User login
How to have a safer and more joyful holiday season
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
This holiday season, I am looking forward to spending some time with family, as I have in the past. As I have chatted with others, many friends are looking forward to events that are potentially larger and potentially returning to prepandemic type gatherings.
Gathering is important and can bring joy, sense of community, and love to the lives of many. Unfortunately, the risks associated with gathering are not over. as our country faces many cases of respiratory syncytial virus (RSV), COVID-19, and influenza at the same time.
During the first week of December, cases of influenza were rising across the country1 and were rising faster than in previous years. Although getting the vaccine is an important method of influenza prevention and is recommended for everyone over the age of 6 months with rare exception, many have not gotten their vaccine this year.
Influenza
Thus far, “nearly 50% of reported flu-associated hospitalizations in women of childbearing age have been in women who are pregnant.” We are seeing this at a time with lower-than-average uptake of influenza vaccine leaving both the pregnant persons and their babies unprotected. In addition to utilizing vaccines as prevention, isolating when ill, cleaning surfaces, and practicing good hand hygiene can all decrease transmission.
RSV
In addition to rises of influenza, there are currently high rates of RSV in various parts of the country. Prior to 2020, RSV typically started in the fall and peaked in the winter months. However, since the pandemic, the typical seasonal pattern has not returned, and it is unclear when it will. Although RSV hits the very young, the old, and the immunocompromised the most, RSV can infect anyone. Unfortunately, we do not currently have a vaccine for everyone against this virus. Prevention of transmission includes, as with flu, isolating when ill, cleaning surfaces, and washing hands.2
COVID-19
Of course, the effects of the COVID-19 pandemic are also still here as well. During the first week of December, the CDC reported rising cases of COVID across the country. Within the past few months, there have been several developments, though, for protection. There are now bivalent vaccines available as either third doses or booster doses approved for all persons over 6 months of age. As of the first week of December, only 13.5% of those aged 5 and over had received an updated booster.
There is currently wider access to rapid testing, including at-home testing, which can allow individuals to identify if COVID positive. Additionally, there is access to medication to decrease the likelihood of severe disease – though this does not take the place of vaccinations.
If anyone does test positive for COVID, they should follow the most recent quarantine guidelines including wearing a well-fitted mask when they do begin returning to activities.3
With rising cases of all three of these viruses, some may be asking how we can safely gather. There are several things to consider and do to enjoy our events. The first thing everyone can do is to receive updated vaccinations for both influenza and COVID-19 if eligible. Although it may take some time to be effective, vaccination is still one of our most effective methods of disease prevention and is important this winter season. Vaccinations can also help decrease the risk of severe disease.
Although many have stopped masking, as cases rise, it is time to consider masking particularly when community levels of any of these viruses are high. Masks help with preventing and spreading more than just COVID-19. Using them can be especially important for those going places such as stores and to large public gatherings and when riding on buses, planes, or trains.
In summary
Preventing exposure by masking can help keep individuals healthy prior to celebrating the holidays with others. With access to rapid testing, it makes sense to consider testing prior to gathering with friends and family. Most importantly, although we all are looking forward to spending time with our loved ones, it is important to stay home if not feeling well. Following these recommendations will allow us to have a safer and more joyful holiday season.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Influenza (flu). [Online] Dec. 1, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/flu/index.htm.
2. Respiratory syncytial virus. Respiratory syncytial virus infection (RSV). [Online] Oct. 28, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/rsv/index.html.
3. COVID-19. [Online] Dec. 7, 2022. [Cited: 2022 Dec 10.] https://www.cdc.gov/coronavirus/2019-ncov/index.html.
Ohio measles outbreak grows, fueled by vaccine hesitancy
The Ohio measles outbreak continues to expand, with cases now totaling 81 – a 37% increase in the course of just 2 weeks.
. Most of the children infected were unvaccinated but were old enough to get the measles, mumps, and rubella (MMR) shot, which is 97% effective at preventing measles.
“I think these are individuals who are making a decision not to protect their children against vaccine-preventable diseases, and some of them are making a specific decision not to use the MMR vaccine,” Columbus Public Health Commissioner Mysheika W. Roberts, MD, told JAMA.
She said that parents’ refusal to vaccinate their children was due to a misconception that the vaccine causes autism.
“We’re sounding the alarm that if your child is of age and not vaccinated, they should get vaccinated ASAP,” Dr. Roberts said, noting that she hasn’t seen that happening more.
Health officials have predicted the outbreak, which started in November, will last at least several months. Measles is so contagious that 9 out of 10 unvaccinated people in a room will become infected if exposed.
All of the infections have been in children. According to the Columbus Public Health measles dashboard, of the 81 confirmed cases:
- 29 children have been hospitalized.
- 22 cases are among children under 1 year old.
- No deaths have been reported.
Dr. Roberts said the hospitalized children have had symptoms including dehydration, diarrhea, and pneumonia. Some have had to go to the intensive care unit.
Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, and even death, the CDC says.
One of the most recent cases was an infant too young to be vaccinated who lives 45 miles away from where the outbreak began, the Dayton Daily News reported. That’s the first case in Clark County in more than 20 years. At least 10% of kindergartners’ parents in the region’s elementary schools opted out of vaccines because of religious or moral objections.
“We knew this was coming. It was a matter of when, not if,” Yamini Teegala, MD, chief medical officer at Rocking Horse Community Health Center in Springfield, told the Dayton Daily News.
This is the second measles outbreak this year. Minnesota tallied 22 cases since June in an unrelated outbreak, JAMA reported.
A version of this article first appeared on WebMD.com.
The Ohio measles outbreak continues to expand, with cases now totaling 81 – a 37% increase in the course of just 2 weeks.
. Most of the children infected were unvaccinated but were old enough to get the measles, mumps, and rubella (MMR) shot, which is 97% effective at preventing measles.
“I think these are individuals who are making a decision not to protect their children against vaccine-preventable diseases, and some of them are making a specific decision not to use the MMR vaccine,” Columbus Public Health Commissioner Mysheika W. Roberts, MD, told JAMA.
She said that parents’ refusal to vaccinate their children was due to a misconception that the vaccine causes autism.
“We’re sounding the alarm that if your child is of age and not vaccinated, they should get vaccinated ASAP,” Dr. Roberts said, noting that she hasn’t seen that happening more.
Health officials have predicted the outbreak, which started in November, will last at least several months. Measles is so contagious that 9 out of 10 unvaccinated people in a room will become infected if exposed.
All of the infections have been in children. According to the Columbus Public Health measles dashboard, of the 81 confirmed cases:
- 29 children have been hospitalized.
- 22 cases are among children under 1 year old.
- No deaths have been reported.
Dr. Roberts said the hospitalized children have had symptoms including dehydration, diarrhea, and pneumonia. Some have had to go to the intensive care unit.
Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, and even death, the CDC says.
One of the most recent cases was an infant too young to be vaccinated who lives 45 miles away from where the outbreak began, the Dayton Daily News reported. That’s the first case in Clark County in more than 20 years. At least 10% of kindergartners’ parents in the region’s elementary schools opted out of vaccines because of religious or moral objections.
“We knew this was coming. It was a matter of when, not if,” Yamini Teegala, MD, chief medical officer at Rocking Horse Community Health Center in Springfield, told the Dayton Daily News.
This is the second measles outbreak this year. Minnesota tallied 22 cases since June in an unrelated outbreak, JAMA reported.
A version of this article first appeared on WebMD.com.
The Ohio measles outbreak continues to expand, with cases now totaling 81 – a 37% increase in the course of just 2 weeks.
. Most of the children infected were unvaccinated but were old enough to get the measles, mumps, and rubella (MMR) shot, which is 97% effective at preventing measles.
“I think these are individuals who are making a decision not to protect their children against vaccine-preventable diseases, and some of them are making a specific decision not to use the MMR vaccine,” Columbus Public Health Commissioner Mysheika W. Roberts, MD, told JAMA.
She said that parents’ refusal to vaccinate their children was due to a misconception that the vaccine causes autism.
“We’re sounding the alarm that if your child is of age and not vaccinated, they should get vaccinated ASAP,” Dr. Roberts said, noting that she hasn’t seen that happening more.
Health officials have predicted the outbreak, which started in November, will last at least several months. Measles is so contagious that 9 out of 10 unvaccinated people in a room will become infected if exposed.
All of the infections have been in children. According to the Columbus Public Health measles dashboard, of the 81 confirmed cases:
- 29 children have been hospitalized.
- 22 cases are among children under 1 year old.
- No deaths have been reported.
Dr. Roberts said the hospitalized children have had symptoms including dehydration, diarrhea, and pneumonia. Some have had to go to the intensive care unit.
Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, and even death, the CDC says.
One of the most recent cases was an infant too young to be vaccinated who lives 45 miles away from where the outbreak began, the Dayton Daily News reported. That’s the first case in Clark County in more than 20 years. At least 10% of kindergartners’ parents in the region’s elementary schools opted out of vaccines because of religious or moral objections.
“We knew this was coming. It was a matter of when, not if,” Yamini Teegala, MD, chief medical officer at Rocking Horse Community Health Center in Springfield, told the Dayton Daily News.
This is the second measles outbreak this year. Minnesota tallied 22 cases since June in an unrelated outbreak, JAMA reported.
A version of this article first appeared on WebMD.com.
FROM JAMA
Children and COVID: New-case counts offer dueling narratives
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
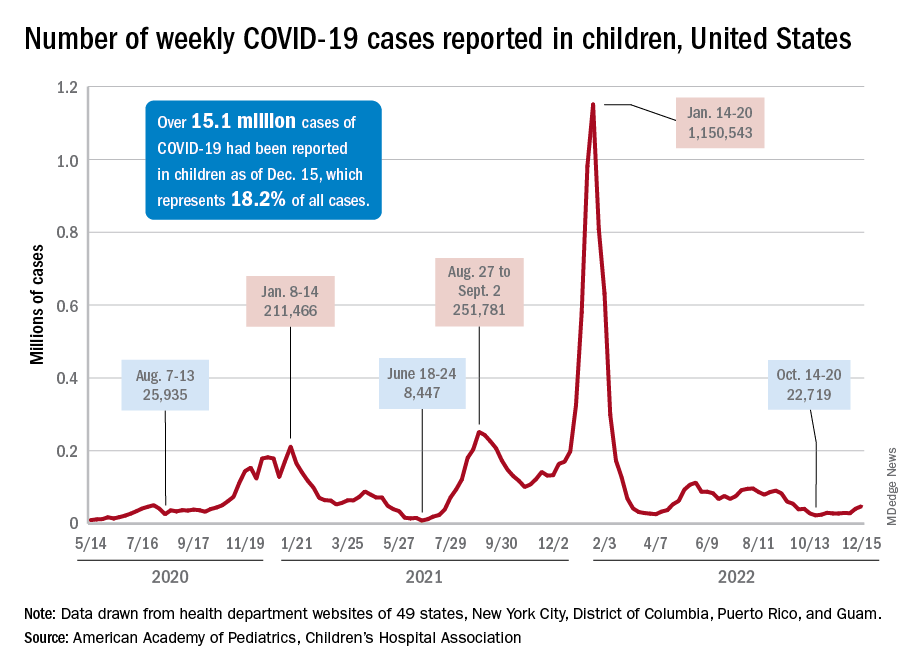
and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
Vaccinating pregnant women protects infants against severe RSV infection
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
An investigational vaccine against respiratory syncytial virus (RSV) in pregnant women has been shown to help protect infants against severe disease, according to the vaccine’s manufacturer.
Pfizer recently announced that in the course of a randomized, double-blind, placebo-controlled phase 3 study, the vaccine RSVpreF had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life, according to a company press release.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. A total of 7,400 women had received a single dose of 120 mcg RSVpreF in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Due to the good results, the enrollment in the study was halted on the recommendation of the study’s Data Monitoring Committee after achieving a primary endpoint. The company plans to apply for marketing authorization to the U.S. Food and Drug Administration by the end of 2022 and to other regulatory agencies in 2023.
“The directness of the strategy, to vaccinate expectant mothers during pregnancy so that their newborn is then later protected, is new and a very interesting approach,” commented Prof. Ortwin Adams, MD, head of virologic diagnostics at the Institute for Virology of the University Hospital of Düsseldorf (Germany) to the Science Media Centre (SMC).
In terms of the RSV vaccination strategy presented, “the unborn child has taken center stage from the outset.” Because the vaccination route is the placental transfer of antibodies from mother to child (“passive immunity”), “... the medical points of contact for this vaccination will be the gynecologists, not the pediatricians,” Dr. Adams said.
“This concept imitates the natural process, since the mother normally passes immune defenses she acquired through infections to the child via the umbilical cord and her breast milk before and after birth. This procedure is long-proven and practiced worldwide, especially in nonindustrialized countries, for a variety of diseases, including tetanus, whooping cough (pertussis), and viral flu (influenza),” explained Markus Rose, MD, PhD, head of Pediatric Pulmonology at the Olgahospital, Stuttgart, Germany.
The development of an RSV vaccine had ground to a halt for many decades: A tragedy in the 1960s set the whole field of research back. Using the model of the first polio vaccine, scientists had manufactured an experimental vaccine with inactivated viruses. However, tests showed that the vaccine did not protect the children vaccinated, but it actually infected them with RSV, they then fell ill, and two children died. Today, potential RSV vaccines are first tested on adults and not on children.
Few treatment options
RSV causes seasonal epidemics, can lead to bronchiolitis and pneumonia in infants, and is one of the main causes of hospital stays in young children. Monoclonal antibodies are currently the only preventive option, since there is still no vaccine. Usually, 60%-70% of infants and nearly all children younger than 2 years are infected with RSV, but the virus can also trigger pneumonia in adults.
“RSV infections constitute a major public health challenge: It is the most dangerous respiratory virus for young infants, it is also a threat to the chronically ill and immunocompromised of all ages, and [it] is the second most common cause of death worldwide (after malaria) in young children,” stated Dr. Rose.
Recently, pandemic-related measures (face masks, more intense disinfection) meant that the “normal” RSV infections in healthy adults, which usually progress like a mild cold, were prevented, and mothers were unable to pass on as much RSV immune defense to their children. “This was presumably responsible in part for the massive wave of RSV infections in fall and winter of 2021/22,” explained Dr. Rose.
Thomas Mertens, MD, PhD, chair of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO) and former director of the Institute for Virology at Ulm University Hospital, Germany, also noted: “It would be an important and potentially achievable goal to significantly reduce the incidence rate of hospitalizations. In this respect, RSV poses a significant problem for young children, their parents, and the burden on pediatric clinics.”
Final evaluation pending
“I am definitely finding the data interesting, but the original data are needed,” Dr. Mertens said. Once the data are published at a conference or published in a peer-reviewed journal, physicians will be able to better judge the data for themselves, he said.
Dr. Rose characterized the new vaccine as “novel,” including in terms of its composition. Earlier RSV vaccines used the so-called postfusion F protein as their starting point. But it has become known in the meantime that the key to immunogenicity is the continued prefusion state of the apical epitope: Prefusion F-specific memory B cells in adults naturally infected with RSV produce potent neutralizing antibodies.
The new vaccine is bivalent and protects against both RSV A and RSV B.
To date, RSV vaccination directly in young infants have had only had a weak efficacy and were sometimes poorly tolerated. The vaccine presented here is expected to be tested in young adults first, then in school children, then young children.
Through successful vaccination of the entire population, the transfer of RS viruses to young children could be prevented. “To what extent this, or any other RSV vaccine still to be developed on the same basis, will also be effective and well tolerated in young infants is still difficult to assess,” said Dr. Rose.
Dr. Mertens emphasized that all of the study data now needs to be seen as quickly as possible: “This is also a general requirement for transparency from the pharmaceutical companies, which is also rightly criticized.”
This article was originally published in Medscape’s German edition and a version appeared on Medscape.com.
Systematic review supports preferred drugs for HIV in youths
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE INTERNATIONAL AIDS SOCIETY
Scientists use mRNA technology for universal flu vaccine
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
COVID booster shot poll: People ‘don’t think they need one’
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
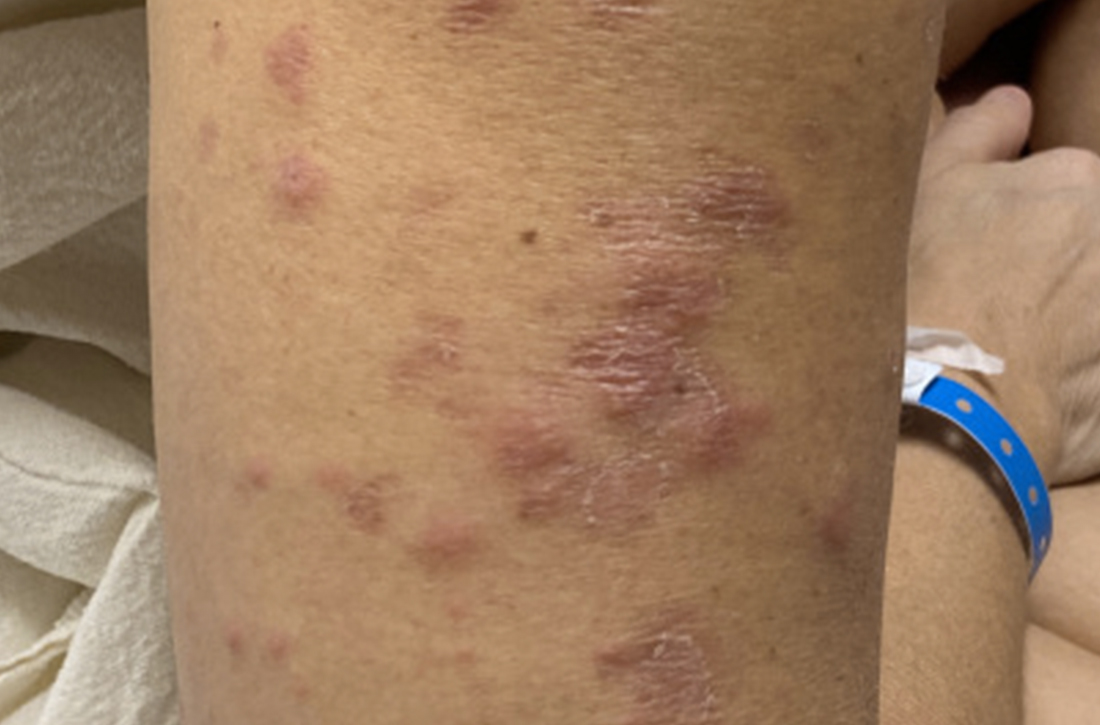
40-year-old woman • fever • rash • arthralgia • Dx?
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8
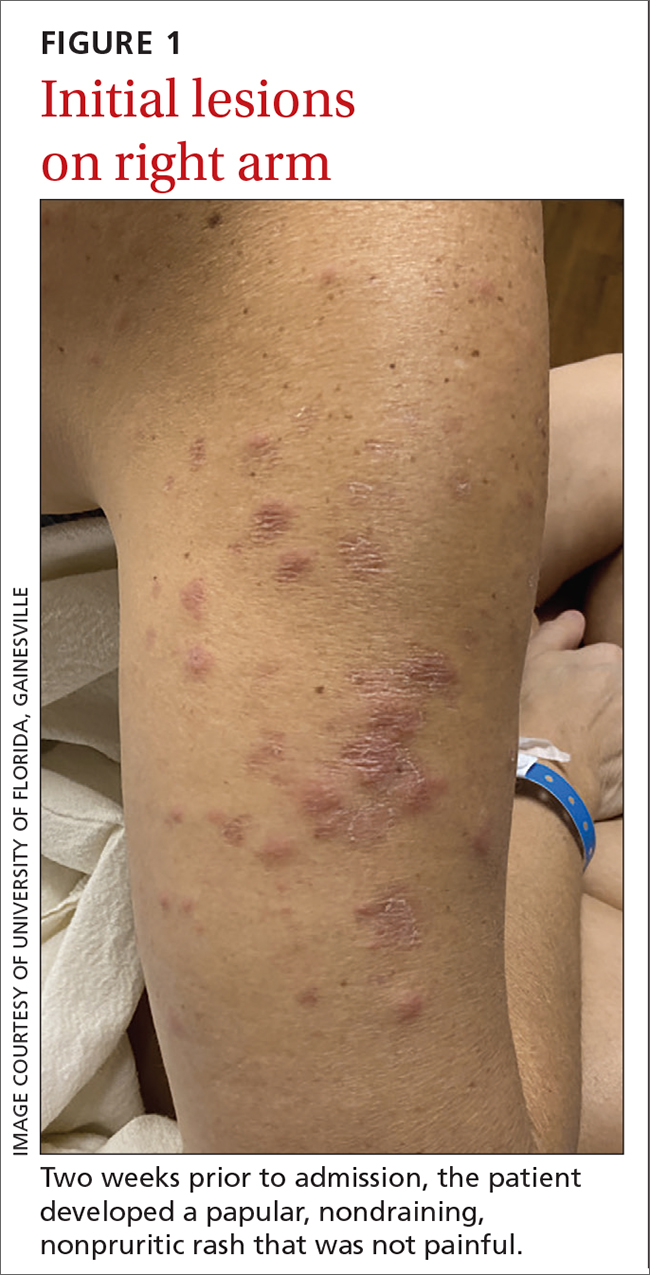
The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.
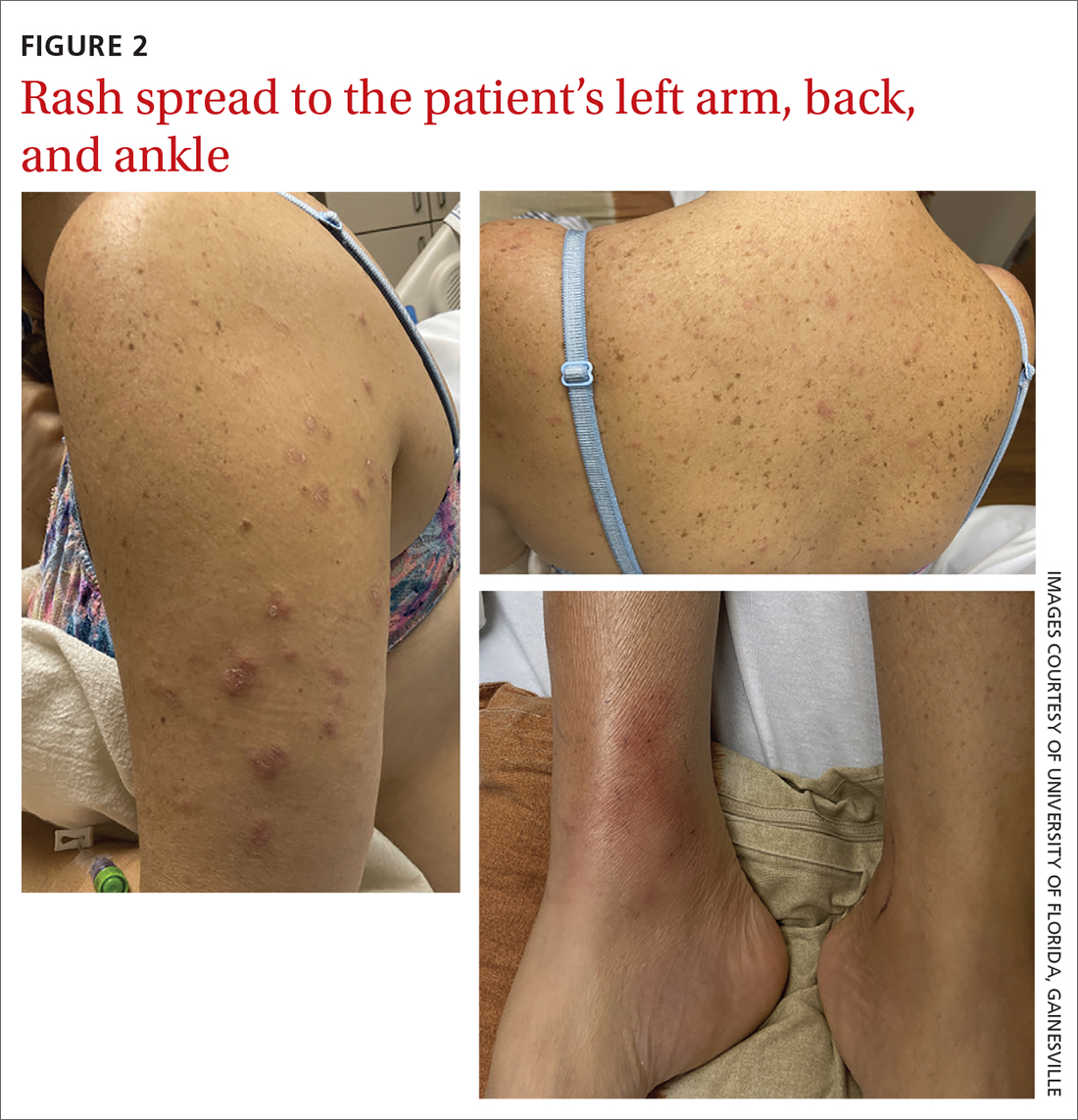
At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome. Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; [email protected]
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
What length antibiotic course for prostatitis?
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Flu hospitalizations drop amid signs of an early peak
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.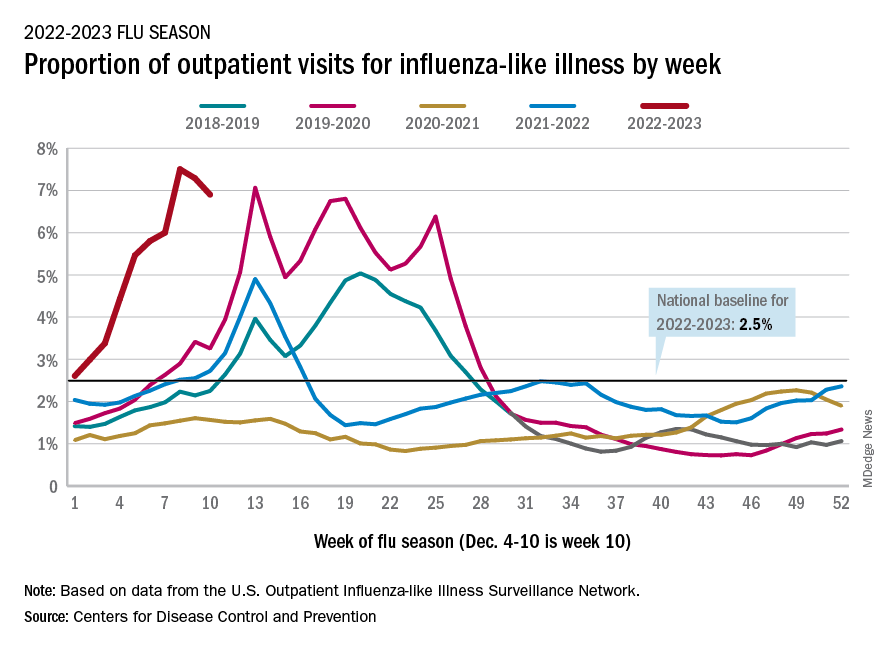
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.