User login
Chronic insomnia afflicts one in three perimenopausal women
LAS VEGAS – Sleeplessness is both common and distressing for women in midlife, according to a new analysis of data from a large observational study.
One-third of women at all stages of the menopausal transition reported sleep disturbance that met the clinical criteria for insomnia, regardless of many markers of health and socioeconomic status, Colleen L. Ciano, Ph.D., reported at the NAMS 2015 annual meeting.
Acute insomnia is prevalent in adults, and women are known to suffer more than men from chronic insomnia. Perimenopausal women, in particular, may have increased sleep disturbances.
Dr. Ciano, a postdoctoral fellow at the University of Pennsylvania, Philadelphia, and her colleagues used data from the large, longitudinal Study of Women’s Health Across the Nation (SWAN) to ascertain insomnia’s prevalence in perimenopausal and surgically menopausal women. Using SWAN study data, she also attempted to tease out factors that might increase a woman’s risk for chronic insomnia.
SWAN, said Dr. Ciano, was designed to examine the health of midlife women, gathering data from 3,302 women with a mean age of 45.9 years. Ten years of study data are now available from an ethnically diverse sample of women participating at seven sites across the United States.
Four questions from the SWAN questionnaire were identified for analysis as dependent variables. The questions related to trouble falling asleep, nighttime wakings, early-morning wakings, and a subjective rating of sleep quality over 2 weeks. Women were stratified into early or late menopause, or surgical menopause.
More than one-third of women at all stages of the midlife transition, or who were in surgical menopause, reported diagnosable insomnia. On the whole, insomnia increased for women over the study period. Waking after sleep onset also increased for perimenopausal women as they moved through menopause, from 26% to 36%. Sleep quality, as a bifurcated measure of sleep quality derived from a four-point scale, also dropped over the study period.
During the SWAN data collection period, the number of perimenopausal women who met at least one of the American Academy of Sleep Medicine criteria for insomnia rose from just over 30% to just over 40%, while the number of women who reported no insomnia symptoms fell steeply, dropping from just under 70% to approximately 50%.
When Dr. Ciano analyzed the prevalence of insomnia by perimenopausal stage, she found more insomnia in late-perimenopausal women (prevalence 31%-48%), compared with those in early menopause (31%-59%).
Women in late perimenopause had an odds ratio of 1.3 of reporting any symptom of insomnia, compared with those in early menopause, according to Dr. Ciano.
Women who were heavier, older, or depressed, as well as those having night sweats, were all more likely to suffer from insomnia on multivariable analysis (all P=0.002 or less). Factors that did not influence insomnia included marital status, tobacco or alcohol use, race/ethnicity, socioeconomic status, and many comorbidities including diabetes, hypertension, and cardiovascular disease.
Dr. Ciano advised clinicians to pay attention to these findings and initiate insomnia screenings. Routine evaluation of perimenopausal women should include careful questioning and an assessment of sleep patterns and problems. This is especially important since obesity, diabetes, and cardiovascular disease are all prevalent chronic illnesses that can be impacted by insomnia, she said.
Future studies should include physiologic measures and objective sleep data, which could be correlated with subjective reports, said Dr. Ciano. Sleep diaries can be an adjunct to observational and objective data, and can be correlated with such objective measures as endogenous hormone levels and objective vasomotor symptom monitoring.
Dr. Ciano’s research was supported by the National Institute for Nursing Research, Pennsylvana State University, and the Beta Sigma chapter of Sigma Theta Tau. She reported having no relevant financial disclosures.
On Twitter @karioakes
LAS VEGAS – Sleeplessness is both common and distressing for women in midlife, according to a new analysis of data from a large observational study.
One-third of women at all stages of the menopausal transition reported sleep disturbance that met the clinical criteria for insomnia, regardless of many markers of health and socioeconomic status, Colleen L. Ciano, Ph.D., reported at the NAMS 2015 annual meeting.
Acute insomnia is prevalent in adults, and women are known to suffer more than men from chronic insomnia. Perimenopausal women, in particular, may have increased sleep disturbances.
Dr. Ciano, a postdoctoral fellow at the University of Pennsylvania, Philadelphia, and her colleagues used data from the large, longitudinal Study of Women’s Health Across the Nation (SWAN) to ascertain insomnia’s prevalence in perimenopausal and surgically menopausal women. Using SWAN study data, she also attempted to tease out factors that might increase a woman’s risk for chronic insomnia.
SWAN, said Dr. Ciano, was designed to examine the health of midlife women, gathering data from 3,302 women with a mean age of 45.9 years. Ten years of study data are now available from an ethnically diverse sample of women participating at seven sites across the United States.
Four questions from the SWAN questionnaire were identified for analysis as dependent variables. The questions related to trouble falling asleep, nighttime wakings, early-morning wakings, and a subjective rating of sleep quality over 2 weeks. Women were stratified into early or late menopause, or surgical menopause.
More than one-third of women at all stages of the midlife transition, or who were in surgical menopause, reported diagnosable insomnia. On the whole, insomnia increased for women over the study period. Waking after sleep onset also increased for perimenopausal women as they moved through menopause, from 26% to 36%. Sleep quality, as a bifurcated measure of sleep quality derived from a four-point scale, also dropped over the study period.
During the SWAN data collection period, the number of perimenopausal women who met at least one of the American Academy of Sleep Medicine criteria for insomnia rose from just over 30% to just over 40%, while the number of women who reported no insomnia symptoms fell steeply, dropping from just under 70% to approximately 50%.
When Dr. Ciano analyzed the prevalence of insomnia by perimenopausal stage, she found more insomnia in late-perimenopausal women (prevalence 31%-48%), compared with those in early menopause (31%-59%).
Women in late perimenopause had an odds ratio of 1.3 of reporting any symptom of insomnia, compared with those in early menopause, according to Dr. Ciano.
Women who were heavier, older, or depressed, as well as those having night sweats, were all more likely to suffer from insomnia on multivariable analysis (all P=0.002 or less). Factors that did not influence insomnia included marital status, tobacco or alcohol use, race/ethnicity, socioeconomic status, and many comorbidities including diabetes, hypertension, and cardiovascular disease.
Dr. Ciano advised clinicians to pay attention to these findings and initiate insomnia screenings. Routine evaluation of perimenopausal women should include careful questioning and an assessment of sleep patterns and problems. This is especially important since obesity, diabetes, and cardiovascular disease are all prevalent chronic illnesses that can be impacted by insomnia, she said.
Future studies should include physiologic measures and objective sleep data, which could be correlated with subjective reports, said Dr. Ciano. Sleep diaries can be an adjunct to observational and objective data, and can be correlated with such objective measures as endogenous hormone levels and objective vasomotor symptom monitoring.
Dr. Ciano’s research was supported by the National Institute for Nursing Research, Pennsylvana State University, and the Beta Sigma chapter of Sigma Theta Tau. She reported having no relevant financial disclosures.
On Twitter @karioakes
LAS VEGAS – Sleeplessness is both common and distressing for women in midlife, according to a new analysis of data from a large observational study.
One-third of women at all stages of the menopausal transition reported sleep disturbance that met the clinical criteria for insomnia, regardless of many markers of health and socioeconomic status, Colleen L. Ciano, Ph.D., reported at the NAMS 2015 annual meeting.
Acute insomnia is prevalent in adults, and women are known to suffer more than men from chronic insomnia. Perimenopausal women, in particular, may have increased sleep disturbances.
Dr. Ciano, a postdoctoral fellow at the University of Pennsylvania, Philadelphia, and her colleagues used data from the large, longitudinal Study of Women’s Health Across the Nation (SWAN) to ascertain insomnia’s prevalence in perimenopausal and surgically menopausal women. Using SWAN study data, she also attempted to tease out factors that might increase a woman’s risk for chronic insomnia.
SWAN, said Dr. Ciano, was designed to examine the health of midlife women, gathering data from 3,302 women with a mean age of 45.9 years. Ten years of study data are now available from an ethnically diverse sample of women participating at seven sites across the United States.
Four questions from the SWAN questionnaire were identified for analysis as dependent variables. The questions related to trouble falling asleep, nighttime wakings, early-morning wakings, and a subjective rating of sleep quality over 2 weeks. Women were stratified into early or late menopause, or surgical menopause.
More than one-third of women at all stages of the midlife transition, or who were in surgical menopause, reported diagnosable insomnia. On the whole, insomnia increased for women over the study period. Waking after sleep onset also increased for perimenopausal women as they moved through menopause, from 26% to 36%. Sleep quality, as a bifurcated measure of sleep quality derived from a four-point scale, also dropped over the study period.
During the SWAN data collection period, the number of perimenopausal women who met at least one of the American Academy of Sleep Medicine criteria for insomnia rose from just over 30% to just over 40%, while the number of women who reported no insomnia symptoms fell steeply, dropping from just under 70% to approximately 50%.
When Dr. Ciano analyzed the prevalence of insomnia by perimenopausal stage, she found more insomnia in late-perimenopausal women (prevalence 31%-48%), compared with those in early menopause (31%-59%).
Women in late perimenopause had an odds ratio of 1.3 of reporting any symptom of insomnia, compared with those in early menopause, according to Dr. Ciano.
Women who were heavier, older, or depressed, as well as those having night sweats, were all more likely to suffer from insomnia on multivariable analysis (all P=0.002 or less). Factors that did not influence insomnia included marital status, tobacco or alcohol use, race/ethnicity, socioeconomic status, and many comorbidities including diabetes, hypertension, and cardiovascular disease.
Dr. Ciano advised clinicians to pay attention to these findings and initiate insomnia screenings. Routine evaluation of perimenopausal women should include careful questioning and an assessment of sleep patterns and problems. This is especially important since obesity, diabetes, and cardiovascular disease are all prevalent chronic illnesses that can be impacted by insomnia, she said.
Future studies should include physiologic measures and objective sleep data, which could be correlated with subjective reports, said Dr. Ciano. Sleep diaries can be an adjunct to observational and objective data, and can be correlated with such objective measures as endogenous hormone levels and objective vasomotor symptom monitoring.
Dr. Ciano’s research was supported by the National Institute for Nursing Research, Pennsylvana State University, and the Beta Sigma chapter of Sigma Theta Tau. She reported having no relevant financial disclosures.
On Twitter @karioakes
AT THE NAMS 2015 ANNUAL MEETING
Key clinical point: One in three perimenopausal women has persistent insomnia.
Major finding: An average of one-third of perimenopausal women have significant symptoms of insomnia, higher than age-matched prevalence in the general population.
Data source: Analysis of the Study of Women’s Health Across the Nation (SWAN), a multi-site longitudinal study of 3,302 ethnically diverse midlife-aged women.
Disclosures: The research was supported by the National Institute for Nursing Research, Pennsylvana State University, and the Beta Sigma chapter of Sigma Theta Tau. Dr. Ciano reported having no relevant financial disclosures.
Ask, listen, help: Pearls to treat sexual dysfunction in menopause
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – The first step is to ask. A menopausal woman may be struggling with a female sexual disorder (FSD), but unless her clinician asks, the patient may never volunteer information about her sexual health.
Susan Kellogg Spadt, Ph.D., offered that advice, along with a toolkit of tips, treatments, and pearls for physicians and others caring for the menopausal woman’s sexual health.
Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, addressed FSD in the context of the complicated psychosocial landscape of midlife.
Women at this stage of life may be experiencing life stress as children move out, retirement looms, and aging parents require time and attention. Also, as women age, they are more likely to require medications that can negatively affect sexual health. Body image issues, depression, anxiety, and discrepancy with partner desire levels can all be prevalent in women aged 45-64 years, the group most likely to experience distress from sexual problems, she said at the NAMS 2015 annual meeting.
This is important in the context of a relationship, said Dr. Kellogg Spadt. She pointed out that “when sex is good, it adds a little bit – like icing on the cupcake – to a good relationship.” But when sex is bad or nonexistent, she said, it plays an inordinately negative role, reducing the quality of the relationship by 50%-70% in some studies.
Dr. Kellogg Spadt said a good opening approach should confirm the ubiquity of sexual problems in midlife and normalize concerns. Clinicians can ask: “With menopause, many women have changes in their sexual response. These concerns are very common. Tell me – are you feeling well and complete in your sex life?”
If questioning reveals unsatisfying or nonexistent sex, many problems can be addressed in the office. First, careful questioning and an exam can tease out the extent to which dyspareunia and vaginal dryness may be limiting sexual pleasure. In that case, lubricants, moisturizers, and topical estrogen can be considered.
Office sessions with physical therapists certified in pelvic issues, combined with home use of dilators, can help overcome physical contributors to an uncomfortable sexual experience, she said.
Clinicians can also provide brief office-based counseling using the “PLISSIT” model, which. gives permission for the patient to speak openly about sexual issues; provides limited information to educate the patient about her anatomy and resources available; offers specific suggestions, for example, positioning tips or moisturizer recommendations; and offers intensive therapy, when indicated, such as referring for adjunctive psychotherapy.
Dr. Kellogg Stadt concluded with her top clinical pearls for sexual health in menopausal women:
• Add moisture daily. Using a water-based, bioadhesive lubricant several times a week regardless of sexual frequency can significantly ease comfort and satisfaction with sex and make it easier to have an orgasm.
• Nourish. A Mediterranean diet has been shown to promote sexual function, and regular exercise improves mood and overall health.
• Talk. Partners can use “I” language to talk about sex honestly and in a nonaccusatory way. Clinicians can help provide the vocabulary and communication tips to facilitate this.
• Prioritize pleasure. Intimate time together won’t just happen; even a 20-minute block of time, scheduled weekly, for touching and intimate conversation can clear the way to better sex.
• Think. Reading or watching erotica, being mindful of erotic thoughts as they occur, and focusing on sensation rather than distractions during arousal are all important.
• Stimulate. After menopause, some women need more intense stimulation to reach orgasm, so vibrators can be incorporated into sex play. Women who are uncomfortable with this can use the “doctor’s orders” approach with their partners.
• Try. Just opening up and talking about sex problems shows that a woman is committed to her partner, and taking action shows her level of care and concern for the relationship.
Dr. Kellogg Stadt reported being a consultant or on the advisory board of Neogyn and Nuelle, and on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE NAMS 2015 ANNUAL MEETING
Does hormone therapy reduce mortality in recently menopausal women?
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
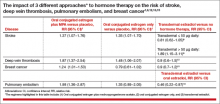
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
Individualizing treatment of menopausal symptoms
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Menopause experts Andrew M. Kaunitz, MD, and JoAnn E. Manson, MD, DrPH, provide a comprehensive review of various treatments for menopausal symptoms in an article recently published ahead of print in Obstetrics and Gynecology.1 They discuss hormonal and nonhormonal options to treat vasomotor symptoms, genitourinary syndrome of menopause (GSM), and considerations for the use of hormone therapy in special populations: women with early menopause, women with a history of breast cancer and those who carry the BRCA gene mutation, and women with a history of venous thrombosis.1
The authors write that, “given the lower rates of adverse events on HT among women close to menopause onset and at lower baseline risk of cardiovascular disease, risk stratification and personalized risk assessment appear to represent a sound strategy for optimizing the benefit–risk profile and safety of HT.”1 They suggest that instead of stopping systemic HT at age 65 years, the length of treatment be individualized based on a woman’s risk profile and preferences. The authors encourage gynecologists and other clinicians to use benefit–risk profile tools for both hormonal and nonhormonal options to help women make sound decisions on treating menopausal symptoms.1
Readthe full Clinical Expert Series here.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Reference
- Kaunitz AM, Manson JE. Management of menopausal symptoms [published online ahead of print September 3, 2015]. Obstet Gynecol. doi: 10.1097/AOG.0000000000001058. Accessed September 18, 2015.
Turn down the androgens to treat female pattern hair loss
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Early intervention may forestall menopause-related skin aging
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Osteoporosis trends collide for Mexican American women
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?
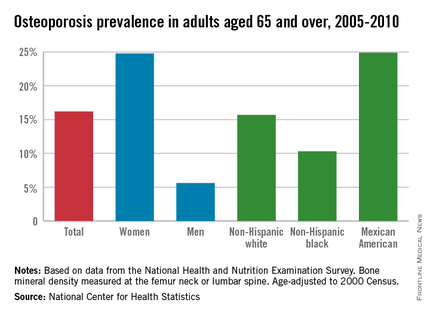
First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?

First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
Among adults aged 65 years and over, women were 4.4 times as likely as men to have osteoporosis, and Mexican Americans were 2.4 times more likely than were blacks to have osteoporosis from 2005 to 2010, the National Center for Health Statistics reported.
So where does leave those who are both women and Mexican American?

First, a little background: The age-adjusted prevalence of osteoporosis measured at either the lumbar spine or femur neck among adults aged 65 years and over was 24.8% for women and 5.6% for men, for an overall prevalence of 16.2%. Adults aged 65-79 years had an unadjusted prevalence of 12.8%, compared with 25.7% for those aged 80 years and over, according to data from the 2005-2010 National Health and Nutrition Examination Survey.
Age-adjusted prevalence over that time period for Mexican Americans aged 65 years and older was 24.9%, compared with 15.7% for non-Hispanic whites and 10.3% for non-Hispanic blacks.
Mexican American women, who find themselves at the intersection of these two trends, had an adjusted osteoporosis rate of 36.8%, the NCHS reported.
Osteoporosis was defined as a bone mineral density value that was more than 2.5 standard deviations below the mean value for young, non-Hispanic white females.
Progesterone, estrogen benefit postmenopausal cognition
Twelve weeks of estradiol or progesterone yielded distinct cognitive benefits in recently menopausal women, compared with placebo, according to a randomized, double-blind study reported in Psychoneuroendocrinology.
“Despite uncertainty about the potential cognitive benefits of postmenopausal hormone use, as women continue to live longer and healthier lives beyond the end of their reproductive years, hormones will continue to be prescribed for symptomatic indications,” said Dr. Alison Berent-Spillson of the University of Michigan, Ann Arbor, and her associates. “In contrast to previous studies that have found negative cognitive effects of treatment with synthetic progestins, our results point to potential cognitive benefits of both estrogen and progesterone.”
Few studies have examined unopposed progesterone treatment in postmenopausal women, and none have included functional neuroimaging (fMRI), even though fMRI can detect neurobiologic differences before patients exhibit measurable behavioral changes on task assessments, the investigators said. Past studies of postmenopausal hormone therapy and cognition have reported inconsistent results, they noted. Therefore, the investigators carried out a pilot study of 29 women who were within 38 months of their final menstrual period, and thus were inside the “critical window” when hormone therapy is thought to offer maximum benefit. Participants were randomized to 12 weeks of 1 mg oral estradiol or 200 mg oral progesterone. Each therapy was counterbalanced with placebo for the same amount of time, separated by a 12-week washout period. At the end of each 12-week treatment period, the women underwent verbal and visual cognitive function and working memory tests, as well as functional MRI of verbal and visual working memory processing (Psychoneuroendocrinology 2015; 59:25-36).
Compared with placebo, postmenopausal progesterone therapy was associated with a greater verbal working memory composite score and with greater fMRI activation of the right prefrontal cortex during the verbal working memory task (P = .014), the investigators reported. Women on progesterone also had significantly greater activation of the left prefrontal cortex (P = .001) and the right hippocampus (P = .003) during the visual working memory tasks, compared with the placebo group. Estradiol therapy and placebo did not differ significantly in terms of verbal or visual learning, recall, or working memory composite scores. However, estradiol was associated with increased activation of the left prefrontal cortex (P = .006) and the left hippocampus (P = .037) compared with placebo during the verbal working memory tasks, the researchers said.
Progesterone might affect the hippocampus by promoting neurogenesis and neuron survival, although its effects on the postmenopausal brain remain largely unstudied, the investigators said. Estrogen seems to offer cognitive effects during menopause through its actions on the hippocampus, but a longer treatment period might be needed for such benefits to emerge during testing, they said. “While we did not detect differences in verbal ability between the estrogen and placebo treatment arms, we saw increased activation after estrogen treatment in the left prefrontal cortex during the verbal processing task, a region associated with verbal processing,” they emphasized. “This may reflect more efficient encoding, as women remembered more words from the verbal task after estrogen than placebo, although this difference did not reach statistical significance.”
Although the study was small, its crossover design maximized the statistical power, said the researchers. They recommended larger studies with longer treatment durations in order to better detect emergent differences in cognitive ability between treatment groups and relationships between performance on tasks and regional brain activation patterns.
The National Institutes of Health and the Phil F. Jenkins Foundation funded the study. The investigators declared no conflicts of interest.
Twelve weeks of estradiol or progesterone yielded distinct cognitive benefits in recently menopausal women, compared with placebo, according to a randomized, double-blind study reported in Psychoneuroendocrinology.
“Despite uncertainty about the potential cognitive benefits of postmenopausal hormone use, as women continue to live longer and healthier lives beyond the end of their reproductive years, hormones will continue to be prescribed for symptomatic indications,” said Dr. Alison Berent-Spillson of the University of Michigan, Ann Arbor, and her associates. “In contrast to previous studies that have found negative cognitive effects of treatment with synthetic progestins, our results point to potential cognitive benefits of both estrogen and progesterone.”
Few studies have examined unopposed progesterone treatment in postmenopausal women, and none have included functional neuroimaging (fMRI), even though fMRI can detect neurobiologic differences before patients exhibit measurable behavioral changes on task assessments, the investigators said. Past studies of postmenopausal hormone therapy and cognition have reported inconsistent results, they noted. Therefore, the investigators carried out a pilot study of 29 women who were within 38 months of their final menstrual period, and thus were inside the “critical window” when hormone therapy is thought to offer maximum benefit. Participants were randomized to 12 weeks of 1 mg oral estradiol or 200 mg oral progesterone. Each therapy was counterbalanced with placebo for the same amount of time, separated by a 12-week washout period. At the end of each 12-week treatment period, the women underwent verbal and visual cognitive function and working memory tests, as well as functional MRI of verbal and visual working memory processing (Psychoneuroendocrinology 2015; 59:25-36).
Compared with placebo, postmenopausal progesterone therapy was associated with a greater verbal working memory composite score and with greater fMRI activation of the right prefrontal cortex during the verbal working memory task (P = .014), the investigators reported. Women on progesterone also had significantly greater activation of the left prefrontal cortex (P = .001) and the right hippocampus (P = .003) during the visual working memory tasks, compared with the placebo group. Estradiol therapy and placebo did not differ significantly in terms of verbal or visual learning, recall, or working memory composite scores. However, estradiol was associated with increased activation of the left prefrontal cortex (P = .006) and the left hippocampus (P = .037) compared with placebo during the verbal working memory tasks, the researchers said.
Progesterone might affect the hippocampus by promoting neurogenesis and neuron survival, although its effects on the postmenopausal brain remain largely unstudied, the investigators said. Estrogen seems to offer cognitive effects during menopause through its actions on the hippocampus, but a longer treatment period might be needed for such benefits to emerge during testing, they said. “While we did not detect differences in verbal ability between the estrogen and placebo treatment arms, we saw increased activation after estrogen treatment in the left prefrontal cortex during the verbal processing task, a region associated with verbal processing,” they emphasized. “This may reflect more efficient encoding, as women remembered more words from the verbal task after estrogen than placebo, although this difference did not reach statistical significance.”
Although the study was small, its crossover design maximized the statistical power, said the researchers. They recommended larger studies with longer treatment durations in order to better detect emergent differences in cognitive ability between treatment groups and relationships between performance on tasks and regional brain activation patterns.
The National Institutes of Health and the Phil F. Jenkins Foundation funded the study. The investigators declared no conflicts of interest.
Twelve weeks of estradiol or progesterone yielded distinct cognitive benefits in recently menopausal women, compared with placebo, according to a randomized, double-blind study reported in Psychoneuroendocrinology.
“Despite uncertainty about the potential cognitive benefits of postmenopausal hormone use, as women continue to live longer and healthier lives beyond the end of their reproductive years, hormones will continue to be prescribed for symptomatic indications,” said Dr. Alison Berent-Spillson of the University of Michigan, Ann Arbor, and her associates. “In contrast to previous studies that have found negative cognitive effects of treatment with synthetic progestins, our results point to potential cognitive benefits of both estrogen and progesterone.”
Few studies have examined unopposed progesterone treatment in postmenopausal women, and none have included functional neuroimaging (fMRI), even though fMRI can detect neurobiologic differences before patients exhibit measurable behavioral changes on task assessments, the investigators said. Past studies of postmenopausal hormone therapy and cognition have reported inconsistent results, they noted. Therefore, the investigators carried out a pilot study of 29 women who were within 38 months of their final menstrual period, and thus were inside the “critical window” when hormone therapy is thought to offer maximum benefit. Participants were randomized to 12 weeks of 1 mg oral estradiol or 200 mg oral progesterone. Each therapy was counterbalanced with placebo for the same amount of time, separated by a 12-week washout period. At the end of each 12-week treatment period, the women underwent verbal and visual cognitive function and working memory tests, as well as functional MRI of verbal and visual working memory processing (Psychoneuroendocrinology 2015; 59:25-36).
Compared with placebo, postmenopausal progesterone therapy was associated with a greater verbal working memory composite score and with greater fMRI activation of the right prefrontal cortex during the verbal working memory task (P = .014), the investigators reported. Women on progesterone also had significantly greater activation of the left prefrontal cortex (P = .001) and the right hippocampus (P = .003) during the visual working memory tasks, compared with the placebo group. Estradiol therapy and placebo did not differ significantly in terms of verbal or visual learning, recall, or working memory composite scores. However, estradiol was associated with increased activation of the left prefrontal cortex (P = .006) and the left hippocampus (P = .037) compared with placebo during the verbal working memory tasks, the researchers said.
Progesterone might affect the hippocampus by promoting neurogenesis and neuron survival, although its effects on the postmenopausal brain remain largely unstudied, the investigators said. Estrogen seems to offer cognitive effects during menopause through its actions on the hippocampus, but a longer treatment period might be needed for such benefits to emerge during testing, they said. “While we did not detect differences in verbal ability between the estrogen and placebo treatment arms, we saw increased activation after estrogen treatment in the left prefrontal cortex during the verbal processing task, a region associated with verbal processing,” they emphasized. “This may reflect more efficient encoding, as women remembered more words from the verbal task after estrogen than placebo, although this difference did not reach statistical significance.”
Although the study was small, its crossover design maximized the statistical power, said the researchers. They recommended larger studies with longer treatment durations in order to better detect emergent differences in cognitive ability between treatment groups and relationships between performance on tasks and regional brain activation patterns.
The National Institutes of Health and the Phil F. Jenkins Foundation funded the study. The investigators declared no conflicts of interest.
FROM PSYCHONEUROENDOCRINOLOGY
Key clinical point: Compared with placebo, progesterone was associated with a greater verbal working memory composite score and greater activation of the right prefrontal cortex during that task.
Major finding: Women on progesterone also had significantly greater activation of the left prefrontal cortex and the right hippocampus during the visual working memory tasks, compared with the placebo group.
Data source: Double-blind placebo-controlled randomized pilot study of 29 recently postmenopausal women.
Disclosures: The National Institutes of Health and the Phil F. Jenkins Foundation supported the research. The investigators declared no conflicts of interest.
Genitourinary syndrome of menopause: Current and emerging therapies
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 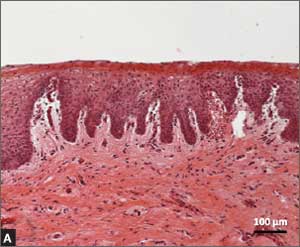
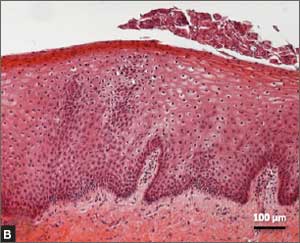
|
FIGURE 2: Atrophic vaginitis 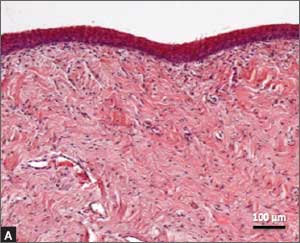
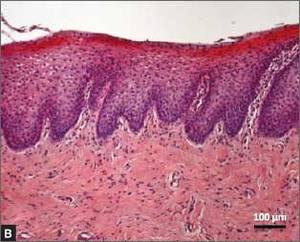
This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 

|
FIGURE 2: Atrophic vaginitis 

This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Genitourinary syndrome of menopause (GSM) is the new terminology to describe symptoms occurring secondary to vulvovaginal atrophy.1 The recent change in terminology originated with a consensus panel comprising the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of the North American Menopause Society (NAMS). At a terminology consensus conference in May 2013, these groups determined that the term GSM is medically more accurate and all encompassing than vulvovaginal atrophy. It is also more publicly acceptable.
The symptoms of GSM derive from the hypoestrogenic state most commonly associated with menopause and its effects on the genitourinary tract.2 Vaginal symptoms associated with GSM include vaginal or vulvar dryness, discharge, itching, and dyspareunia.3 Histologically, a loss of superficial epithelial cells in the genitourinary tract leads to thinning of the tissue. There is then a loss of vaginal rugae and elasticity, leading to narrowing and shortening of the vagina.
In addition, the vaginal epithelium becomes much more fragile, which can lead to tears, bleeding, and fissures. There is also a loss of the subcutaneous fat of the labia majora, a change that can result in narrowing of the introitus, fusion of the labia majora, and shrinkage of the clitoral prepuce and urethra. The vaginal pH level becomes more alkaline, which may alter vaginal flora and increase the risk of urogenital infections—specifically, urinary tract infection (UTI). Vaginal secretions, largely transudate, from the vaginal vasculature also decrease over time. These changes lead to significant dyspareunia and impairment of sexual function.
In this article, we survey the therapies available for GSM, focusing first on proven treatments such as local estrogen administration and use of ospemifene (Osphena), and then describing an emerging treatment involving the use of fractional CO2 laser.
How prevalent is GSM?
Approximately half of all postmenopausal women in the United States report atrophy-related symptoms and a significant negative effect on quality of life.4–6 Few women with these symptoms seek medical attention.
The Vaginal Health: Insights, Views, and Attitudes (VIVA) survey found that 80% of women with genital atrophy considered its impact on their lives to be negative, 75% reported negative consequences in their sexual life, 68% reported that it made them feel less sexual, 33% reported negative effects on their marriage or relationship, and 26% reported a negative impact on their self-esteem.7
Another review of the impact of this condition by Nappi and Palacios estimated that, by the year 2025, there will be 1.1 billion women worldwide older than age 50 with specific needs related to GSM.8 Nappi and Palacios cite 4 recent surveys that suggest that health care providers need to be more proactive in helping patients disclose their symptoms. The same can be said of other symptoms of the urinary tract, such as urinary frequency, urgency, and incontinence, as well as pelvic floor relaxation.
A recently published international survey on vaginal atrophy not only depicts the extremely high prevalence of the condition but also describes fairly significant differences in attitudes toward symptoms between countries in Europe and North America.9 Overall, 77% of respondents, who included more than 4,000 menopausal women, believed that women were uncomfortable discussing symptoms of vaginal atrophy.9
Pastore and colleagues, using data from the Women’s Health Initiative (WHI), found the most prevalent urogenital symptoms to be vaginal dryness (27%), vaginal irritation or itching (18.6%), vaginal discharge (11.1%), and dysuria (5.2%).4 Unlike vasomotor symptoms of menopause, which tend to decrease over time, GSM does not spontaneously remit and commonly recurs when hormone therapy—the dominant treatment—is withdrawn.
What can we offer our patients?
Vaginal estrogen
The most common therapy used to manage GSM is estrogen. Most recommendations state that if the primary menopausal symptoms are related to vaginal atrophy, then local estrogen administration should be the primary mode of therapy. The Society of Gynecologic Surgeons Systematic Review Group recently concluded that all commercially available vaginal estrogens effectively can relieve common vulvovaginal atrophy−related symptoms and have additional utility in women with urinary urgency, frequency, stress incontinence, urge incontinence, and recurrent UTIs.10 Although their meta-analysis clearly demonstrated that estrogen therapy improves the symptoms of GSM, investigators acknowledged that a clearer understanding is needed of the exact risk to the endometrium with sustained use of vaginal estrogen, as well as a more precise assessment of changes in serum estradiol levels.10
A recent Cochrane review concluded that all forms of local estrogen appear to be equally effective for symptoms of vaginal atrophy.11 One trial cited in the review found significant adverse effects following administration of cream, compared with tablets, causing uterine bleeding, breast pain, and perineal pain.11
Another trial cited in the Cochrane review found significant endometrial overstimulation following use of cream, compared with the vaginal ring. As a treatment of choice, women appeared to favor the estradiol-releasing vaginal ring for ease of use, comfort of product, and overall satisfaction.11
After the release of the WHI data, the US Food and Drug Administration (FDA) released a “black box” warning on postmenopausal hormone use in women, which has significantly reduced the use of both local and systemic estrogen in eligible women. NAMS has recommended that the FDA revisit this warning, calling specifically for an independent commission to scrutinize every major WHI paper to determine whether the data justify the conclusions drawn.12
Most data back local estrogen as treatment for GSM
In 2013, the North American Menopause Society (NAMS) issued a position statement noting that the choice of therapy for genitourinary syndrome of menopause (GSM) depends on the severity of symptoms, the efficacy and safety of therapy for the individual patient, and patient preference.1
To date, estrogen therapy is the most effective treatment for moderate to severe GSM, although a direct comparison of estrogen and ospemifene is lacking. Nonhormonal therapies available without a prescription provide sufficient relief for most women with mild symptoms. When low-dose estrogen is administered locally, a progestin is not indicated for women without a uterus—and generally is not indicated for women with an intact uterus. However, endometrial safety has not been studied in clinical trials beyond 1 year. Data are insufficient to confirm the safety of local estrogen in women with breast cancer.
Future research on the use of the fractional CO2 laser, which seems to be a promising emerging therapy, may provide clinicians with another option to treat the common and distressing problem of GSM.
Reference
1. Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888–902.
Ospemifene
This estrogen agonist and antagonist selectively stimulates or inhibits estrogen receptors of different target tissues, making it a selective estrogen receptor modulator (SERM). In a study involving 826 postmenopausal women randomly allocated to 30 mg or 60 mg of ospemifene, the 60-mg dose proved to be more effective for improving vulvovaginal atrophy.13 Long-term safety studies revealed that ospemifene 60 mg given daily for 52 weeks was well tolerated and not associated with any endometrial- or breast-related safety issues.13,14 Common adverse effects of ospemifene reported during clinical trials included hot flashes, vaginal discharge, muscle spasms, general discharge, and excessive sweating.12
Vaginal lubricants and moisturizers
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may alleviate vaginal symptoms related to menopause. These products may be particularly helpful for women who do not wish to use hormone therapies.
Vaginal lubricants are intended to relieve friction and dyspareunia related to vaginal dryness during intercourse, with the ultimate goal of trapping moisture and providing long-term relief of vaginal dryness.
Although data are limited on the efficacy of these products, prospective studies have demonstrated that vaginal moisturizers improve vaginal dryness, pH balance, and elasticity and reduce vaginal itching, irritation, and dyspareunia.
Data are insufficient to support the use of herbal remedies or soy products for the treatment of vaginal symptoms.
An emerging therapy: fractional CO2 laser
In September 2014, the FDA cleared for use the SmartXide2 CO2 laser system (DEKA Medical) for “incision, excision, vaporization and coagulation of body soft tissues” in medical specialties that include gynecology and genitourinary surgery.15 The system, also marketed by Cynosure as the MonaLisa Touch treatment, was not approved specifically for treatment of GSM—and it is important to note that the path to device clearance by the FDA is much less cumbersome than the route to drug approval. As NAMS notes in an article about the fractional CO2 laser, “Device clearance does not require the large, double-blind, randomized, placebo-controlled trials with established efficacy and safety endpoints required for the approval of new drugs.”16 Nevertheless, this laser system appears to be poised to become a new treatment for the symptoms of GSM.
This laser supplies energy with a specific pulse to the vaginal wall to rapidly and superficially ablate the epithelial component of atrophic mucosa, which is characterized by low water content. Ablation is followed by tissue coagulation, stimulated by laser energy penetrating into deeper tissues, triggering the synthesis of new collagen and other components of the ground substance of the matrix.
The supraphysiologic level of heat generated by the CO2 laser induces a rapid and transient heat-shock response that temporarily alters cellular metabolism and activates a small family of proteins referred to as the “heat shock proteins” (HSPs). HSP 70, which is overexpressed following laser treatment, stimulates transforming growthfactor‑beta, triggering an inflammatory response that stimulates fibroblasts, which produce new collagen and extracellular matrix.
The laser has emissions characteristics aligned for the transfer of the energy load to the mucosa while avoiding excessive localized damage. This aspect of its design allows for restoration of the permeability of the connective tissue, enabling the physiologic transfer of various nutrients from capillaries to tissues. When there is a loss of estrogen, as during menopause, vaginal atrophy develops, with the epithelium deteriorating and thinning. The fractional CO2 laser therapy improves the state of the epithelium by restoring epithelial cell trophism.
The vaginal dryness that occurs with atrophy is due to poor blood flow, as well as reduced activity of the fibroblasts in the deeper tissue. The increased lubrication that occurs after treatment is usually a vaginal transudate from blood outflow through the capillaries that supply blood to the vaginal epithelium. The high presence of water molecules increases permeability, allowing easier transport of metabolites and nutrients from capillaries to tissue, as well as the drainage of waste products from tissues to blood and lymph vessels.
With atrophy, the glycogen in the epithelial cells decreases. Because lactobacilli need glycogen to thrive and are responsible for maintaining the acidity of the vagina, the pH level increases. With the restoration of trophism, glycogen levels increase, furthering colonization of vaginal lactobacilli as well as vaginal acidity, reducing the pH level. This effect also may protect against the development of recurrent UTIs.
A look at the data
To date, more than 2,000 women in Italy and more than 10,000 women worldwide with GSM have been treated with fractional CO2 laser therapy, and several peer-reviewed publications have documented its efficacy and safety.17–21
In published studies, however, the populations have been small and the investigations have been mostly short term (12 weeks).17–21
A pilot study reported that a treatment cycle of 3 laser applications significantly improved the most bothersome symptoms of vulvovaginal atrophy and improved scores of vaginal health at 12 weeks’ follow-up in 50 women who had not responded to or were unsatisfied with local estrogen therapy.17 This investigation was followed by 2 additional studies involving another 92 women that specifically addressed the impact of fractional CO2 laser therapy on dyspareunia and female sexual function.19,20 Both studies showed statistically significant improvement in dyspareunia as well as Female Sexual Function Index (FSFI) scores. All women in these studies were treated in an office setting with no pretreatment anesthesia. No adverse events were reported.
Recently published histology data highlight significant changes 1 month after fractional CO2 laser treatment that included a much thicker epithelium with wide columns of large epithelial cells rich in glycogen.21 Also noted was a significant reorganization of connective tissue, both in the lamina propria and the core of the papillae (FIGURES 1 and 2).
| FIGURE 1: Early-stage vaginal atrophy 

|
FIGURE 2: Atrophic vaginitis 

This histologic preparation of vaginal mucosa sections shows untreated atrophic vaginitis (A) and the same mucosa 1 month after treatment with fractional CO2 laser therapy (B). Reprinted with permission from DEKA M.E.L.A. Srl (Calenzano, Italy) and Professor A. Calligaro, University of Pavia, Italy. |
Caveats
No International Classification of Diseases (ICD) 9 or 10 code has been assigned to the procedure to date, and the cost to the patient ranges from $600 to $1,000 per procedure.16
NAMS position. A review of the technology by NAMS noted the need for large, long-term, randomized, sham-controlled studies “to further evaluate the safety and efficacy of this procedure.”16
NAMS also notes that “lasers have become a very costly option for the treatment of symptomatic [GSM], without a single trial comparing active laser treatment to sham laser treatment and no information on long-term safety. In all published trials to date, only several hundred women have been studied and most studies are only 12 weeks in duration.”16
Not a new concept. The concept of treating skin with a microablative CO2 laser is not new. This laser has been safely used on the skin of the face, neck, and chest to produce new collagen and elastin fibers with remodeling of tissue.22,23
Preliminary data on the use of a fractionated CO2 microablative laser to treat symptoms associated with GSM suggest that the therapy is feasible, effective, and safe in the short term. If these findings are confirmed by larger, longer-term, well-controlled studies, this laser will be an additional safe and effective treatment for this very common and distressing disorder.
Two authors (Mickey Karram, MD, and Eric Sokol, MD) are performing a study of the fractional CO2 laser for treatment of genitourinary syndrome of menopause (GSM) in the United States. To date, 30 women with GSM have been treated with 3 cycles and followed for 3 months. Preliminary data show significant improvement in all symptoms, with all patients treated in an office setting with no pretreatment or posttreatment analgesia required.
The laser settings for treatment included a power of 30 W, a dwell time of 1,000 µs, spacing between 2 adjacent treated spots of 1,000 µs, and a stack parameter for pulses from 1 to 3.
Laser energy is delivered through a specially designed scanner and a vaginal probe. The probe is slowly inserted to the top of the vaginal canal and then gradually withdrawn, treating the vaginal epithelium at increments of almost 1 cm (FIGURE 3). The laser beam projects onto a 45° mirror placed at the tip of the probe, which reflects it at 90°, thereby ensuring that only the vaginal wall is treated, and not the uterine cervix.
A treatment cycle included 3 laser treatments at 6-week intervals. Each treatment lasted 3 to 5 minutes. Initial improvement was noted in most patients, including increased lubrication within 1 week after the first treatment, with further improvement after each session. To date, the positive results have persisted, and all women in the trial now have been followed for 3 months—all have noted improvement in symptoms. They will continue periodic assessment, with a final subjective and objective evaluation 1 year after their first treatment.
Bottom line
Although preliminary studies of the fractional CO2 laser as a treatment for GSM are promising, local estrogen is backed by a large body of reliable data. Ospemifene also has FDA approval for treatment of this disorder.
For women who cannot or will not use a hormone-based therapy, vaginal lubricants and moisturizer may offer at least some relief.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1–6.
- Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–285.
- Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51(3):549–555.
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303.
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Nappi RE, Kokot-Kierepa M. Vaginal Health Insights, Views and Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36–44.
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 2014;17(1):3–9.
- Nappi RE, Kokot-Kierepa M. Women’s voices in menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233–238.
- Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Suckling J, Lethaby A, Kennedy R. Local estrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;Oct 18(4):CD001500.
- Utian WH. A decade post WHI, menopausal hormone therapy comes full circle—need for independent commission. Climacteric 2012;15(4):320–325.
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Ospemifene Study Group. Menopause. 2010;17(3):480–486.
- Wurz GT, Kao CT, Degregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging. 2014;9:1939–1950.
- Letter to Paolo Peruzzi. US Food and Drug Administration; September 5, 2014. http://www.accessdata.fda.gov/cdrh_docs/pdf13/K133895.pdf. Accessed July 8, 2015.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society Menopause e-Consult: Laser Treatment Safe for Vulvovaginal Atrophy? The North American Menopause Society (NAMS). 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed July 8, 2015.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363–369.
- Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometriosis Pelvic Pain Disorders. 2014;6(3):121–162.
- Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–225.
- Salvatore S, Maggiore LR, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue; an ex vivo study. Menopause. 2015;22(8):845–849.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–436.
- Tierney EP, Hanke CW. Ablative fractionated CO2, laser resurfacing for the neck: prospective study and review of the literature. J Drugs Dermatol. 2009;8(8):723–731.
- Peterson JD, Goldman MP. Rejuvenation of the aging chest: a review and our experience. Dermatol Surg. 2011;37(5):555–571.
In this article
- What can we offer our patients?
- Fractional CO2 laser: a study in progress
- Bottom line
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Case: Disabling vasomotor symptoms in a BRCA1 mutation carrier
Christine is a 39-year-old mother of 2 who underwent risk-reducing, minimally invasive bilateral salpingo-oophorectomy with hysterectomy 4 months ago for a BRCA1 mutation (with benign findings on pathology). Eighteen months before that surgery, she had risk-reducing bilateral mastectomy with reconstruction (implants) and was advised by her surgeon that she no longer needs breast imaging.
Today she reports disabling hot flashes, insomnia, vaginal dryness, and painful sex. Her previous ObGyn, who performed the hysterectomy, was unwilling to prescribe hormone therapy (HT) due to safety concerns. Christine tried venlafaxine at 37 to 75 mg but noted little relief of her vasomotor symptoms.
In discussing her symptoms with you during this initial visit, Christine, a practicing accountant, also reveals that she does not feel as intellectually “sharp” as she did before her gynecologic surgery.
What can you offer for relief of her symptoms?
More BRCA mutation carriers are being identified and choosing to undergo risk-reducing salpingo-oophorectomy and bilateral mastectomy. Accordingly, clinicians are likely to face more questions about the use of systemic HT in this population. Because mutation carriers may worry about the safety of HT, given their BRCA status, some may delay or avoid salpingo-oophorectomy—a surgery that not only reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 80% but also decreases the risk of breast cancer by 48%.1
Surgically menopausal women in their 30s or 40s who are not treated with HT appear to have an elevated risk for dementia and Parkinsonism.2 In addition, vasomotor symptoms are often more severe, and the risks for osteoporosis and, likely, cardiovascular disease are elevated in women with early menopause who are not treated with HT. For these reasons, systemic HT is recommended for women with early menopause, and generally should be continued at least until the normal age of menopause unless specific contraindications are present.3
Because Christine not only has had risk-reducing gynecologic surgery but also risk-reducing bilateral mastectomy, her current risk for breast cancer is very low whether or not she uses HT. Because she does not have a uterus, her symptoms can effectively and safely be treated with systemic estrogen-only therapy.
Among clinicians with special expertise in the management of BRCA mutation carriers, the use of systemic HT would be considered appropriate—and not controversial—in this setting.4
Angelina Jolie details her surgeries
In March 2015, 39-year-old Oscar-winning actress and filmmaker Angelina Jolie Pitt published an opinion piece in the New York Times detailing her recent laparoscopic salpingo-oophorectomy and initiation of HT.5 Ms. Jolie Pitt, who carries the BRCA1 mutation, lost her mother, grandmother, and aunt to hereditary breast/ovarian cancer. Two years earlier, Ms. Jolie Pitt made news by describing her decision to move ahead with risk-reducing bilateral mastectomy.
Following her risk-reducing salpingo-oophorectomy, she initiated systemic HT using transdermal estradiol and off-label use of a levonorgestrel-releasing intrauterine system for endometrial protection.
Her courageous decision to publicly describe her surgery and subsequent initiation of systemic HT will likely encourage women with ominous family histories to seek out genetic counseling and testing. Her decision to “go public” regarding surgery should help mutation carriers without a history of cancer (known in the BRCA community as “previvors”) who have completed their families to move forward with risk-reducing gynecologic surgery and, when appropriate, use of systemic HT.6
The outlook for previvors with intact breasts
Three studies address the risk of breast cancer with use of systemic HT among previvors with intact breasts. A 2005 study followed a cohort of BRCA1 and BRCA2 carriers with intact breasts, 155 of whom had undergone risk-reducing salpingo-oophorectomy, for a mean of 3.6 years. Of these women, 60% and 7%, respectively, of those who had and had not undergone salpingo-oophorectomy used HT. The authors noted that bilateral salpingo-oophorectomy reduced the risk of breast cancer by some 60%, whether or not women used HT.7
A 2008 case-control study focused on 472 menopausal BRCA1 carriers, half of whom had been diagnosed with breast cancer (cases); the other half had not received this diagnosis (controls). A 43% reduction in the risk of breast cancer was associated with prior use of HT.8
A 2011 presentation described a cohort study in which 1,299 BRCA1 and BRCA2 carriers with intact breasts who had undergone salpingo-oophorectomy were followed for a mean of 5.4 years postoperatively. In this population, use of HT was not associated with an increased risk of breast cancer. Among women with BRCA1 mutations, use of systemic HT was associated with a reduced risk of breast cancer.9
Viewed in aggregate, these studies reassure us that short-term use of systemic HT does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.
Dr. Simon
Nevertheless, I think it is important to point out that a properly powered study to assess actual risk in this setting is not available in the literature.
When a patient refuses HT
Dr. Pinkerton
Some BRCA mutation carriers may refuse HT despite reassurance that it is safe. Nonhormonal therapies are not as effective at relieving severe menopausal symptoms. Almost all selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) can be offered, although only low-dose paroxetine salt is approved for treatment of postmenopausal hot flashes.10,11 Gabapentin also has shown efficacy in relieving hot flashes.
For genitourinary syndrome of menopause (GSM; formerly known as vulvovaginal atrophy), lubricants and moisturizers may provide some benefit, but they don’t improve the vaginal superficial cells and, therefore, are not as effective as hormonal options. There is now a selective estrogen receptor modulator (SERM) approved to treat GSM—ospemifene. However, in clinical trials, ospemifene has been shown to increase hot flashes, so it would not be a good option for our patient.12
Case: Continued
Christine follows up 3 months after initiating estrogen therapy (oral estradiol 2 mg daily). She reports significant improvement in her hot flashes, with improved sleep and fewer sleep disruptions. In addition, she feels that her “mental sharpness” has returned.
Dr. Pinkerton
What if this patient had an intact uterus? Then she would not be a candidate for estrogen-only therapy because she would need continued endometrial protection. Options then would include low-dose continuous or cyclic progestogen therapy, often starting with micronized progesterone, as the E3N Study suggested it has a less negative effect on the uterus.13 Or she could use a levonorgestrel-releasing intrauterine system off label, as Ms. Jolie Pitt elected to do.
Another option would be combining estrogen with a SERM. The only estrogen/SERM combination currently approved by the US Food and Drug Administration (FDA) is conjugated estrogen/bazedoxefine, which showed no increase in breast tenderness, breast density, or bleeding rates, compared with placebo, in multiple trials up to 5 years in duration.14
Case: Resolved
Christine says she would like to continue HT, although she still experiences dryness and discomfort when sexually active with her husband, despite use of a vaginal lubricant. A pelvic examination is consistent with early changes of GSM.15
You discuss GSM with Christine and suggest that she consider 1 of 2 strategies:
- Switch from daily use of oral estradiol to the 3-month systemic 0.1-mg estradiol ring (Femring), which would address both her vasomotor symptoms and her GSM.
- Continue oral estradiol and add low-dose vaginal estrogen (cream, tablets, or Estring 2 mg).
Christine chooses Option 2. When she returns 6 months later for her well-woman visit, she reports that all of her menopausal symptoms have resolved, and a pelvic examination no longer reveals changes of GSM.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
1. Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553.
2. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
3. North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.
4. Finch AP, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl). 2012;8(5):543–555.
5. Pitt AJ. Angelina Jolie Pitt: Diary of a Surgery. New York Times. http://www.nytimes.com/2015/03/24/opinion/angelina-jolie-pitt-diary-of-a-surgery.html. Published March 24, 2015. Accessed July 9, 2015.
6. Holman L, Brandt A, Daniels M, et al. Risk-reducing salpingo-oophorectomy and prophylactic mastectomy among BRCA mutation “previvors.” Gynecol Oncol. 2012;127(1 suppl):S17.
7. Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810.
8. Eisen A, Lubinski J, Gronwald J, et al; Hereditary Breast Cancer Clinical Study Group. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100(19):1361–1367.
9. Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29(32):4224–4226.
10. Krause MS, Nakajima ST. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am. 2015;42(1):163–179.
11. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1030.
12. Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
13. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor–defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
14. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
15. Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
Angelina Jolie, previvors, systemic HT, nonhormonal therapy, selective serotonin reuptake inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, SNRIs, paroxetine salt, gabapentin, genitourinary syndrome of menopause, GSM, vaginal lubricants, vaginal moisturizers, selective estrogen receptor modulator, SERM, ospemifene, estrogen therapy, oral estradiol, endometrial protection, progestogen therapy, micronized progesterone,
In This Article
- Angelina Jolie describes her surgeries
- Hormone therapy for previvors with intact breasts?
- When a patient refuses hormone therapy