User login
Monkeypox largely a mystery for pregnant people
With monkeypox now circulating in the United States, expecting mothers may worry about what might happen if they contract the infection while pregnant.
As of today, 25 cases of monkeypox have been confirmed in the United States since the outbreak began in early May, according to the U.S. Centers for Disease Control and Prevention. Although none of those cases has involved a pregnant person, the World Health Organization says monkeypox can pass from mother to fetus before delivery or to newborns by close contact during and after birth.
The case count could grow as the agency continues to investigate potential infections of the virus. In a conference call Friday, health officials stressed the importance of contact tracing, testing, and vaccine treatment.
As physicians in the United States are scrambling for information on ways to treat patients, a new study, published in Ultrasound in Obstetrics & Gynecology, could help clinicians better care for pregnant people infected with monkeypox. The authors advise consistently monitoring the fetus for infection and conducting regular ultrasounds, among other precautions.
Asma Khalil, MBBCh, MD, a professor of obstetrics and fetal medicine at St. George’s University, London, and lead author of the new study, said the monkeypox outbreak outside Africa caught many clinicians by surprise.
“We quickly realized very few physicians caring for pregnant women knew anything at all about monkeypox and how it affects pregnancy,” Dr. Khalil told this news organization. “Clinicians caring for pregnant women are likely to be faced soon with pregnant women concerned they may have the infection – because they have a rash, for example – or indeed pregnant women who do have the infection.”
According to the CDC, monkeypox can be transmitted through direct contact with the rash, sores, or scabs caused by the virus, as well as contact with clothing, bedding, towels, or other surfaces used by an infected person. Respiratory droplets and oral fluids from a person with monkeypox have also been linked to spread of the virus, as has sexual activity.
Although the condition is rarely fatal, infants and young children are at the greatest risk of developing severe symptoms, health officials said.
The U.S. Food and Drug Administration has approved a monkeypox vaccine, Jynneos (Bavarian Nordic A/S), for general use, but it has not been specifically approved for pregnant people. However, a study of 300 pregnant women who received the vaccine reported no adverse reactions or failed pregnancies linked to the shots.
The new review suggests that women who have a confirmed infection during pregnancy should have a doctor closely monitor the fetus until birth.
If the fetus is over 26 weeks or if the mother is unwell, the fetus should be cared for with heart monitoring, either by a doctor or remotely every 2-3 days. Ultrasounds should be performed regularly to confirm that the fetus is still growing well and that the placenta is functioning properly.
Further into the pregnancy, monitoring should include measurements of the fetus and detailed assessment of the fetal organs and the amniotic fluid. Once the infection is resolved, the risk to the fetus is small, according to Dr. Khalil. However, since data are limited, she recommended an ultrasound scan every 2-4 weeks. At birth, for the protection of the infant and the mother, the baby should be isolated until infection is no longer a risk.
The Royal College of Obstetricians & Gynaecologists is preparing guidance on the management of monkeypox in pregnant people, Dr. Khalil said. The American College of Obstetricians and Gynecologists said it is “relying on the CDC for the time being,” according to a spokesperson for ACOG.
“There is a clear need for further research in this area,” Dr. Khalil said. “The current outbreak is an ideal opportunity to make this happen.”
Dr. Khalil has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With monkeypox now circulating in the United States, expecting mothers may worry about what might happen if they contract the infection while pregnant.
As of today, 25 cases of monkeypox have been confirmed in the United States since the outbreak began in early May, according to the U.S. Centers for Disease Control and Prevention. Although none of those cases has involved a pregnant person, the World Health Organization says monkeypox can pass from mother to fetus before delivery or to newborns by close contact during and after birth.
The case count could grow as the agency continues to investigate potential infections of the virus. In a conference call Friday, health officials stressed the importance of contact tracing, testing, and vaccine treatment.
As physicians in the United States are scrambling for information on ways to treat patients, a new study, published in Ultrasound in Obstetrics & Gynecology, could help clinicians better care for pregnant people infected with monkeypox. The authors advise consistently monitoring the fetus for infection and conducting regular ultrasounds, among other precautions.
Asma Khalil, MBBCh, MD, a professor of obstetrics and fetal medicine at St. George’s University, London, and lead author of the new study, said the monkeypox outbreak outside Africa caught many clinicians by surprise.
“We quickly realized very few physicians caring for pregnant women knew anything at all about monkeypox and how it affects pregnancy,” Dr. Khalil told this news organization. “Clinicians caring for pregnant women are likely to be faced soon with pregnant women concerned they may have the infection – because they have a rash, for example – or indeed pregnant women who do have the infection.”
According to the CDC, monkeypox can be transmitted through direct contact with the rash, sores, or scabs caused by the virus, as well as contact with clothing, bedding, towels, or other surfaces used by an infected person. Respiratory droplets and oral fluids from a person with monkeypox have also been linked to spread of the virus, as has sexual activity.
Although the condition is rarely fatal, infants and young children are at the greatest risk of developing severe symptoms, health officials said.
The U.S. Food and Drug Administration has approved a monkeypox vaccine, Jynneos (Bavarian Nordic A/S), for general use, but it has not been specifically approved for pregnant people. However, a study of 300 pregnant women who received the vaccine reported no adverse reactions or failed pregnancies linked to the shots.
The new review suggests that women who have a confirmed infection during pregnancy should have a doctor closely monitor the fetus until birth.
If the fetus is over 26 weeks or if the mother is unwell, the fetus should be cared for with heart monitoring, either by a doctor or remotely every 2-3 days. Ultrasounds should be performed regularly to confirm that the fetus is still growing well and that the placenta is functioning properly.
Further into the pregnancy, monitoring should include measurements of the fetus and detailed assessment of the fetal organs and the amniotic fluid. Once the infection is resolved, the risk to the fetus is small, according to Dr. Khalil. However, since data are limited, she recommended an ultrasound scan every 2-4 weeks. At birth, for the protection of the infant and the mother, the baby should be isolated until infection is no longer a risk.
The Royal College of Obstetricians & Gynaecologists is preparing guidance on the management of monkeypox in pregnant people, Dr. Khalil said. The American College of Obstetricians and Gynecologists said it is “relying on the CDC for the time being,” according to a spokesperson for ACOG.
“There is a clear need for further research in this area,” Dr. Khalil said. “The current outbreak is an ideal opportunity to make this happen.”
Dr. Khalil has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With monkeypox now circulating in the United States, expecting mothers may worry about what might happen if they contract the infection while pregnant.
As of today, 25 cases of monkeypox have been confirmed in the United States since the outbreak began in early May, according to the U.S. Centers for Disease Control and Prevention. Although none of those cases has involved a pregnant person, the World Health Organization says monkeypox can pass from mother to fetus before delivery or to newborns by close contact during and after birth.
The case count could grow as the agency continues to investigate potential infections of the virus. In a conference call Friday, health officials stressed the importance of contact tracing, testing, and vaccine treatment.
As physicians in the United States are scrambling for information on ways to treat patients, a new study, published in Ultrasound in Obstetrics & Gynecology, could help clinicians better care for pregnant people infected with monkeypox. The authors advise consistently monitoring the fetus for infection and conducting regular ultrasounds, among other precautions.
Asma Khalil, MBBCh, MD, a professor of obstetrics and fetal medicine at St. George’s University, London, and lead author of the new study, said the monkeypox outbreak outside Africa caught many clinicians by surprise.
“We quickly realized very few physicians caring for pregnant women knew anything at all about monkeypox and how it affects pregnancy,” Dr. Khalil told this news organization. “Clinicians caring for pregnant women are likely to be faced soon with pregnant women concerned they may have the infection – because they have a rash, for example – or indeed pregnant women who do have the infection.”
According to the CDC, monkeypox can be transmitted through direct contact with the rash, sores, or scabs caused by the virus, as well as contact with clothing, bedding, towels, or other surfaces used by an infected person. Respiratory droplets and oral fluids from a person with monkeypox have also been linked to spread of the virus, as has sexual activity.
Although the condition is rarely fatal, infants and young children are at the greatest risk of developing severe symptoms, health officials said.
The U.S. Food and Drug Administration has approved a monkeypox vaccine, Jynneos (Bavarian Nordic A/S), for general use, but it has not been specifically approved for pregnant people. However, a study of 300 pregnant women who received the vaccine reported no adverse reactions or failed pregnancies linked to the shots.
The new review suggests that women who have a confirmed infection during pregnancy should have a doctor closely monitor the fetus until birth.
If the fetus is over 26 weeks or if the mother is unwell, the fetus should be cared for with heart monitoring, either by a doctor or remotely every 2-3 days. Ultrasounds should be performed regularly to confirm that the fetus is still growing well and that the placenta is functioning properly.
Further into the pregnancy, monitoring should include measurements of the fetus and detailed assessment of the fetal organs and the amniotic fluid. Once the infection is resolved, the risk to the fetus is small, according to Dr. Khalil. However, since data are limited, she recommended an ultrasound scan every 2-4 weeks. At birth, for the protection of the infant and the mother, the baby should be isolated until infection is no longer a risk.
The Royal College of Obstetricians & Gynaecologists is preparing guidance on the management of monkeypox in pregnant people, Dr. Khalil said. The American College of Obstetricians and Gynecologists said it is “relying on the CDC for the time being,” according to a spokesperson for ACOG.
“There is a clear need for further research in this area,” Dr. Khalil said. “The current outbreak is an ideal opportunity to make this happen.”
Dr. Khalil has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ECDC gives guidance on prevention and treatment of monkeypox
In a new risk-assessment document, the European Centre for Disease Prevention and Control summarizes what we currently know about monkeypox and recommends that European countries focus on the identification and management of the disease as well as contract tracing and prompt reporting of new cases of the virus.
Recent developments
From May 15 to May 23, in eight European Union member states (Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, and Sweden) a total of 85 cases of monkeypox were reported; they were acquired through autochthonous transmission. Current diagnosed cases of monkeypox have mainly been recorded in men who have sexual relations with other men, suggesting that transmission may occur during sexual intercourse, through infectious material coming into contact with mucosa or damaged skin, or via large respiratory droplets during prolonged face-to-face contact.
Andrea Ammon, MD, director of the ECDC, stated that “most current cases have presented with mild symptoms of the disease, and for the general population, the chance of diffusion is very low. However, the likelihood of a further spread of the virus through close contact, for example during sexual activities among people with multiple sexual partners, is considerably increased.”
Stella Kyriakides, European commissioner for health and food safety, added, “I am worried about the increase of cases of monkeypox in the EU and worldwide. We are currently monitoring the situation and, although, at the moment, the probability of it spreading to the general population is low, the situation is evolving. We should all remain alert, making sure that contact tracing and a sufficient diagnostic capacity are in place and guarantee that vaccines and antiviral drugs are available, as well as sufficient personal protective equipment [PPE] for health care professionals.”
Routes of transmission
Monkeypox is not easily spread among people. Person-to-person transmission occurs through close contact with infectious material, coming from skin lesions of an infected person, through air droplets in the case of prolonged face-to-face contact, and through fomites. So far, diagnosed cases suggest that transmission can occur through sexual intercourse.
The incubation period is 5-21 days, and patients are symptomatic for 2-4 weeks.
According to the ECDC, the likelihood of this infection spreading is increased among people who have more than one sexual partner. Although most current cases present with mild symptoms, monkeypox can cause severe disease in some groups (such as young children, pregnant women, and immunosuppressed people). However, the probability of severe disease cannot yet be estimated precisely.
The overall risk is considered moderate for people who have multiple sexual partners and low for the general population.
Clinical course
The disease initially presents with fever, myalgia, fatigue, and headache. Within 3 days of the onset of the prodromal symptoms, a centrifugal maculopapular rash appears on the site of primary infection and rapidly spreads to other parts of the body. The palms of the hands and bottoms of the feet are involved in cases where the rash has spread, which is a characteristic of the disease. Usually within 12 days, the lesions progress, simultaneously changing from macules to papules, blisters, pustules, and scabs before falling off. The lesions may have a central depression and be extremely itchy.
If the patient scratches them, a secondary bacterial infection may take hold (for which treatment with oral antihistamines is indicated). Lesions may also be present in the oral or ocular mucous membrane. Either before or at the same time as onset of the rash, patients may experience swelling of the lymph nodes, which usually is not seen with smallpox or chickenpox.
The onset of the rash is considered the start of the infectious period; however, people with prodromal symptoms may also transmit the virus.
Most cases in people present with mild or moderate symptoms. Complications seen in endemic countries include encephalitis, secondary bacterial skin infections, dehydration, conjunctivitis, keratitis, and pneumonia. The death rate ranges from 0% to 11% in endemic areas, with fatalities from the disease mostly occurring in younger children.
There is not a lot of information available on the disease in immunosuppressed individuals. In the 2017 Nigerian epidemic, patients with a concomitant HIV infection presented with more severe disease, with a greater number of skin lesions and genital ulcers, compared with HIV-negative individuals. No deaths were reported among seropositive patients. The main sequelae from the disease are usually disfiguring scars and permanent corneal lesions.
Treatment
No smallpox vaccines are authorized for use against monkeypox, however the third-generation smallpox vaccine Imvanex (Modified Vaccinia Ankara) has been authorized by the European Medicines Agency (EMA) for the EU market against smallpox and has demonstrated to provide protection in primates.
Old-generation smallpox vaccines have significant side effects, are no longer authorized, and should no longer be used. It is also important to note the lack of safety data for the use of Imvanex in immunocompromised people.
For this reason, National Immunization Technical Advisory Groups have been asked to develop specific guidelines for vaccination in close contacts of patients with monkeypox. The use of a smallpox vaccine for preexposure prophylaxis cannot be considered now, when taking into account the risk-benefit ratio.
In regard to treatment, tecovirimat is the only antiviral drug with an EMA-authorized indication for orthopoxvirus infection.
Brincidofovir is not authorized in the EU but has been authorized by the US Food and Drug Administration. However, availability on the European market is limited somewhat by the number of doses.
According to the ECDC, health care authorities should provide information about which groups should have priority access to treatment.
The use of antivirals for postexposure prophylaxis should be investigated further. Cidofovir is active in vitro for smallpox but has a pronounced nephrotoxicity profile that makes it unsuitable for first-line treatment.
The ECDC document also proposes an interim case definition for epidemiologic reporting. Further indications will also be provided for the management of monkeypox cases and close contacts. Those infected should remain in isolation until the scabs have fallen off and should, above all, avoid close contact with at-risk or immunosuppressed people as well as pets.
Most infected people can remain at home with supportive care.
Prevention
Close contacts for cases of monkeypox should monitor the development of their symptoms until 21 days have passed from their most recent exposure to the virus.
Health care workers should wear appropriate PPE (gloves, water-resistant gowns, FFP2 masks) during screening for suspected cases or when working with confirmed cases. Laboratory staff should also take precautions to avoid exposure in the workplace.
Close contacts of an infected person should not donate blood, organs, or bone marrow for at least 21 days from the last day of exposure.
Finally, the ECDC recommends increasing proactive communication of the risks to increase awareness and provide updates and indications to individuals who are at a greater risk, as well as to the general public. These messages should highlight that monkeypox is spread through close person-to-person contact, especially within the family unit, and also potentially through sexual intercourse. A balance, however, should be maintained between informing the individuals who are at greater risk and communicating that the virus is not easily spread and that the risk for the general population is low.
Human-to-animal transmission
A potential risk for human-to-animal transmission exists in Europe; therefore, a close collaboration is required between human and veterinary health care authorities, working together to manage domestic animals exposed to the virus and to prevent transmission of the disease to wildlife. To date, the European Food Safety Authority is not aware of any reports of animal infections (domestic or wild) within the EU.
There are still many unknown factors about this outbreak. The ECDC continues to closely monitor any developments and will update the risk assessment as soon as new data and information become available.
If human-to-animal transmission occurs and the virus spreads among animal populations, there is a risk that the disease could become an endemic in Europe. Therefore, human and veterinary health care authorities should work together closely to manage cases of domestic animals exposed to the virus and prevent transmission of the disease to wildlife.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
In a new risk-assessment document, the European Centre for Disease Prevention and Control summarizes what we currently know about monkeypox and recommends that European countries focus on the identification and management of the disease as well as contract tracing and prompt reporting of new cases of the virus.
Recent developments
From May 15 to May 23, in eight European Union member states (Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, and Sweden) a total of 85 cases of monkeypox were reported; they were acquired through autochthonous transmission. Current diagnosed cases of monkeypox have mainly been recorded in men who have sexual relations with other men, suggesting that transmission may occur during sexual intercourse, through infectious material coming into contact with mucosa or damaged skin, or via large respiratory droplets during prolonged face-to-face contact.
Andrea Ammon, MD, director of the ECDC, stated that “most current cases have presented with mild symptoms of the disease, and for the general population, the chance of diffusion is very low. However, the likelihood of a further spread of the virus through close contact, for example during sexual activities among people with multiple sexual partners, is considerably increased.”
Stella Kyriakides, European commissioner for health and food safety, added, “I am worried about the increase of cases of monkeypox in the EU and worldwide. We are currently monitoring the situation and, although, at the moment, the probability of it spreading to the general population is low, the situation is evolving. We should all remain alert, making sure that contact tracing and a sufficient diagnostic capacity are in place and guarantee that vaccines and antiviral drugs are available, as well as sufficient personal protective equipment [PPE] for health care professionals.”
Routes of transmission
Monkeypox is not easily spread among people. Person-to-person transmission occurs through close contact with infectious material, coming from skin lesions of an infected person, through air droplets in the case of prolonged face-to-face contact, and through fomites. So far, diagnosed cases suggest that transmission can occur through sexual intercourse.
The incubation period is 5-21 days, and patients are symptomatic for 2-4 weeks.
According to the ECDC, the likelihood of this infection spreading is increased among people who have more than one sexual partner. Although most current cases present with mild symptoms, monkeypox can cause severe disease in some groups (such as young children, pregnant women, and immunosuppressed people). However, the probability of severe disease cannot yet be estimated precisely.
The overall risk is considered moderate for people who have multiple sexual partners and low for the general population.
Clinical course
The disease initially presents with fever, myalgia, fatigue, and headache. Within 3 days of the onset of the prodromal symptoms, a centrifugal maculopapular rash appears on the site of primary infection and rapidly spreads to other parts of the body. The palms of the hands and bottoms of the feet are involved in cases where the rash has spread, which is a characteristic of the disease. Usually within 12 days, the lesions progress, simultaneously changing from macules to papules, blisters, pustules, and scabs before falling off. The lesions may have a central depression and be extremely itchy.
If the patient scratches them, a secondary bacterial infection may take hold (for which treatment with oral antihistamines is indicated). Lesions may also be present in the oral or ocular mucous membrane. Either before or at the same time as onset of the rash, patients may experience swelling of the lymph nodes, which usually is not seen with smallpox or chickenpox.
The onset of the rash is considered the start of the infectious period; however, people with prodromal symptoms may also transmit the virus.
Most cases in people present with mild or moderate symptoms. Complications seen in endemic countries include encephalitis, secondary bacterial skin infections, dehydration, conjunctivitis, keratitis, and pneumonia. The death rate ranges from 0% to 11% in endemic areas, with fatalities from the disease mostly occurring in younger children.
There is not a lot of information available on the disease in immunosuppressed individuals. In the 2017 Nigerian epidemic, patients with a concomitant HIV infection presented with more severe disease, with a greater number of skin lesions and genital ulcers, compared with HIV-negative individuals. No deaths were reported among seropositive patients. The main sequelae from the disease are usually disfiguring scars and permanent corneal lesions.
Treatment
No smallpox vaccines are authorized for use against monkeypox, however the third-generation smallpox vaccine Imvanex (Modified Vaccinia Ankara) has been authorized by the European Medicines Agency (EMA) for the EU market against smallpox and has demonstrated to provide protection in primates.
Old-generation smallpox vaccines have significant side effects, are no longer authorized, and should no longer be used. It is also important to note the lack of safety data for the use of Imvanex in immunocompromised people.
For this reason, National Immunization Technical Advisory Groups have been asked to develop specific guidelines for vaccination in close contacts of patients with monkeypox. The use of a smallpox vaccine for preexposure prophylaxis cannot be considered now, when taking into account the risk-benefit ratio.
In regard to treatment, tecovirimat is the only antiviral drug with an EMA-authorized indication for orthopoxvirus infection.
Brincidofovir is not authorized in the EU but has been authorized by the US Food and Drug Administration. However, availability on the European market is limited somewhat by the number of doses.
According to the ECDC, health care authorities should provide information about which groups should have priority access to treatment.
The use of antivirals for postexposure prophylaxis should be investigated further. Cidofovir is active in vitro for smallpox but has a pronounced nephrotoxicity profile that makes it unsuitable for first-line treatment.
The ECDC document also proposes an interim case definition for epidemiologic reporting. Further indications will also be provided for the management of monkeypox cases and close contacts. Those infected should remain in isolation until the scabs have fallen off and should, above all, avoid close contact with at-risk or immunosuppressed people as well as pets.
Most infected people can remain at home with supportive care.
Prevention
Close contacts for cases of monkeypox should monitor the development of their symptoms until 21 days have passed from their most recent exposure to the virus.
Health care workers should wear appropriate PPE (gloves, water-resistant gowns, FFP2 masks) during screening for suspected cases or when working with confirmed cases. Laboratory staff should also take precautions to avoid exposure in the workplace.
Close contacts of an infected person should not donate blood, organs, or bone marrow for at least 21 days from the last day of exposure.
Finally, the ECDC recommends increasing proactive communication of the risks to increase awareness and provide updates and indications to individuals who are at a greater risk, as well as to the general public. These messages should highlight that monkeypox is spread through close person-to-person contact, especially within the family unit, and also potentially through sexual intercourse. A balance, however, should be maintained between informing the individuals who are at greater risk and communicating that the virus is not easily spread and that the risk for the general population is low.
Human-to-animal transmission
A potential risk for human-to-animal transmission exists in Europe; therefore, a close collaboration is required between human and veterinary health care authorities, working together to manage domestic animals exposed to the virus and to prevent transmission of the disease to wildlife. To date, the European Food Safety Authority is not aware of any reports of animal infections (domestic or wild) within the EU.
There are still many unknown factors about this outbreak. The ECDC continues to closely monitor any developments and will update the risk assessment as soon as new data and information become available.
If human-to-animal transmission occurs and the virus spreads among animal populations, there is a risk that the disease could become an endemic in Europe. Therefore, human and veterinary health care authorities should work together closely to manage cases of domestic animals exposed to the virus and prevent transmission of the disease to wildlife.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
In a new risk-assessment document, the European Centre for Disease Prevention and Control summarizes what we currently know about monkeypox and recommends that European countries focus on the identification and management of the disease as well as contract tracing and prompt reporting of new cases of the virus.
Recent developments
From May 15 to May 23, in eight European Union member states (Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, and Sweden) a total of 85 cases of monkeypox were reported; they were acquired through autochthonous transmission. Current diagnosed cases of monkeypox have mainly been recorded in men who have sexual relations with other men, suggesting that transmission may occur during sexual intercourse, through infectious material coming into contact with mucosa or damaged skin, or via large respiratory droplets during prolonged face-to-face contact.
Andrea Ammon, MD, director of the ECDC, stated that “most current cases have presented with mild symptoms of the disease, and for the general population, the chance of diffusion is very low. However, the likelihood of a further spread of the virus through close contact, for example during sexual activities among people with multiple sexual partners, is considerably increased.”
Stella Kyriakides, European commissioner for health and food safety, added, “I am worried about the increase of cases of monkeypox in the EU and worldwide. We are currently monitoring the situation and, although, at the moment, the probability of it spreading to the general population is low, the situation is evolving. We should all remain alert, making sure that contact tracing and a sufficient diagnostic capacity are in place and guarantee that vaccines and antiviral drugs are available, as well as sufficient personal protective equipment [PPE] for health care professionals.”
Routes of transmission
Monkeypox is not easily spread among people. Person-to-person transmission occurs through close contact with infectious material, coming from skin lesions of an infected person, through air droplets in the case of prolonged face-to-face contact, and through fomites. So far, diagnosed cases suggest that transmission can occur through sexual intercourse.
The incubation period is 5-21 days, and patients are symptomatic for 2-4 weeks.
According to the ECDC, the likelihood of this infection spreading is increased among people who have more than one sexual partner. Although most current cases present with mild symptoms, monkeypox can cause severe disease in some groups (such as young children, pregnant women, and immunosuppressed people). However, the probability of severe disease cannot yet be estimated precisely.
The overall risk is considered moderate for people who have multiple sexual partners and low for the general population.
Clinical course
The disease initially presents with fever, myalgia, fatigue, and headache. Within 3 days of the onset of the prodromal symptoms, a centrifugal maculopapular rash appears on the site of primary infection and rapidly spreads to other parts of the body. The palms of the hands and bottoms of the feet are involved in cases where the rash has spread, which is a characteristic of the disease. Usually within 12 days, the lesions progress, simultaneously changing from macules to papules, blisters, pustules, and scabs before falling off. The lesions may have a central depression and be extremely itchy.
If the patient scratches them, a secondary bacterial infection may take hold (for which treatment with oral antihistamines is indicated). Lesions may also be present in the oral or ocular mucous membrane. Either before or at the same time as onset of the rash, patients may experience swelling of the lymph nodes, which usually is not seen with smallpox or chickenpox.
The onset of the rash is considered the start of the infectious period; however, people with prodromal symptoms may also transmit the virus.
Most cases in people present with mild or moderate symptoms. Complications seen in endemic countries include encephalitis, secondary bacterial skin infections, dehydration, conjunctivitis, keratitis, and pneumonia. The death rate ranges from 0% to 11% in endemic areas, with fatalities from the disease mostly occurring in younger children.
There is not a lot of information available on the disease in immunosuppressed individuals. In the 2017 Nigerian epidemic, patients with a concomitant HIV infection presented with more severe disease, with a greater number of skin lesions and genital ulcers, compared with HIV-negative individuals. No deaths were reported among seropositive patients. The main sequelae from the disease are usually disfiguring scars and permanent corneal lesions.
Treatment
No smallpox vaccines are authorized for use against monkeypox, however the third-generation smallpox vaccine Imvanex (Modified Vaccinia Ankara) has been authorized by the European Medicines Agency (EMA) for the EU market against smallpox and has demonstrated to provide protection in primates.
Old-generation smallpox vaccines have significant side effects, are no longer authorized, and should no longer be used. It is also important to note the lack of safety data for the use of Imvanex in immunocompromised people.
For this reason, National Immunization Technical Advisory Groups have been asked to develop specific guidelines for vaccination in close contacts of patients with monkeypox. The use of a smallpox vaccine for preexposure prophylaxis cannot be considered now, when taking into account the risk-benefit ratio.
In regard to treatment, tecovirimat is the only antiviral drug with an EMA-authorized indication for orthopoxvirus infection.
Brincidofovir is not authorized in the EU but has been authorized by the US Food and Drug Administration. However, availability on the European market is limited somewhat by the number of doses.
According to the ECDC, health care authorities should provide information about which groups should have priority access to treatment.
The use of antivirals for postexposure prophylaxis should be investigated further. Cidofovir is active in vitro for smallpox but has a pronounced nephrotoxicity profile that makes it unsuitable for first-line treatment.
The ECDC document also proposes an interim case definition for epidemiologic reporting. Further indications will also be provided for the management of monkeypox cases and close contacts. Those infected should remain in isolation until the scabs have fallen off and should, above all, avoid close contact with at-risk or immunosuppressed people as well as pets.
Most infected people can remain at home with supportive care.
Prevention
Close contacts for cases of monkeypox should monitor the development of their symptoms until 21 days have passed from their most recent exposure to the virus.
Health care workers should wear appropriate PPE (gloves, water-resistant gowns, FFP2 masks) during screening for suspected cases or when working with confirmed cases. Laboratory staff should also take precautions to avoid exposure in the workplace.
Close contacts of an infected person should not donate blood, organs, or bone marrow for at least 21 days from the last day of exposure.
Finally, the ECDC recommends increasing proactive communication of the risks to increase awareness and provide updates and indications to individuals who are at a greater risk, as well as to the general public. These messages should highlight that monkeypox is spread through close person-to-person contact, especially within the family unit, and also potentially through sexual intercourse. A balance, however, should be maintained between informing the individuals who are at greater risk and communicating that the virus is not easily spread and that the risk for the general population is low.
Human-to-animal transmission
A potential risk for human-to-animal transmission exists in Europe; therefore, a close collaboration is required between human and veterinary health care authorities, working together to manage domestic animals exposed to the virus and to prevent transmission of the disease to wildlife. To date, the European Food Safety Authority is not aware of any reports of animal infections (domestic or wild) within the EU.
There are still many unknown factors about this outbreak. The ECDC continues to closely monitor any developments and will update the risk assessment as soon as new data and information become available.
If human-to-animal transmission occurs and the virus spreads among animal populations, there is a risk that the disease could become an endemic in Europe. Therefore, human and veterinary health care authorities should work together closely to manage cases of domestic animals exposed to the virus and prevent transmission of the disease to wildlife.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
Pfizer asks FDA to authorize COVID vaccine for children younger than 5
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
TDF use in HBV-HIV coinfection linked with kidney, bone issues
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Patients coinfected with hepatitis B virus (HBV) and human immunodeficiency virus who take tenofovir disoproxil fumarate (TDF) may have worsening renal function and bone turnover, according to a small, prospective cohort study in HIV Medicine.
“In this HBV-HIV cohort of adults with high prevalence of tenofovir use, several biomarkers of renal function and bone turnover indicated worsening status over approximately 4 years, highlighting the importance of clinicians’ awareness,” lead author Richard K. Sterling, MD, MSc, assistant chair of research in the department of internal medicine of Virginia Commonwealth University, Richmond, told this news organization in an email.
TDF is a common component of antiretroviral therapy (ART) in adults coinfected with HBV and HIV. The drug is known to adversely affect kidney function and bone turnover, but few studies have evaluated these issues, the authors write.
Dr. Sterling and colleagues enrolled adults coinfected with HBV and HIV who were taking any type of ART in their study at eight sites in North America.
The authors assessed demographics, medical history, current health status reports, physical exams, and blood and urine tests. They extracted clinical, laboratory, and radiologic data from medical records, and they processed whole blood, stored serum at -70 °C (-94 °F) at each site, and tested specimens in central laboratories.
The researchers assessed the participants at baseline and every 24 weeks for up to 192 weeks (3.7 years). They tested bone markers from stored serum at baseline, week 96, and week 192. And they recorded changes in renal function markers and bone turnover over time.
At baseline, the median age of the 115 patients was 49 years; 91% were male, and 52% were non-Hispanic Black. Their median body mass index was 26 kg/m2, with 6.3% of participants underweight and 59% overweight or obese. The participants had been living with HIV for a median of about 20 years.
Overall, 84% of participants reported tenofovir use, 3% reported no HBV therapy, and 80% had HBV/HIV suppression. In addition, 13% had stage 2 liver fibrosis and 23% had stage 3 to 4 liver fibrosis. No participants reported using immunosuppressants, 4% reported using an anticoagulant, 3% reported taking calcium plus vitamin D, and 33% reported taking multivitamins.
Throughout the follow-up period, TDF use ranged from 80% to 92%. Estimated glomerular filtration rate (eGFR) dropped from 87.1 to 79.9 ml/min/1.73m2 over 192 weeks (P < .001); but eGFR prevalence < 60 ml/min/1.73m2 did not appear to change over time (always < 16%; P = .43).
From baseline to week 192, procollagen type 1 N-terminal propeptide (P1NP) dropped from 146.7 to 130.5 ng/ml (P = .001), osteocalcin dropped from 14.4 to 10.2 ng/ml (P < .001), and C-terminal telopeptides of type I collagen (CTX-1) dropped from 373 to 273 pg/ml (P < .001).
Predictors of decrease in eGFR included younger age, male sex, and overweight or obesity. Predictors of worsening bone turnover included Black race, healthy weight, advanced fibrosis, undetectable HBV DNA, and lower parathyroid hormone level.
Monitor patients with HBV and HIV closely
“The long-term effects of TDF on renal and bone health are important to monitor,” Dr. Sterling advised. “For renal health, physicians should monitor GFR as well as creatinine. For bone health, monitoring serum calcium, vitamin D, parathyroid hormone, and phosphate may not catch increased bone turnover.”
“We knew that TDF can cause renal dysfunction; however, we were surprised that we did not observe significant rise in serum creatinine but did observe decline in glomerular filtration rate and several markers of increased bone turnover,” he added.
Dr. Sterling acknowledged that limitations of the study include its small cohort, short follow-up, and lack of control participants who were taking TDF while mono-infected with either HBV or HIV. He added that strengths include close follow-up, use of bone turnover markers, and control for severity of liver disease.
Joseph Alvarnas, MD, a hematologist and oncologist in the department of hematology & hematopoietic cell transplant at City of Hope Comprehensive Cancer Center, Duarte, California, told this news organization that he welcomes the rigor of the study. “This study provides an important reminder of the complexities of taking a comprehensive management approach to the care of patients with long-term HIV infection,” Dr. Alvarnas wrote in an email. He was not involved in the study.
“More than 6 million people worldwide live with coinfection,” he added. “Patients coinfected with HBV and HIV have additional care needs over those living with only chronic HIV infection. With more HIV-infected patients becoming long-term survivors who are managed through the use of effective ART, fully understanding the differentiated long-term care needs of this population is important.”
Debika Bhattacharya, MD, a specialist in HIV and viral hepatitis coinfection in the Division of Infectious Diseases at UCLA Health, Los Angeles, joined Dr. Sterling and Dr. Alvarnas in advising clinicians to regularly evaluate the kidney and bone health of their coinfected patients.
“While this study focuses the very common antiretroviral agent TDF, it will be important to see the impact of a similar drug, tenofovir alafenamide (TAF) – which has been associated with less impact on bone and kidney health – on clinical outcomes in HBV-HIV coinfection,” Dr. Bhattacharya, who also was not involved in the study, wrote in an email.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Sterling has served on boards for Pfizer and AskBio, and he reports research grants from Gilead, Abbott, AbbVie, and Roche to his institution. Most other authors report financial relationships with pharmaceutical companies. Dr. Alvarnas reports no relevant financial relationships. Dr. Bhattacharya has received a research grant from Gilead Sciences, paid to her institution.
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: IVIG in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia
Children & COVID: Rise in new cases slows
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
C. diff.: How did a community hospital cut infections by 77%?
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Teamwork by a wide range of professional staff, coupled with support from leadership, enabled one academic community hospital to cut its rate of hospital-onset Clostridioides difficile infections (HO-CDIs) by almost two-thirds in 1 year and by over three-quarters in 3 years, a study published in the American Journal of Infection Control reports.
C. diff. is a major health threat. According to the U.S. Centers for Disease Control and Prevention, CDIs, mainly linked with hospitals, caused an estimated 223,900 cases in hospitalized patients and 12,800 deaths in the United States in 2017.
“The interventions and outcomes of the project improved patient care by ensuring early testing, diagnosis, treatment if warranted, and proper isolation, which helped reduce C. diff. transmission to staff and other patients,” lead study author Cherith Walter, MSN, RN, a clinical nurse specialist at Emory Saint Joseph’s Hospital, Atlanta, told this news organization. “Had we not worked together as a team, we would not have had the ability to carry out such a robust project,” she added in an email.
Each HO-CDI case costs a health care system an estimated $12,313, and high rates of HO-CDIs incur fines from the Hospital-Acquired Condition Reduction Program of the Centers for Medicare & Medicaid Services (CMS), the authors write.
A diverse staff team collaborated
Emory Saint Joseph’s, a 410-bed hospital in Atlanta, had a history of being above the national CMS benchmark for HO-CDIs. To reduce these infections, comply with CMS requirements, and avoid fines, Ms. Walter and colleagues launched a quality improvement project between 2015 and 2020.
With the approval of the chief nursing officer, chief quality officer, and hospital board, researchers mobilized a diverse team of professionals: a clinical nurse specialist, a physician champion, unit nurse champions, a hospital epidemiologist, an infection preventionist, a clinical microbiologist, an antimicrobial stewardship pharmacist, and an environmental services representative.
The team investigated what caused their hospital’s HO-CDIs from 2014 through 2016 and developed appropriate, evidence-based infection prevention interventions. The integrated approach involved:
- Diagnostic stewardship, including a diarrhea decision-tree algorithm that enabled nurses to order tests of any loose or unformed stool for C. diff. during the first 3 days of admission.
- Enhanced environmental cleaning, which involved switching from sporicidal disinfectant only in isolation rooms to using a more effective Environmental Protection Agency–approved sporicidal disinfectant containing hydrogen peroxide and peracetic acid in all patient rooms for daily cleaning and after discharge. Every day, high-touch surfaces in C. diff. isolation rooms were cleaned and shared equipment was disinfected with bleach wipes. After patient discharge, staff cleaned mattresses on all sides, wiped walls with disinfectant, and used ultraviolet light.
- Antimicrobial stewardship. Formulary fluoroquinolones were removed as standalone orders and made available only through order sets with built-in clinical decision support.
- Education of staff on best practices, through emails, flyers, meetings, and training sessions. Two nurses needed to approve the appropriateness of testing specific specimens for CDI. All HO-CDIs were reviewed and findings presented at CDI team meetings.
- Accountability. Staff on the team and units received emailed notices about compliance issues and held meetings to discuss how to improve compliance.
After 1 year, HO-CDI incidence dropped 63% from baseline, from above 12 cases per 10,000 patient-days to 4.72 per 10,000 patient-days. And after 3 years, infections dropped 77% to 2.80 per 10,000 patient-days.
The hospital’s HO-CDI standardized infection ratio – the total number of infections divided by the National Healthcare Safety Network’s risk-adjusted predicted number of infections – dropped below the national benchmark, from 1.11 in 2015 to 0.43 in 2020.
The hospital also increased testing of appropriate patients for CDI within the first 3 days of admission, from 54% in 2014 to 81% in late 2019.
“By testing patients within 3 days of admission, we discovered that many had acquired C. diff. before admission,” Ms. Walter said. “I don’t think we realized how prevalent C. diff. was in the community.”
Benjamin D. Galvan, MLS(ASCP), CIC, an infection preventionist at Tampa General Hospital and a member of the Association for Professionals in Infection Control and Epidemiology, welcomed the study’s results.
“Effective collaboration within the health care setting is a highly effective way to implement and sustain evidence-based practices related to infection reduction. When buy-in is obtained from the top, and pertinent stakeholders are engaged for their expertise, we can see sustainable change and improved patient outcomes,” Mr. Galvan, who was not involved in the study, said in an email.
“The researchers did a fantastic job,” he added. “I am grateful to see this important work addressed in the literature, as it will only improve buy-in for improvement efforts aimed at reducing infections moving forward across the health care continuum.”
Douglas S. Paauw, MD, a professor of medicine and chair for patient-centered clinical education at the University of Washington School of Medicine, Seattle, said that the team’s most important interventions were changing the environmental cleaning protocol and using agents that kill C. diff. spores.
“We know that as many as 10%-20% of hospitalized patients carry C. diff. Cleaning only the rooms where you know you have C. diff. (isolation rooms) will miss most of it,” said Dr. Paauw, who was also not involved in the study. “Cleaning every room with cleaners that actually work is very important but costs money.”
Handwashing with soap and water works, alcohol hand gels do not
“We know that handwashing with soap and water is the most important way to prevent hospital C. diff. transmission,” Dr. Paauw noted. “Handwashing protocols implemented prior to the study were probably a big part of the team’s success.”
Handwashing with soap and water works but alcohol hand gels do not, he cautioned.
“C. diff. rates in hospitals went up years ago when we started putting alcohol gels outside patients’ rooms,” Dr. Paauw explained. “Now, instead of washing their hands, staff quickly pump gel before they see patients. Applying gel is easy, but gel does not eliminate C. diff. spores. Handwashing is such a simple way to fix the C. diff. problem, but doctors don’t take the time.”
“We need to take the C. diff. problem seriously. We have enough information, and we know the right things to do. We need to wash our hands. We need to clean the rooms. We need to stop cutting corners if we want to give good care,” he said.
The authors plan to conduct further related research.
The study was not funded. All study authors, as well as Mr. Galvan and Dr. Paauw, have reported no relevant financial interests.
A version of this article first appeared on Medscape.com.
Focus on antivirals, vaccines as monkeypox continues
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the first case of monkeypox on May 6, reports of outbreaks have come from multiple countries, with the United Kingdom, Spain, and Portugal in the lead, followed by Canada, Israel, and Australia, among others. The United States has reported cases in Boston and New York, and presumed cases have occurred in Utah and Florida. As of May 25, close to 350 cases, either suspected (83) or confirmed (265), have been reported globally.
Monkeypox outbreaks have previously been confined to Central and West Africa, except for an impressively large outbreak in the United States in 2003, during which 47 people were infected across six states. The epidemic was traced to a Gambian rat, rope squirrels, and dormice that had been imported from Ghana as pets and that had infected prairie dogs at a large wholesale pet store.
“It’s amazing how many of these viruses – COVID, now monkeypox and others – [exist]. They’re out there in the wild in the animal reservoir,” said Dennis Hruby, PhD, executive VP/chief scientific officer and scientific founder of SIGA Technologies.
“When it comes to the human population, they sometimes behave in ways we’re not expecting. That and a few mutations change those strains and pathogenicity and can be pandemic,” he told this news organization.
Now that the virus is pandemic, there is an urgent interest in medicines and vaccines that might halt its spread.
Smallpox drug tecovirimat
SIGA’s drug is tecovirimat, initially known as ST-246 and now branded as TPOXX. The U.S. Food and Drug Administration approved an oral formulation to treat smallpox in 2018. While smallpox was eradicated by 1980, there have been ongoing concerns about its potential use in a bioterrorism attack.
Tecovirimat is also approved for smallpox in Canada. In Europe, the approval includes treatment of monkeypox, cowpox, and complications from immunization with vaccinia. On May 19, the FDA approved an IV formulation of tecovirimat for those unable to tolerate oral medications.
In a press release, SIGA notes that tecovirimat was “developed through funding and collaboration with the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services, as well as early-stage development supported by the National Institutes of Health, US Centers for Disease Control and Prevention, and the Department of Defense. Tecovirimat is stockpiled by the U.S. Government to mitigate the impact of a potential outbreak or bioterror attack.”
SIGA adds that, under Project Bioshield, “the United States maintains a stockpile of 1.7 million courses in the Strategic National Stockpile.” The drug is only available through the government’s stockpile.
Tecovirimat works by preventing the viruses from reproducing by interfering with a protein, VP37. The virus cannot escape the cell and so cannot infect other cells, Dr. Hruby explained.
Tecovirimat was developed under the FDA’s so-called Animal Rule, which allows approval on the basis of animal studies when human efficacy studies are unethical or impractical.
In a placebo-controlled human pharmacokinetic and safety study, only 2% of the 359 who received TPOXX had to have treatment stopped because of adverse reactions, a rate similar to placebo. The most common reactions (≥2%) were headache, nausea, and abdominal pain. Significant drug interactions were found with the coadministration of repaglinide and midazolam.
Of note is that tecovirimat’s efficacy may be reduced in immunocompromised patients. The smallpox vaccine is contraindicated for those who are immunocompromised. Those people should be offered vaccinia immune globulin.
With monkeypox, “the earlier the disease is recognized and you start treating, [the] more effective,” said Dr. Hruby. “In a monkey model which, much like humans, if we treat early on as the first lesions emerged or even several days after the lesions emerged, we see close to 100% protection.”
The other alternative drug for smallpox and (likely) monkeypox is Chimerix’s brincidofovir (BCV, Tembexa), a lipid conjugate of cidofovir, a drug for cytomegalovirus. Brincidofovir has a better safety profile than cidofovir and was also approved under the Animal Rule.
UpToDate suggests that tecovirimat is the drug of choice for monkeypox. They note that for severely infected patients, it can be combined with brincidofovir after consultation with the CDC or state health department officials.
Two vaccines available
Two vaccines are currently available. The oldest is ACAM2000, a replication-competent vaccine that replaced Dryvax, whose use was stopped in 1977, the last year in which naturally occurring cases of smallpox occurred. ACAM2000 is used to immunize military recruits. It was produced by Sanofi and is now produced by Emergent Biosolutions. Being a live vaccinia vaccine, it is contraindicated for people who are immunocompromised or pregnant, as well as for children and those with eczema, because serious and occasionally fatal reactions have occurred. Because of unexpected cardiac complications in first responders who received Dryvax, having a history of cardiac disease or significant risk factors is considered a contraindication to replication-competent (live) vaccination except in the setting of a bioterrorism event.
ACAM2000 is not FDA approved for monkeypox, but it is readily available. The United States stockpile has more than 100 million doses, according to the CDC.
“ACAM is not very different from Dryvax in terms of safety profile,” Melvin Sanicas, MD, a vaccinologist and health educator, told this news organization.
The newest option is a replication-deficient modified vaccinia Ankara vaccine called Jynneos in the United States (Imvanex in Europe; Imvamune in Canada). The vaccine is made by Denmark-based Bavarian Nordic. The FDA approved Jynneos in 2019. It, too, is available through BARDA’s stockpiles; 1,000 doses are available now and more are on order.
In the current monkeypox outbreak, Jynneos has been offered to higher-risk contacts in the United Kingdom. The CDC is planning to provide it to high-risk contacts of infected persons in the United States. This strategy is called “ring vaccination,” through which only close contacts are immunized initially. The rings are then enlarged to include more people as needed. Ring vaccination works well for easily identified diseases such as monkeypox and in situations in which there are few cases. It has been used very effectively for smallpox and Ebola.
Jynneos is not associated with the same risks as the live vaccine. In solicited reactions, injection-site reactions were common. Other reported systemic symptoms were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%), and chills (10.4%).
Other vaccines are expected to be developed. Moderna has just thrown its hat into the ring, announcing it is beginning preclinical trials for monkeypox.
Prolonged close contact
Monkeypox is spread by large droplets or contact with infected lesions or body fluids. It’s thought to require prolonged close contact. In an email interview, Dr. Sanicas told this news organization that the “contact can be with (1) skin lesions of an infected person, (2) respiratory droplets in prolonged face-to-face contact, (3) fomites. The cases in the United Kingdom are in men having sex with men, but it does not mean the disease is now sexually transmitted. People do not need to have sex to be infected, but of course, sexual contact means there is prolonged contact.” The household transmission rate is less than 10%.
Dr. Sanicas confirmed that, as with smallpox, monkeypox could be transmitted by contact with clothing or bedding that has been contaminated through contact with the infected lesions, as smallpox was transmitted to Native Americans by colonizers. Airborne transmission is a theoretical possibility but is not considered likely. Being a DNA virus, monkeypox is less likely to mutate than COVID. “If it were as infectious as flu or coronavirus, there would be more infections and outbreaks in countries where MPX [monkeypox] is endemic in Western Africa or Congo Basin,” said Dr. Sanicas.
Fortunately, this clade of monkeypox, which appears to have originated in West Africa, is estimated to have a mortality rate of about 1%. In contrast, the Congo Basin clade has a death rate of up to 10%.
Dr. Sanicas concluded, “Be cautious, but there’s no need for further fear and panic on top of what we have for COVID-19. Monkeypox is not COVID and will not cause the same devastation/death/lockdowns as COVID-19.”
Dr. Hruby is an employee and stockholder of SIGA. Dr. Sanicas reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
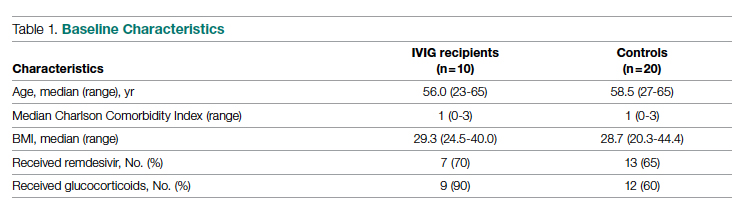
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
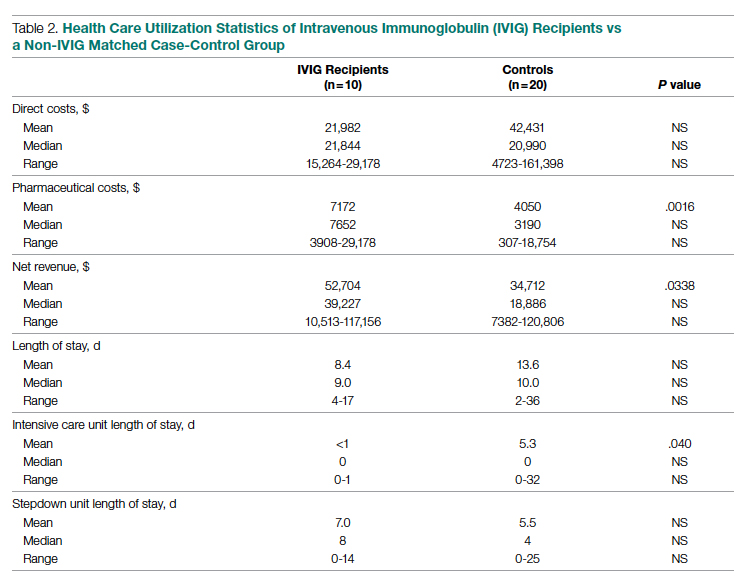
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Manufacturer announces FDA approval for molluscum treatment delayed
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.