, Mondul AM, Lindström S, et al. Circulating vitamin D, vitamin D–related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015; 121(12):1949–1956. doi:10.1002/cncr.29320
- Tacklind J, MacDonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. doi:10.1002/14651858.CD001423.pub3
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675. doi:10.1136/bmj.c4675
- Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother 2008; 42(12):1766–1771. doi:10.1345/aph.1L319
- Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res 2017; 61(1):1377571. doi:10.1080/16546628.2017.1377571
KEY POINTS
- The combination of an alpha-blocker and a 5-alpha reductase inhibitor is an effective regimen for BPH. Withdrawing the alpha-blocker from the combination can be considered if symptoms have been well controlled after 1 year of combination therapy.
- A new look at 2 large trials of prostate-specific antigen screening strengthened evidence that testing in the right patient population can reduce deaths from prostate cancer, but a third recently published trial found no benefit to 1-time screening.
- Magnetic resonance imaging offers a better method than ultrasonography-guided biopsy to triage patients thought to be at high risk of prostate cancer and tends to limit costly overtreatment of disease that likely would not cause death.
- Erectile dysfunction is often associated with chronic disease and may suggest the need to screen for cardiovascular disease.
Bisphosphonate-related atypical femoral fracture: Managing a rare but serious complication
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
KEY POINTS
- The benefits of bisphosphonate therapy in reducing fracture risk outweigh the risk of atypical fracture.
- Bisphosphonate use for longer than 5 years greatly increases the risk of atypical femoral fracture.
- Treatment of atypical femoral fracture varies depending on whether the patient has pain and whether the fracture is complete or incomplete.
How acute pain leads to chronic opioid use
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
PSA screening: Back to the future
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
My urologic career began in the late 1980s, just before prostate-specific antigen (PSA) testing was introduced. Ever since, a busy prostate cancer practice has given me a frontline view of the benefits and possible harms of PSA screening.
In the pre-PSA era, about half of men with newly diagnosed prostate cancer presented with incurable disease, either locally advanced or metastatic. The most common treatment was bilateral orchiectomy, which was the only safe form of androgen deprivation available.
Fast-forward a few years to the mid-1990s. Within 5 years after the introduction of PSA testing, the rate of incurable disease at diagnosis fell to just 5%, and treatment for localized disease skyrocketed, including radical prostatectomy, external beam radiation, and brachytherapy. As a result of earlier diagnosis and improved treatments, the death rate from prostate cancer in US men has fallen more than 30% since 1990.
The first-hand experience of seeing this massive stage migration to curable disease has forever convinced me that PSA screening is beneficial. Robust statistical models lend credence to this belief, with estimates that screening is responsible for 45% to 70% of this decline in mortality.1
Fast-forward again to 2012, when the US Preventive Services Task Force (USPSTF) published a strong recommendation against screening. The recommendation had so much force that as recently as 2014, only 11% of men at highest risk of prostate cancer in the Cleveland Clinic system were screened for it,2 mirroring national trends.
What happened? Colored by the experience in the era before PSA, when men presented frequently with painful metastatic disease and had an average life expectancy of 18 to 24 months, it was widely believed that all detected prostate cancer required treatment. What was not appreciated was that while PSA detects lots of prostate cancer, the most common reason for PSA levels to reach a range worrisome enough to trigger biopsy was actually benign prostatic hypertrophy.
The resulting increase in the number of biopsies resulted in the detection of a substantial number of low-grade cancers that were never destined to cause clinical harm but that got treated anyway, based on the fear that all cancers had metastatic potential. The USPSTF based its recommendation against screening on the harms caused by this overdetection and overtreatment of nonlethal disease, focusing on risks of biopsy such as sepsis, and on treatment-related adverse effects such as changes in urinary, bowel, and sexual function.
RANDOMIZED TRIALS SHOW A BENEFIT FROM SCREENING
As a result of this controversy, several large randomized trials designed to test whether PSA screening was beneficial were organized and begun in the 1990s, with one in the United States and another in Europe.3,4 Mature data from both trials have now established that there is indeed benefit to population-level screening.
The US Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), was initially reported to show no difference in prostate cancer-specific mortality rates in those screened vs not screened, but because more than 90% of the men in the no-screening arm were screened anyway, that conclusion is erroneous.3
With 13-year follow-up and far less PSA contamination in the unscreened arm, the European Randomized Study of Screening for Prostate Cancer (ERSPC) in men ages 55 to 69 demonstrated a 27% reduction in the rate of death and a 35% reduction in the need for palliative treatments (androgen deprivation or radiation, or both) for metastatic disease in those screened vs not screened, clearly establishing substantial clinical benefit to PSA screening.4
A recent analysis of both trials that controlled for PSA drop-ins (comparing those actually screened with those actually not screened) concluded that the benefit of screening in terms of mortality reduction (estimated at about 30%) are equal in both trials.5 A large cohort study from Kaiser Permanente with 16-year follow-up has suggested that PSA screening has both a prostate cancer-specific benefit and an overall mortality benefit.6
ACTIVE SURVEILLANCE CAN REDUCE OVERTREATMENT
In parallel with the design and completion of these trials, there was a significant effort to better identify and manage patients initially overdiagnosed with nonlethal cancers by developing active surveillance regimens.
This management strategy recognizes that most low-grade cancers pose no short-term risk to the patient’s health or longevity, that definitive therapy can be deferred, and that with regular monitoring by digital rectal examination, PSA measurement, and repeat biopsy, cancers that progress can still be cured. The result of this strategy is a marked reduction in the harms caused by overtreatment (ie, the aforementioned adverse effects), as well as the avoidance of unnecessary treatment in many patients.
A randomized trial and 2 large prospective cohort studies have confirmed the long-term safety of this approach,7–9 and the development of commercially available, biopsy-based gene expression profiling tools promises to further improve risk stratification at diagnosis and during follow-up for individual patients.10
NEW USPSTF RECOMMENDATIONS: AN INDIVIDUAL, INFORMED DECISION
Based on the results of the ERSPC and the widespread adoption and safety of active surveillance, which together show benefit to screening and fewer harms in overdetection and overtreatment, in 2018 the USPSTF recast its recommendations. In upgrading the recommendation from “D” to “C,” the recommendation now states that for men ages 55 to 69, PSA screening should be an individual decision after a discussion with an informed provider, although men over 70 are still advised not to undergo screening at all.11
Some may think that this recommendation has arrived just in time, or that it should be made even stronger to actually recommend screening, as recent data from 2 national registries—the Surveillance, Epidemiology, and End Results program and the National Cancer Database—show that the fall in screening after the 2012 USPSTF guidelines has resulted in an increase in men presenting with advanced stage disease.12,13 (All of you Back to the Future fans, please return to the mid to late 1980s to see how that plays out.)
So the pendulum has now swung back in favor of screening, largely supported by solid data showing meaningful clinical benefit, better understanding of PSA and prostate cancer biology, and adoption of active surveillance.
AN IDEAL SCREENING PROGRAM
An ideal screening program would detect only biologically significant cancers, thus eliminating overdetection and overtreatment. There is reason for optimism on this front.
Second-generation PSA tests have better diagnostic accuracy for high-grade disease than earlier tests. Two such tests, the Prostate Health Index (Beckman Coulter) and the 4K-score (Opko Health), are commercially available though not usually covered by commercial insurers.14 A third test, IsoPSA (Cleveland Diagnostics), is under development. Most hospital laboratories will be able to be run this test with no need for a central laboratory.15 All 3 tests have been shown to reduce unnecessary biopsies (because of a low probability of finding a biologically significant cancer) by 30% to 45% and will help reduce overdetection.
Moreover, multiparametric magnetic resonance imaging of the prostate has been shown to improve detection of high-grade cancers,16 and a randomized trial has suggested that its incorporation into a screening strategy is cost-effective and could be better than PSA testing plus transrectal ultrasonography alone (the current standard of care).17
Several risk scores based on germline genomics also hold promise for better identifying those at risk and for helping to de-intensify screening for those unlikely to have high-grade cancer.18
Screening for prostate cancer reduces mortality rates and the burden of metastatic disease, and the paradigm continues to evolve. Men at risk by virtue of age (55 to 69, and healthy men > 70), family history, race, and newly identified factors (germline genetics) all deserve an informed discussion on the benefits and risks of screening
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int 2017; 120(2):257–264. doi:10.1111/bju.13793
- Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med 2016; 374(18):1795–1796. doi:10.1056/NEJMc1515131
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2017; 167(7):449–455. doi:10.7326/M16-2586
- Alpert PF. New evidence for the benefit of prostate-specific antigen screening: data from 400,887 Kaiser Permanente patients. Urology 2018; 118:119–126. doi:10.1016/j.urology.2018.02.049
- Lane JA, Donovan JL, Davis M, et al; ProtecT Study Group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15(10):1109–1118. doi:10.1016/S1470-2045(14)70361-4
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33(3):272–277. doi:10.1200/JCO.2014.55.1192
- Nyame YA, Grimberg DC, Greene DJ, et al. Genomic scores are independent of disease volume in men with favorable risk prostate cancer: implications for choosing men for active surveillance. J Urol 2018; 199(2):438–444. doi:10.1016/j.juro.2017.09.077
- US Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed October 2, 2018.
- Negoita S, Feuer EJ, Mariotto A, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer 2018; 124(13):2801–2814. doi:10.1002/cncr.31549
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19(4):395–397. doi:10.1038/pcan.2016.30
- Loeb S. Biomarkers for prostate biopsy and risk stratification of newly diagnosed prostate cancer patients. Urol Pract 2017; 4(4):315–321. doi:10.1016/j.urpr.2016.08.001
- Klein EA, Chait A, Hafron JM, et al. The single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017; 72(6):942–949. doi:10.1016/j.eururo.2017.03.025
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313(4):390–397. doi:10.1001/jama.2014.17942
- Kasivisvanathan V, Rannikko AS, Borghi M, et al; PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–1777. doi:10.1056/NEJMoa1801993
- Seibert TM, Fan CC, Wang Y, et al. PRACTICAL Consortium. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018; 360:j5757. doi:10.1136/bmj.j5757