User login
Orf Virus in Humans: Case Series and Clinical Review
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
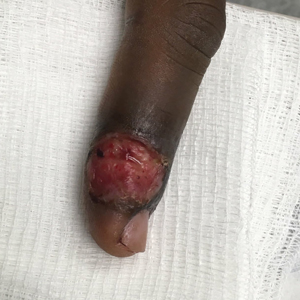
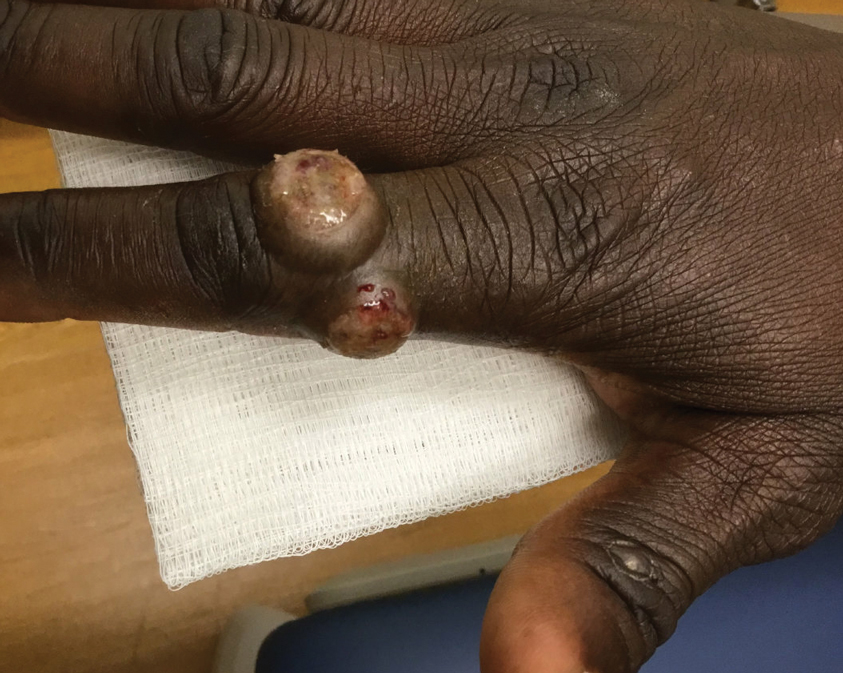
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
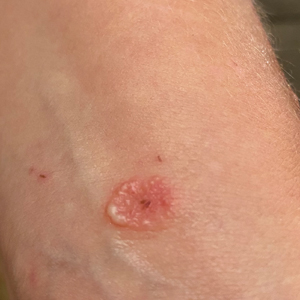
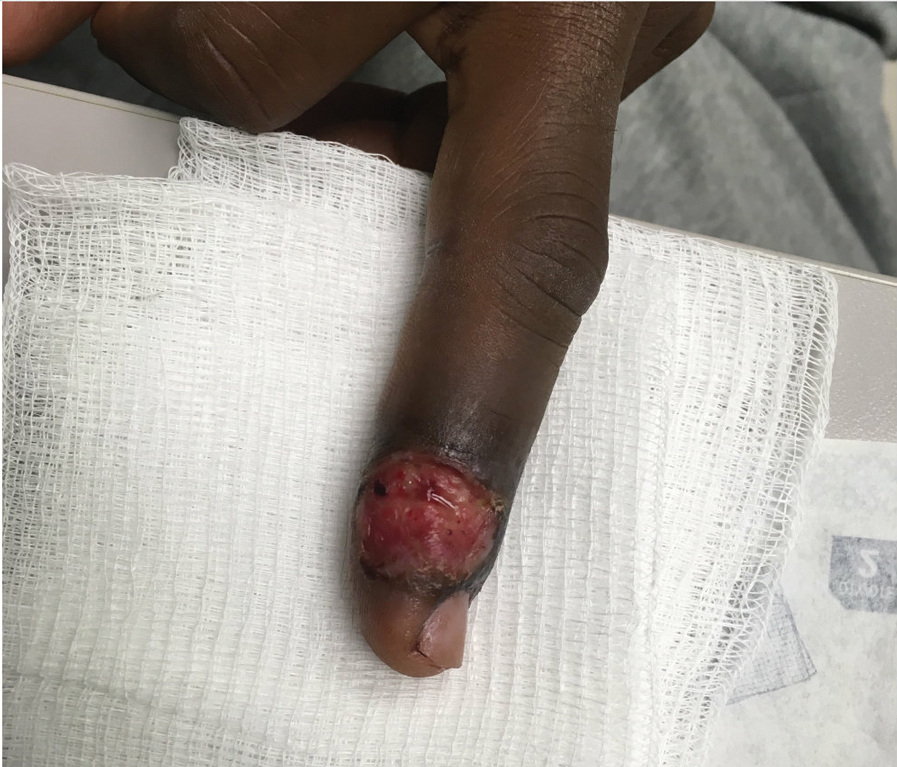
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
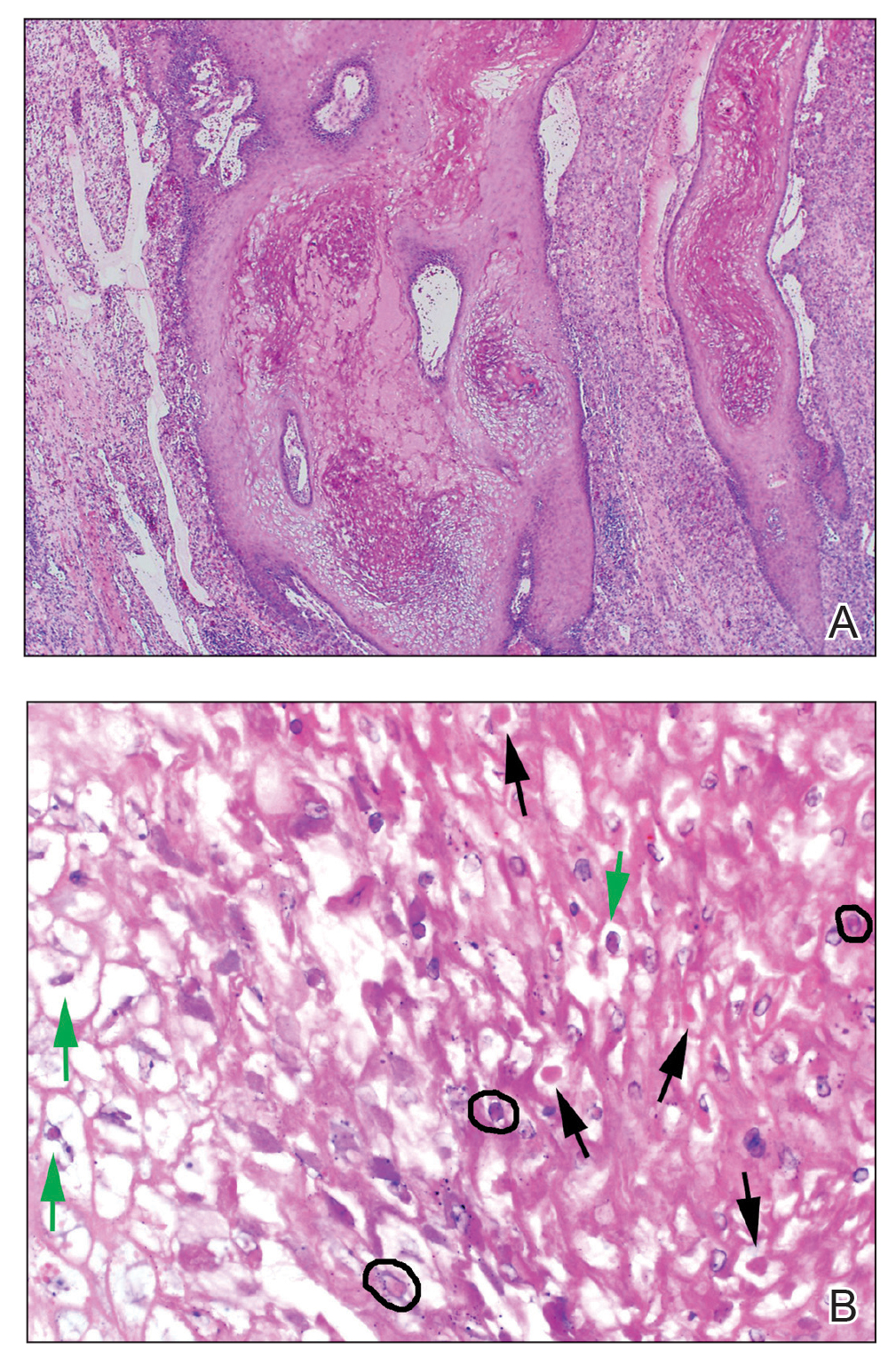
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
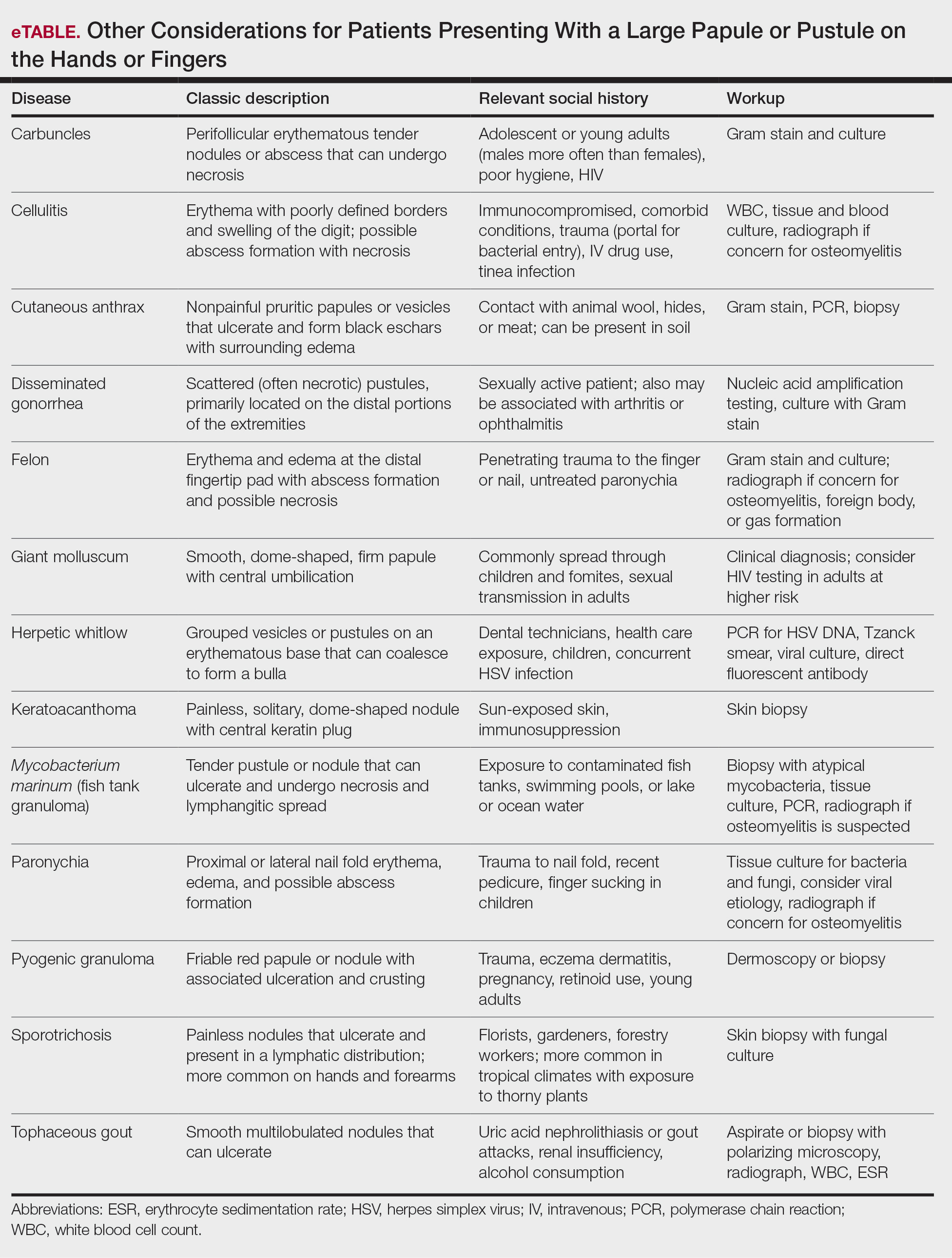
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
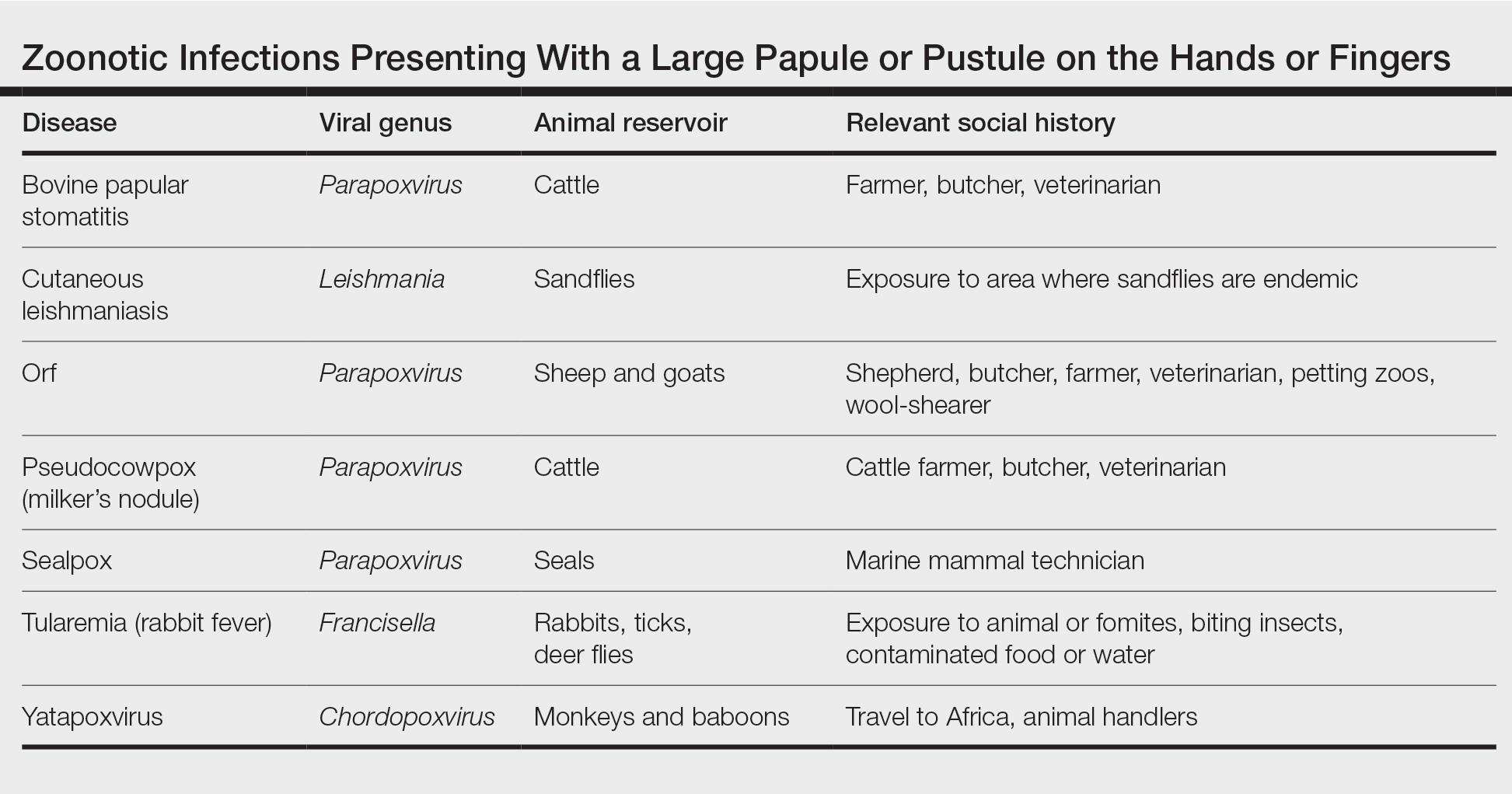
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.
U.K. survey: Dermatologists want training in prescribing antipsychotics for delusional infestation
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT BAD 2022
CDC recommends high-dose flu vaccines for seniors
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
Monkeypox mutating faster than expected
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
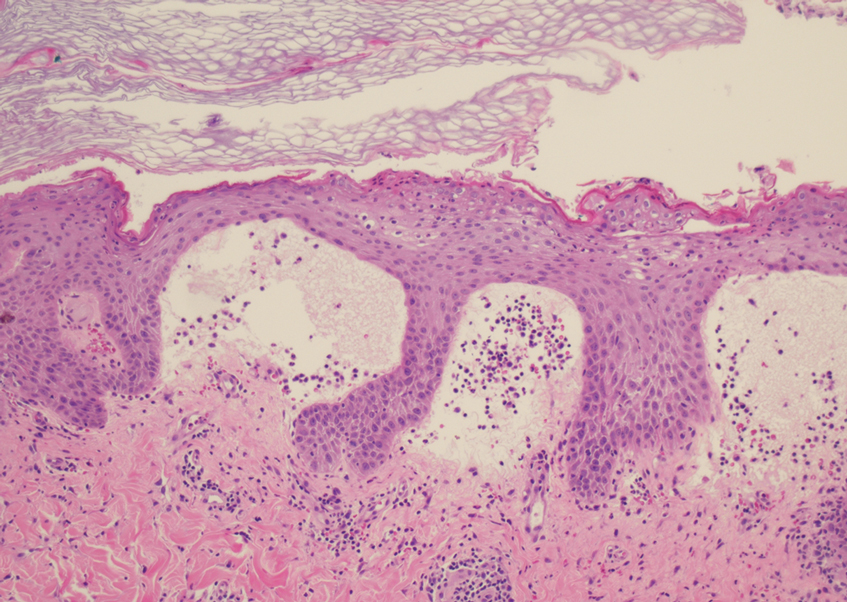
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
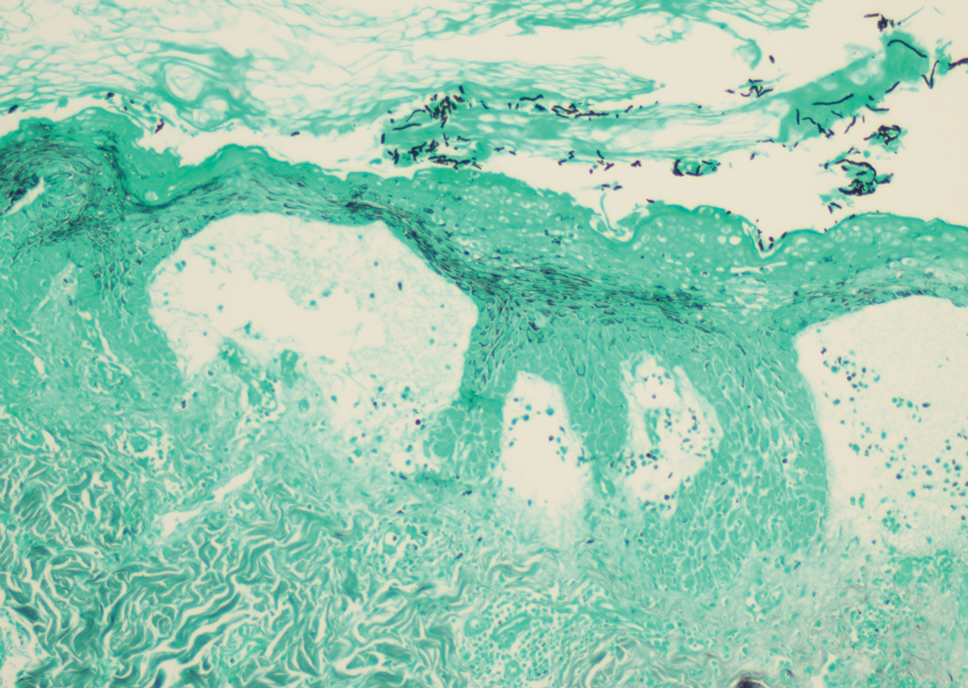
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
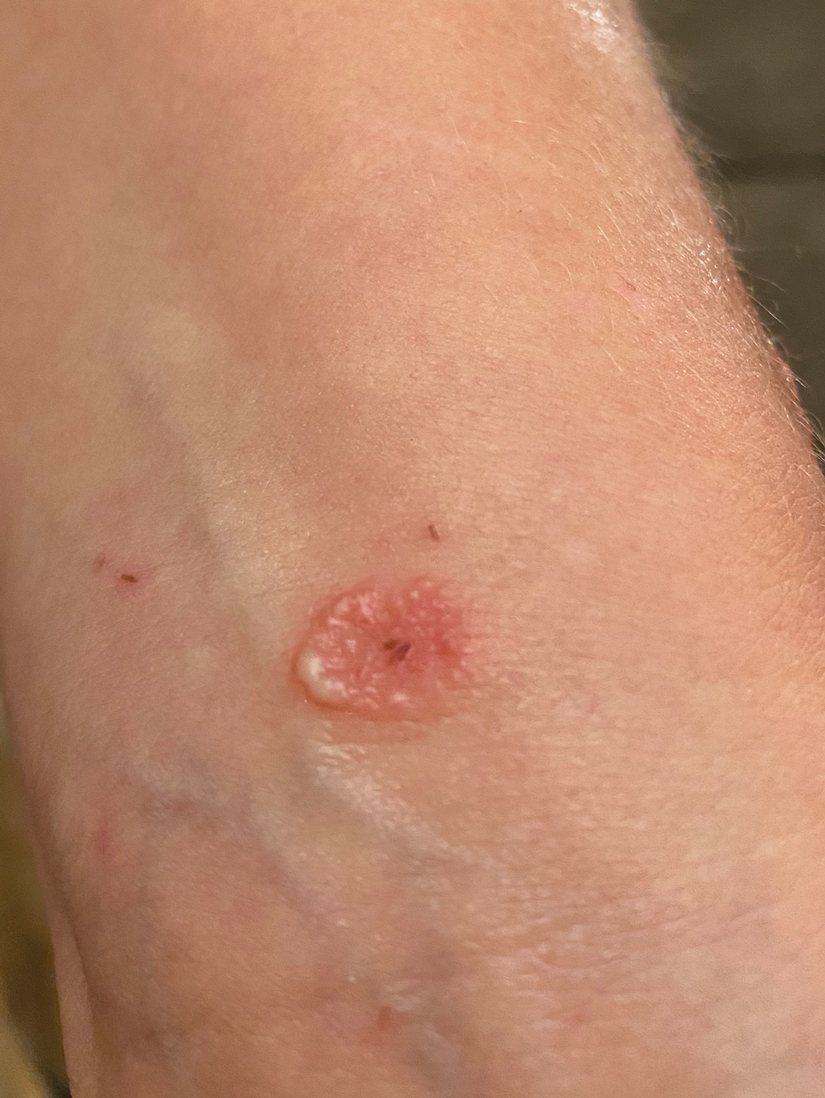
Doctors still overprescribing fluoroquinolones despite risks
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
Monkeypox: What FPs need to know, now
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
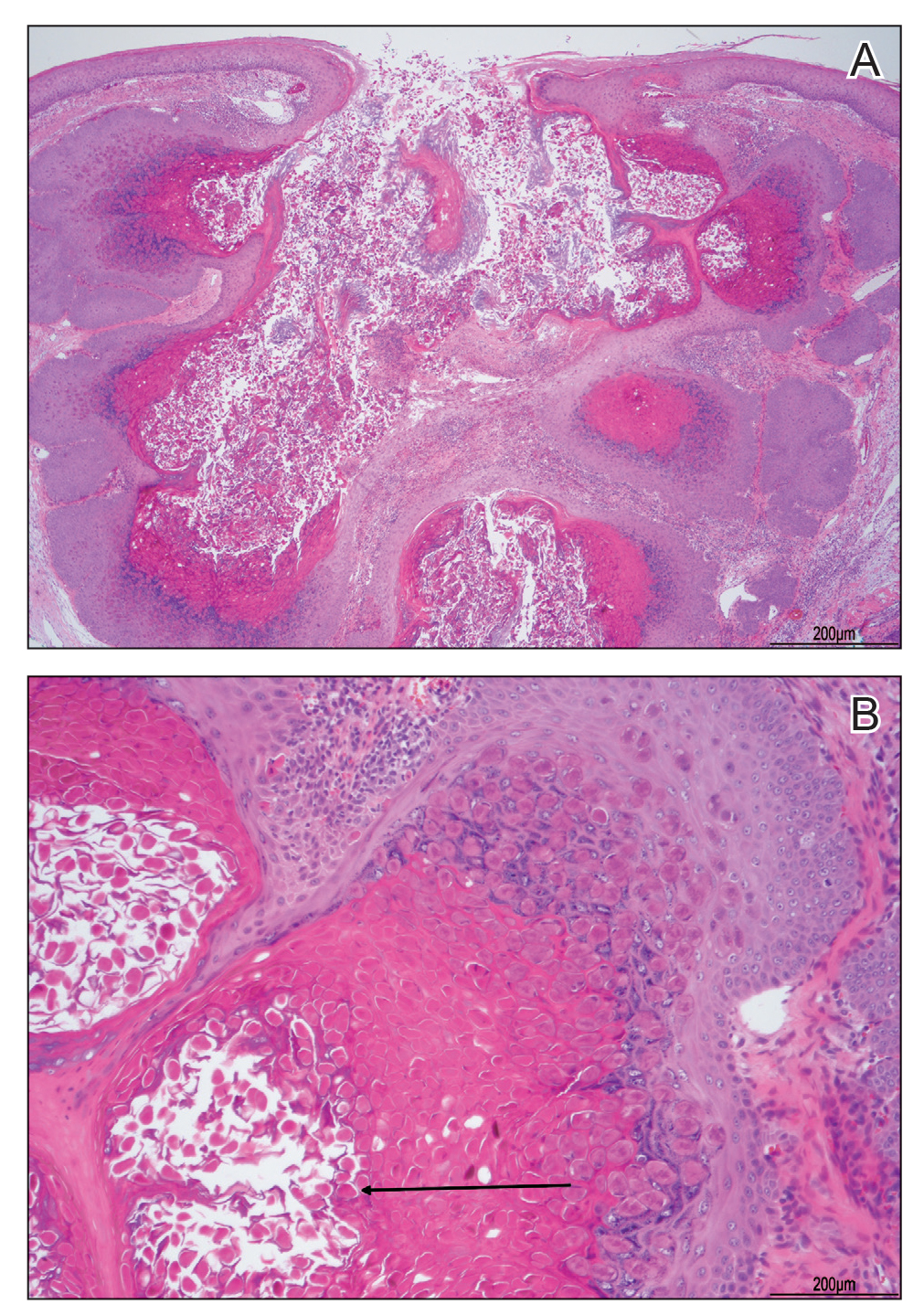
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
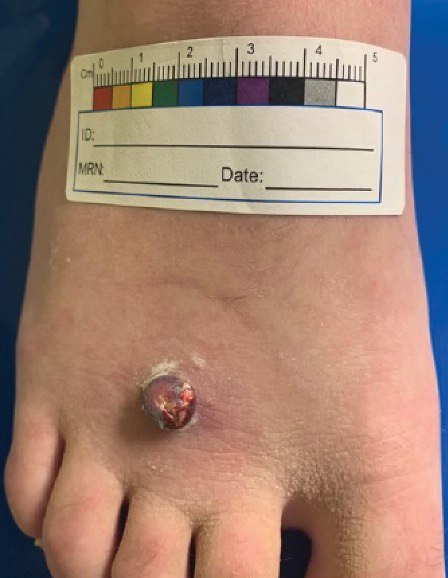
Widespread rash in toddler
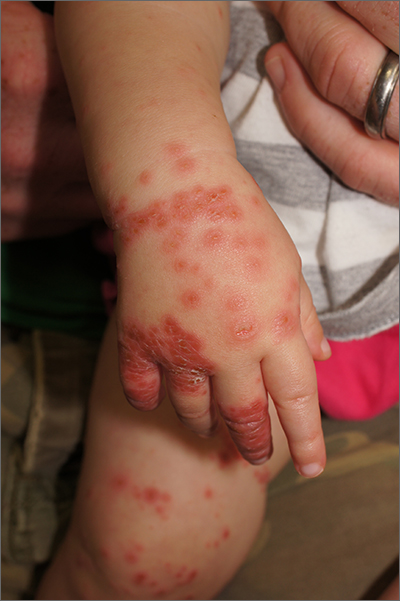
This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071

This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

This patient was given a diagnosis of Gianotti Crosti syndrome (GCS; also called infantile acrodermatitis of childhood), which is a self-resolving (often dramatic) dermatosis triggered by a viral infection or immunization. Patients with this syndrome develop papules, vesicles, and plaques on their face, hands, feet, and extremities a week (or more) after having a viral illness or receiving an immunization. In patients with darker skin types, lesions may appear purple to brown rather than bright red to red/orange. The syndrome typically occurs in children between the ages of 1 to 4 years, but almost all patients are under the age of 15.1 Scratching and sleep disturbance are common. The condition typically resolves on its own after 3 or 4 weeks.
Globally, the hepatitis B virus (HBV) is the most common cause of GCS.1 Other reported triggering viruses include hepatitis A and C, cytomegalovirus, Epstein-Barr virus, enteroviruses, HIV, parainfluenza viruses, parvoviruses, rubella, and COVID-19.2
Since the cause of this patient’s case of GCS was likely linked to a viral infection that produced the loose stools in a population with low-HBV risk, no further serologic testing was performed. Serologic testing may have been necessary if other infections, disease risks, or symptoms were identified. To relieve itching, topical triamcinolone 0.1% cream was prescribed for use once to twice daily on the extremities and hydrocortisone 1% cream was prescribed once to twice daily for use on the child’s face. At the 6-week follow-up visit, the lesions had resolved; light pink discoloration remained but was expected to further fade. In patients with darker skin, post-inflammatory hyperpigmentation may take several months to resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071
1. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-45. doi: 10.1016/j.jaad.2005.09.033
2. Berná-Rico ED, Álvarez-Pinheiro C, Burgos-Blasco P, et al. A Gianotti-Crosti-like eruption in the setting of SARS-CoV-2 infection. Dermatol Ther. 2021;34:e15071. Doi:10.1111/dth.15071
White House expands access to monkeypox vaccines
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.