User login
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.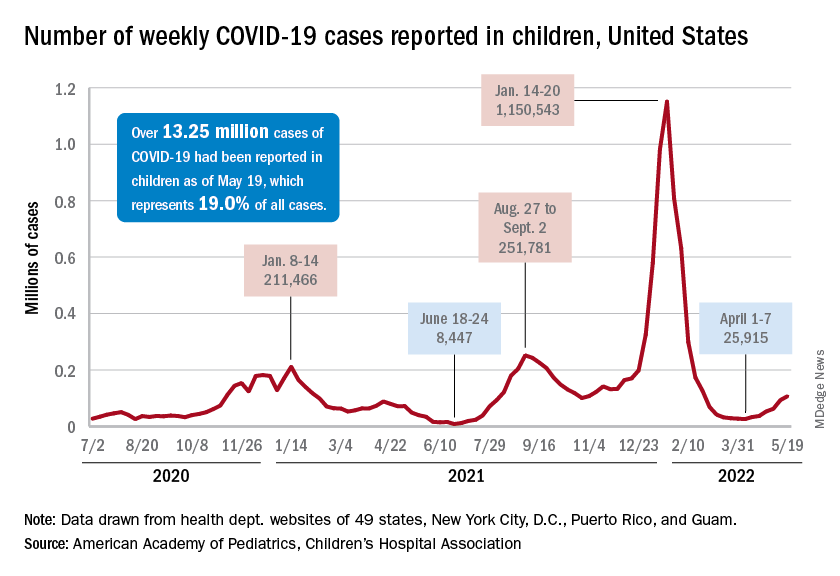
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”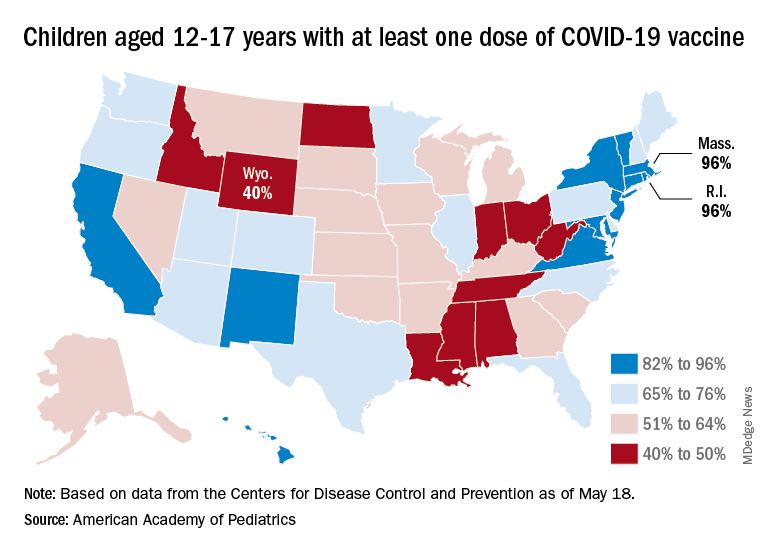
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
Cutaneous Lupus Erythematosus–like Isotopic Response to Herpes Zoster Infection
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
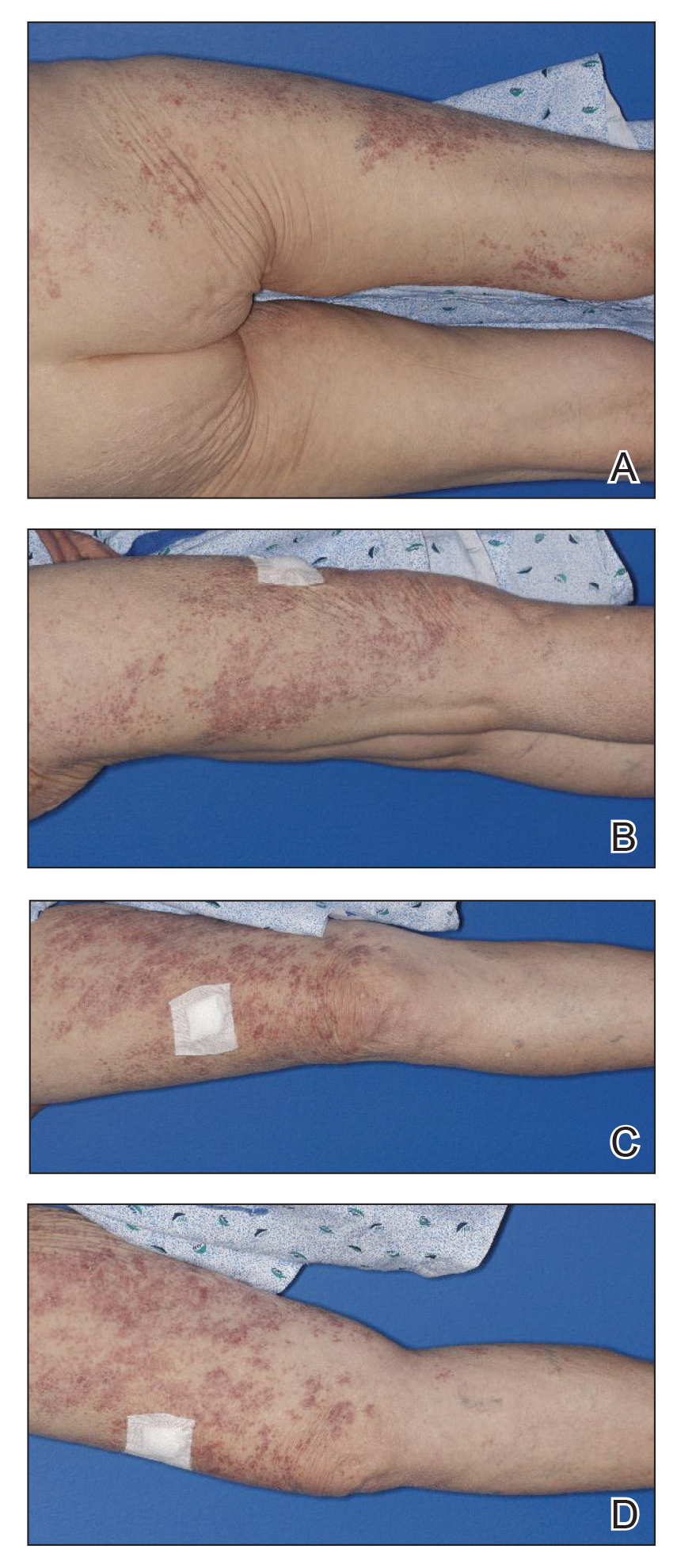
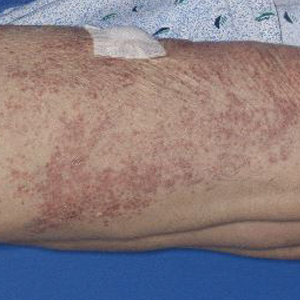
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
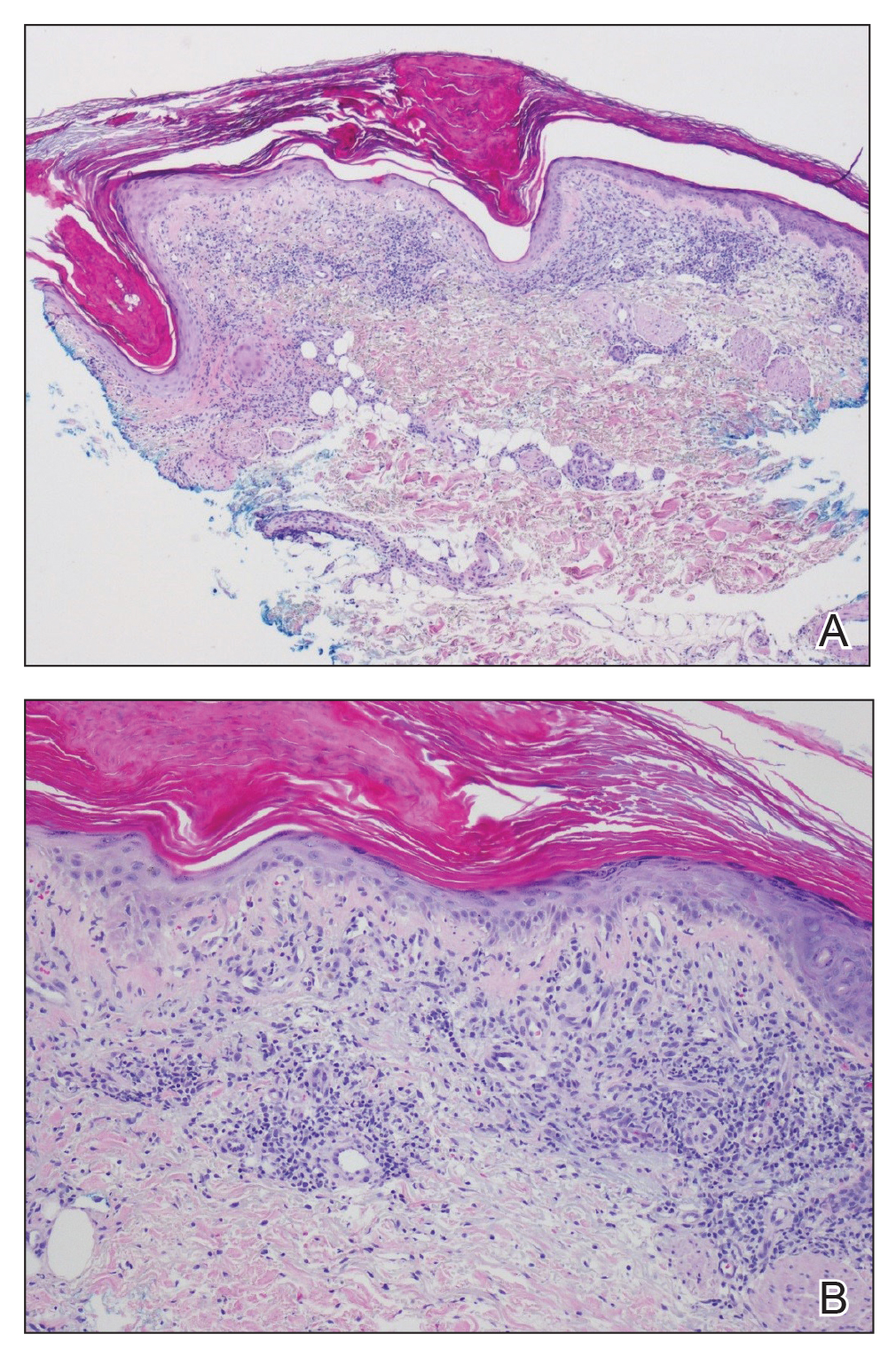
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
Practice Points
- Wolf isotopic response describes the occurrence of a new skin condition at the site of a previously healed and unrelated skin disorder; a granulomatous reaction is a commonly reported isotopic response.
- Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses.
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Monkeypox quarantines not needed in U.S., Biden says
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
Doxycycline bests azithromycin for anorectal chlamydia in women
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
OTC meds, supplements, and other drugs may interact with HIV antiretrovirals
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
Rabies: CDC updates and simplifies preexposure prophylaxis vaccination recommendations
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
RSV kills 100,000 kids under age 5 a year worldwide
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
CDC signs off on COVID boosters in children ages 5-11
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Health care facilities can prevent 35%-70% of infections. Here’s how
Good hand hygiene and other cost-effective infection prevention and control (IPC) practices can eliminate between 35% and 70% of health care–setting infections in all countries regardless of economic status, the World Health Organization reports.
IPC uses a practical, evidence-based approach to help patients, health care workers, and visitors to health care facilities avoid harmful infections, which can range from infections caused by localized antibiotic-resistant bacteria to pandemic viruses. The WHO calls the report the first global analysis of IPC implementation.
“Hospitals across the world saw increased rates of health care–associated infections (HAIs) during the COVID-19 pandemic. This included SARS-CoV-2 infections and other HAIs that increased as our health care systems were stretched to the breaking point and fewer resources were available for HAI prevention,” Daniel Diekema, MD, who was not involved in the report, said in an email.
“As we enter the third year of the pandemic, this WHO report should serve as an urgent call to action,” Dr. Diekema, a clinical professor of internal medicine at University of Iowa Health Care and an associate hospital epidemiologist with University of Iowa Hospitals and Clinics, both in Iowa City, noted. “Investing more resources in IPC programs will not only improve pandemic response, it will reduce morbidity, mortality, and global costs from all HAIs.”
No country or health system is free of HAIs
“Disparities in IPC investments between high- and low-income countries is the greatest challenge outlined in this report,” Dr. Diekema said in an email. “If the pandemic has taught us anything, it is that an infection spread anywhere in the world can soon become a problem everywhere. Thus, it is in everyone’s interest to ensure that IPC resources are more equitably distributed across the world.”
The report notes that HAIs are among the most common adverse events experienced in health care, and many HAIs are caused by multidrug-resistant organisms. The report includes these details:
It is predicted that of every 100 patients in acute-care hospitals, an average of 7 patients in high-income countries and 15 in low- and middle-income countries will acquire at least one HAI while hospitalized; as many as 30% of patients in intensive care encounter HAIs.
Of all cases of hospital-treated sepsis, 23.6% were linked to health care; 48.7% of all sepsis cases involving organ dysfunction treated in adult intensive care were acquired in the hospital; 24.4% of patients and 52.3% of those in intensive care who were affected by health care–associated sepsis died.
The European Centre for Disease Prevention and Control calculated that 4.5 million episodes of HAIs occurred each year among patients in acute-care hospitals in countries of the European Union and the European Economic Area.
The Centers for Disease Control and Prevention estimated that on any day, 1 in 31 hospital patients and 1 in 43 nursing home residents has an HAI.
Up to 41% of hospitalized patients with confirmed COVID-19 were infected with SARS-CoV-2 in health care settings.
Over roughly the first 18 months of the pandemic, COVID-19 killed between 80,000 and 180,000 health care workers worldwide.
The COVID-19 pandemic highlights the need for IPC
Despite the pandemic, high-income countries were eight times more likely to implement more advanced IPC than low-income countries, and IPC national programs in low- and middle-income countries improved only slightly.
Only 4 (3.8%) of the 106 evaluated countries met all the minimum requirements for IPC in place at the national level, and only 15.2% of health care facilities met all IPC minimum requirements.
Libby A. Richards, RN, MSN, PhD, CHES, an associate professor of nursing and the director of the PhD program in the Purdue University School of Nursing in West Lafayette, Ind., welcomed the report.
“While the principles of infection prevention and control have been fundamental for well over a hundred years, the COVID-19 pandemic brought these critical issues to everyone’s attention,” Dr. Richards, who was not involved in the report, said by email. “During the pandemic, the impact on our overburdened and understaffed health care system left little or no room for other acutely ill patients.
“This report brings timely attention to the importance of IPC across health care services,” she added.
Suzanne Wagester, RN, MSN, director of infection prevention at the University of Pittsburgh Medical Center, said in an email, “The pandemic has united us as a society as we recognize that infections impact us all. We struggle with the same universal challenges that directly impact the work of infection prevention.
“IPC programs are vital to facilities, patients, and countries,” Ms. Wagester, who also was not involved in the report, added. “The WHO report highlights the call to action that will hopefully ignite the movement to advance IPC programs across the globe to combat preventable infections.”
The WHO Global IPC Portal helps health care professionals in all countries analyze, track progress, and improve IPC at facility and national levels.
The report was funded by core WHO funds. The authors and Dr. Diekema, Dr. Richards, and Ms. Wagester have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good hand hygiene and other cost-effective infection prevention and control (IPC) practices can eliminate between 35% and 70% of health care–setting infections in all countries regardless of economic status, the World Health Organization reports.
IPC uses a practical, evidence-based approach to help patients, health care workers, and visitors to health care facilities avoid harmful infections, which can range from infections caused by localized antibiotic-resistant bacteria to pandemic viruses. The WHO calls the report the first global analysis of IPC implementation.
“Hospitals across the world saw increased rates of health care–associated infections (HAIs) during the COVID-19 pandemic. This included SARS-CoV-2 infections and other HAIs that increased as our health care systems were stretched to the breaking point and fewer resources were available for HAI prevention,” Daniel Diekema, MD, who was not involved in the report, said in an email.
“As we enter the third year of the pandemic, this WHO report should serve as an urgent call to action,” Dr. Diekema, a clinical professor of internal medicine at University of Iowa Health Care and an associate hospital epidemiologist with University of Iowa Hospitals and Clinics, both in Iowa City, noted. “Investing more resources in IPC programs will not only improve pandemic response, it will reduce morbidity, mortality, and global costs from all HAIs.”
No country or health system is free of HAIs
“Disparities in IPC investments between high- and low-income countries is the greatest challenge outlined in this report,” Dr. Diekema said in an email. “If the pandemic has taught us anything, it is that an infection spread anywhere in the world can soon become a problem everywhere. Thus, it is in everyone’s interest to ensure that IPC resources are more equitably distributed across the world.”
The report notes that HAIs are among the most common adverse events experienced in health care, and many HAIs are caused by multidrug-resistant organisms. The report includes these details:
It is predicted that of every 100 patients in acute-care hospitals, an average of 7 patients in high-income countries and 15 in low- and middle-income countries will acquire at least one HAI while hospitalized; as many as 30% of patients in intensive care encounter HAIs.
Of all cases of hospital-treated sepsis, 23.6% were linked to health care; 48.7% of all sepsis cases involving organ dysfunction treated in adult intensive care were acquired in the hospital; 24.4% of patients and 52.3% of those in intensive care who were affected by health care–associated sepsis died.
The European Centre for Disease Prevention and Control calculated that 4.5 million episodes of HAIs occurred each year among patients in acute-care hospitals in countries of the European Union and the European Economic Area.
The Centers for Disease Control and Prevention estimated that on any day, 1 in 31 hospital patients and 1 in 43 nursing home residents has an HAI.
Up to 41% of hospitalized patients with confirmed COVID-19 were infected with SARS-CoV-2 in health care settings.
Over roughly the first 18 months of the pandemic, COVID-19 killed between 80,000 and 180,000 health care workers worldwide.
The COVID-19 pandemic highlights the need for IPC
Despite the pandemic, high-income countries were eight times more likely to implement more advanced IPC than low-income countries, and IPC national programs in low- and middle-income countries improved only slightly.
Only 4 (3.8%) of the 106 evaluated countries met all the minimum requirements for IPC in place at the national level, and only 15.2% of health care facilities met all IPC minimum requirements.
Libby A. Richards, RN, MSN, PhD, CHES, an associate professor of nursing and the director of the PhD program in the Purdue University School of Nursing in West Lafayette, Ind., welcomed the report.
“While the principles of infection prevention and control have been fundamental for well over a hundred years, the COVID-19 pandemic brought these critical issues to everyone’s attention,” Dr. Richards, who was not involved in the report, said by email. “During the pandemic, the impact on our overburdened and understaffed health care system left little or no room for other acutely ill patients.
“This report brings timely attention to the importance of IPC across health care services,” she added.
Suzanne Wagester, RN, MSN, director of infection prevention at the University of Pittsburgh Medical Center, said in an email, “The pandemic has united us as a society as we recognize that infections impact us all. We struggle with the same universal challenges that directly impact the work of infection prevention.
“IPC programs are vital to facilities, patients, and countries,” Ms. Wagester, who also was not involved in the report, added. “The WHO report highlights the call to action that will hopefully ignite the movement to advance IPC programs across the globe to combat preventable infections.”
The WHO Global IPC Portal helps health care professionals in all countries analyze, track progress, and improve IPC at facility and national levels.
The report was funded by core WHO funds. The authors and Dr. Diekema, Dr. Richards, and Ms. Wagester have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good hand hygiene and other cost-effective infection prevention and control (IPC) practices can eliminate between 35% and 70% of health care–setting infections in all countries regardless of economic status, the World Health Organization reports.
IPC uses a practical, evidence-based approach to help patients, health care workers, and visitors to health care facilities avoid harmful infections, which can range from infections caused by localized antibiotic-resistant bacteria to pandemic viruses. The WHO calls the report the first global analysis of IPC implementation.
“Hospitals across the world saw increased rates of health care–associated infections (HAIs) during the COVID-19 pandemic. This included SARS-CoV-2 infections and other HAIs that increased as our health care systems were stretched to the breaking point and fewer resources were available for HAI prevention,” Daniel Diekema, MD, who was not involved in the report, said in an email.
“As we enter the third year of the pandemic, this WHO report should serve as an urgent call to action,” Dr. Diekema, a clinical professor of internal medicine at University of Iowa Health Care and an associate hospital epidemiologist with University of Iowa Hospitals and Clinics, both in Iowa City, noted. “Investing more resources in IPC programs will not only improve pandemic response, it will reduce morbidity, mortality, and global costs from all HAIs.”
No country or health system is free of HAIs
“Disparities in IPC investments between high- and low-income countries is the greatest challenge outlined in this report,” Dr. Diekema said in an email. “If the pandemic has taught us anything, it is that an infection spread anywhere in the world can soon become a problem everywhere. Thus, it is in everyone’s interest to ensure that IPC resources are more equitably distributed across the world.”
The report notes that HAIs are among the most common adverse events experienced in health care, and many HAIs are caused by multidrug-resistant organisms. The report includes these details:
It is predicted that of every 100 patients in acute-care hospitals, an average of 7 patients in high-income countries and 15 in low- and middle-income countries will acquire at least one HAI while hospitalized; as many as 30% of patients in intensive care encounter HAIs.
Of all cases of hospital-treated sepsis, 23.6% were linked to health care; 48.7% of all sepsis cases involving organ dysfunction treated in adult intensive care were acquired in the hospital; 24.4% of patients and 52.3% of those in intensive care who were affected by health care–associated sepsis died.
The European Centre for Disease Prevention and Control calculated that 4.5 million episodes of HAIs occurred each year among patients in acute-care hospitals in countries of the European Union and the European Economic Area.
The Centers for Disease Control and Prevention estimated that on any day, 1 in 31 hospital patients and 1 in 43 nursing home residents has an HAI.
Up to 41% of hospitalized patients with confirmed COVID-19 were infected with SARS-CoV-2 in health care settings.
Over roughly the first 18 months of the pandemic, COVID-19 killed between 80,000 and 180,000 health care workers worldwide.
The COVID-19 pandemic highlights the need for IPC
Despite the pandemic, high-income countries were eight times more likely to implement more advanced IPC than low-income countries, and IPC national programs in low- and middle-income countries improved only slightly.
Only 4 (3.8%) of the 106 evaluated countries met all the minimum requirements for IPC in place at the national level, and only 15.2% of health care facilities met all IPC minimum requirements.
Libby A. Richards, RN, MSN, PhD, CHES, an associate professor of nursing and the director of the PhD program in the Purdue University School of Nursing in West Lafayette, Ind., welcomed the report.
“While the principles of infection prevention and control have been fundamental for well over a hundred years, the COVID-19 pandemic brought these critical issues to everyone’s attention,” Dr. Richards, who was not involved in the report, said by email. “During the pandemic, the impact on our overburdened and understaffed health care system left little or no room for other acutely ill patients.
“This report brings timely attention to the importance of IPC across health care services,” she added.
Suzanne Wagester, RN, MSN, director of infection prevention at the University of Pittsburgh Medical Center, said in an email, “The pandemic has united us as a society as we recognize that infections impact us all. We struggle with the same universal challenges that directly impact the work of infection prevention.
“IPC programs are vital to facilities, patients, and countries,” Ms. Wagester, who also was not involved in the report, added. “The WHO report highlights the call to action that will hopefully ignite the movement to advance IPC programs across the globe to combat preventable infections.”
The WHO Global IPC Portal helps health care professionals in all countries analyze, track progress, and improve IPC at facility and national levels.
The report was funded by core WHO funds. The authors and Dr. Diekema, Dr. Richards, and Ms. Wagester have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.