User login
H pylori Infection Linked to Increased Alzheimer’s Risk
TOPLINE:
results of a large and lengthy population-based study suggest.
METHODOLOGY:
- Researchers identified all cases with a first-time diagnosis of AD and matched each AD case to up to 40 AD-free control cases on the basis of age, sex, cohort entry date, and duration of follow-up.
- The exposure of interest was CAHPI, defined based on an algorithm using clinical guidelines and recommendations on the management of H pylori (HP) infection, with researchers focusing on infected individuals presenting with symptoms or developing serious complications from the infection.
- Researchers performed several sensitivity analyses, which included repeating the primary analysis using alternate lag periods, restricting the cohort to participants with AD (not vascular, alcoholic, and unspecified dementia), and using salmonellosis, an infection not previously associated with AD, as a negative control exposure.
TAKEAWAY:
- Compared with no exposure to CAHPI, exposure to CAHPI was associated with a moderately increased risk for AD (odds ratio [OR], 1.11; 95% CI, 1.01-1.21), with no major effect modification by demographics or socioeconomic status.
- The increased risk peaked 7.3-10.8 years after CAHPI onset (OR, 1.24; 95% CI, 1.05-1.47) before decreasing.
- Sensitivity analyses yielded findings that were overall consistent with those of the primary analysis.
- The analysis with salmonellosis as a negative control exposure showed no association with the risk for AD (OR, 1.03; 95% CI, 0.82-1.29).
IN PRACTICE:
“These results support the notion of HP infection as a potential modifiable risk factor of AD” and “pave the way for future randomized controlled trials that would assess the impact and cost-effectiveness of population-based targeted interventions such as individualized HP eradication programs, on the development of AD,” the authors write.
SOURCE:
The study was conducted by Antonios Douros, Department of Medicine, and Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Given the observational nature of the study, residual confounding is possible. Because the exposure definition was on the basis of CAHPI recorded by general practitioners, exposure misclassification due to symptomatic patients not seeking primary care is possible, as is outcome misclassification. The authors can’t rule out the possibility of an association between asymptomatic H pylori infection and AD risk.
DISCLOSURES:
The study received funding from the Canadian Institutes of Health Research. Douros has no relevant conflicts of interest; see paper for disclosures of other authors.
Pauline Anderson has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a large and lengthy population-based study suggest.
METHODOLOGY:
- Researchers identified all cases with a first-time diagnosis of AD and matched each AD case to up to 40 AD-free control cases on the basis of age, sex, cohort entry date, and duration of follow-up.
- The exposure of interest was CAHPI, defined based on an algorithm using clinical guidelines and recommendations on the management of H pylori (HP) infection, with researchers focusing on infected individuals presenting with symptoms or developing serious complications from the infection.
- Researchers performed several sensitivity analyses, which included repeating the primary analysis using alternate lag periods, restricting the cohort to participants with AD (not vascular, alcoholic, and unspecified dementia), and using salmonellosis, an infection not previously associated with AD, as a negative control exposure.
TAKEAWAY:
- Compared with no exposure to CAHPI, exposure to CAHPI was associated with a moderately increased risk for AD (odds ratio [OR], 1.11; 95% CI, 1.01-1.21), with no major effect modification by demographics or socioeconomic status.
- The increased risk peaked 7.3-10.8 years after CAHPI onset (OR, 1.24; 95% CI, 1.05-1.47) before decreasing.
- Sensitivity analyses yielded findings that were overall consistent with those of the primary analysis.
- The analysis with salmonellosis as a negative control exposure showed no association with the risk for AD (OR, 1.03; 95% CI, 0.82-1.29).
IN PRACTICE:
“These results support the notion of HP infection as a potential modifiable risk factor of AD” and “pave the way for future randomized controlled trials that would assess the impact and cost-effectiveness of population-based targeted interventions such as individualized HP eradication programs, on the development of AD,” the authors write.
SOURCE:
The study was conducted by Antonios Douros, Department of Medicine, and Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Given the observational nature of the study, residual confounding is possible. Because the exposure definition was on the basis of CAHPI recorded by general practitioners, exposure misclassification due to symptomatic patients not seeking primary care is possible, as is outcome misclassification. The authors can’t rule out the possibility of an association between asymptomatic H pylori infection and AD risk.
DISCLOSURES:
The study received funding from the Canadian Institutes of Health Research. Douros has no relevant conflicts of interest; see paper for disclosures of other authors.
Pauline Anderson has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a large and lengthy population-based study suggest.
METHODOLOGY:
- Researchers identified all cases with a first-time diagnosis of AD and matched each AD case to up to 40 AD-free control cases on the basis of age, sex, cohort entry date, and duration of follow-up.
- The exposure of interest was CAHPI, defined based on an algorithm using clinical guidelines and recommendations on the management of H pylori (HP) infection, with researchers focusing on infected individuals presenting with symptoms or developing serious complications from the infection.
- Researchers performed several sensitivity analyses, which included repeating the primary analysis using alternate lag periods, restricting the cohort to participants with AD (not vascular, alcoholic, and unspecified dementia), and using salmonellosis, an infection not previously associated with AD, as a negative control exposure.
TAKEAWAY:
- Compared with no exposure to CAHPI, exposure to CAHPI was associated with a moderately increased risk for AD (odds ratio [OR], 1.11; 95% CI, 1.01-1.21), with no major effect modification by demographics or socioeconomic status.
- The increased risk peaked 7.3-10.8 years after CAHPI onset (OR, 1.24; 95% CI, 1.05-1.47) before decreasing.
- Sensitivity analyses yielded findings that were overall consistent with those of the primary analysis.
- The analysis with salmonellosis as a negative control exposure showed no association with the risk for AD (OR, 1.03; 95% CI, 0.82-1.29).
IN PRACTICE:
“These results support the notion of HP infection as a potential modifiable risk factor of AD” and “pave the way for future randomized controlled trials that would assess the impact and cost-effectiveness of population-based targeted interventions such as individualized HP eradication programs, on the development of AD,” the authors write.
SOURCE:
The study was conducted by Antonios Douros, Department of Medicine, and Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Given the observational nature of the study, residual confounding is possible. Because the exposure definition was on the basis of CAHPI recorded by general practitioners, exposure misclassification due to symptomatic patients not seeking primary care is possible, as is outcome misclassification. The authors can’t rule out the possibility of an association between asymptomatic H pylori infection and AD risk.
DISCLOSURES:
The study received funding from the Canadian Institutes of Health Research. Douros has no relevant conflicts of interest; see paper for disclosures of other authors.
Pauline Anderson has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
COVID Strain JN.1 Is Now a ‘Variant of Interest,’ WHO Says
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
Reactive Angioendotheliomatosis Following Ad26.COV2.S Vaccination
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.
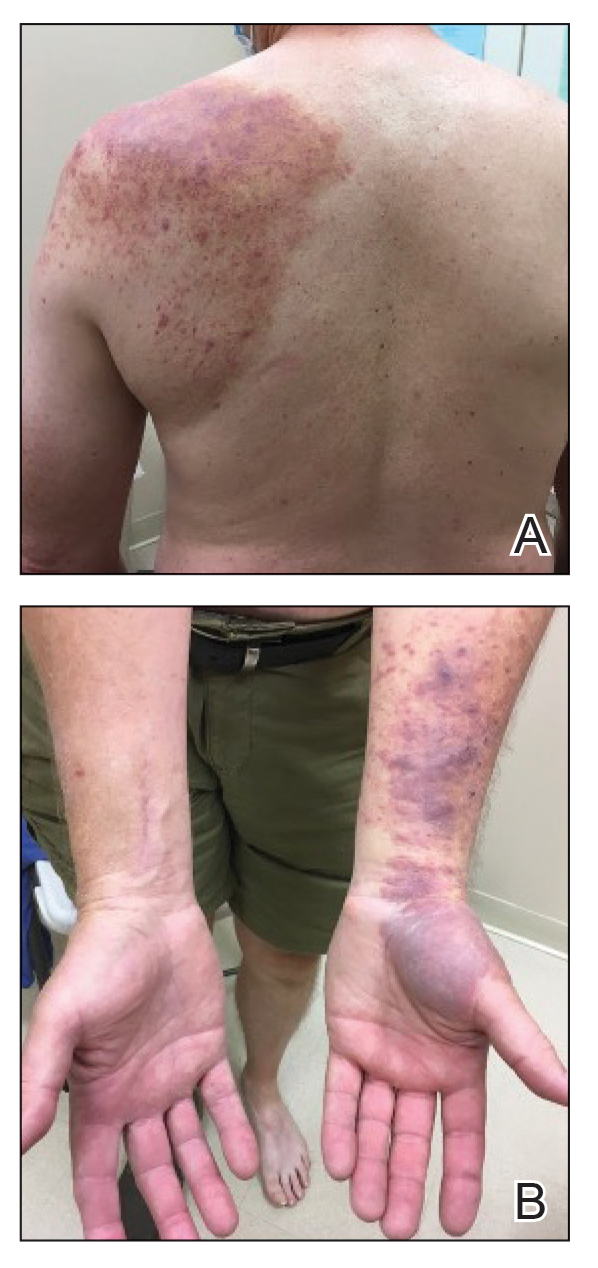
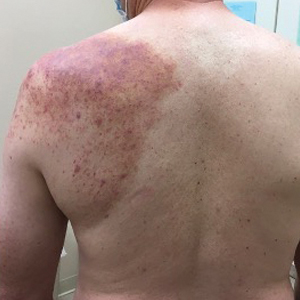
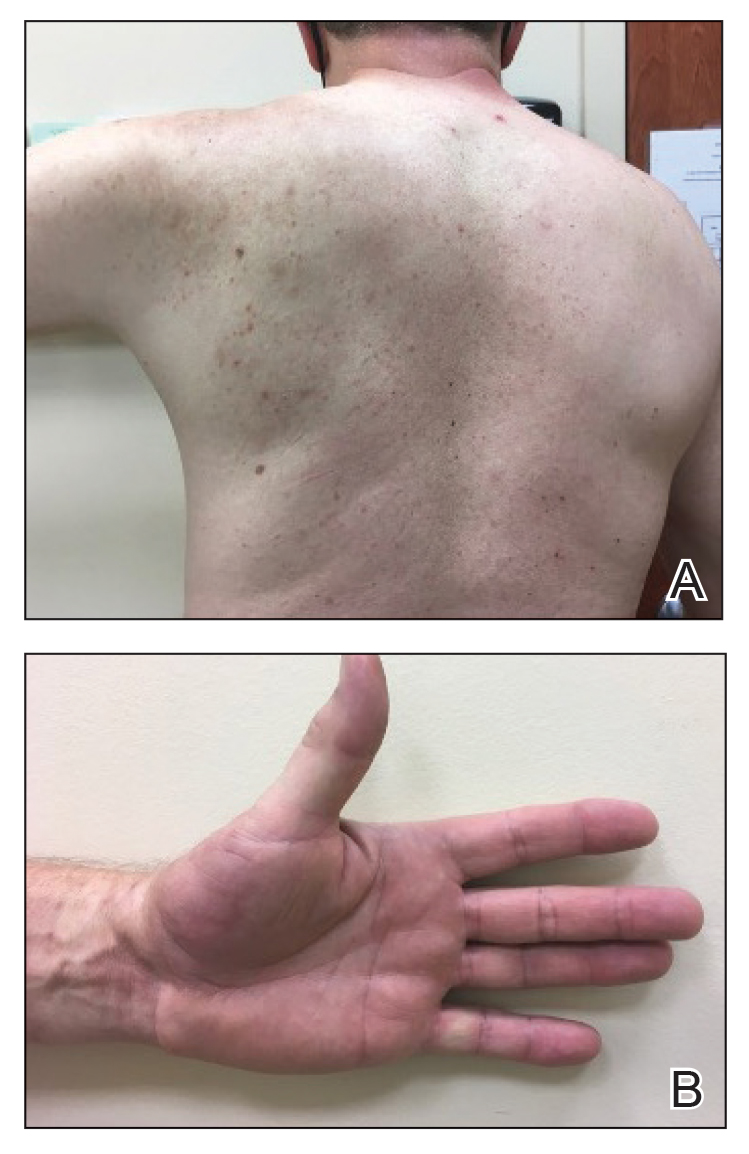
Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.
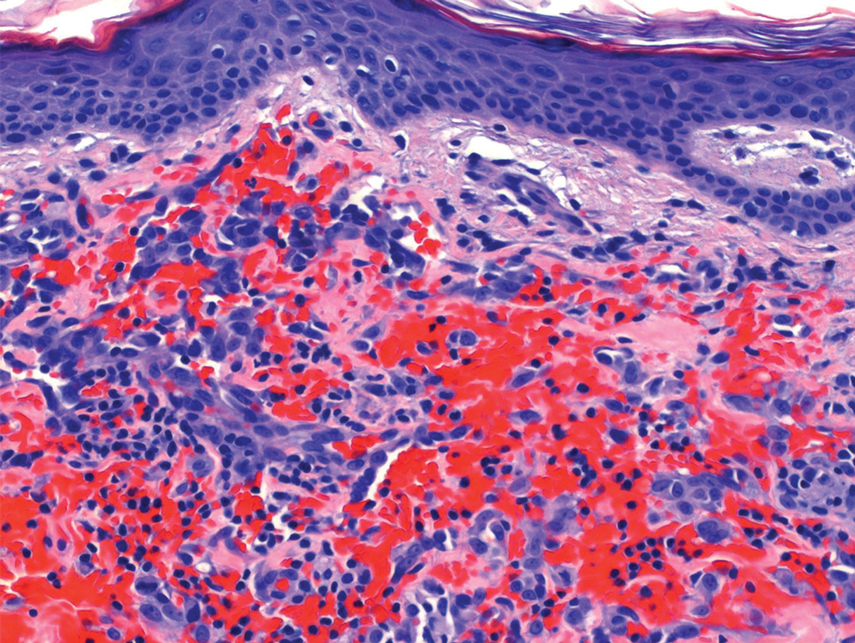
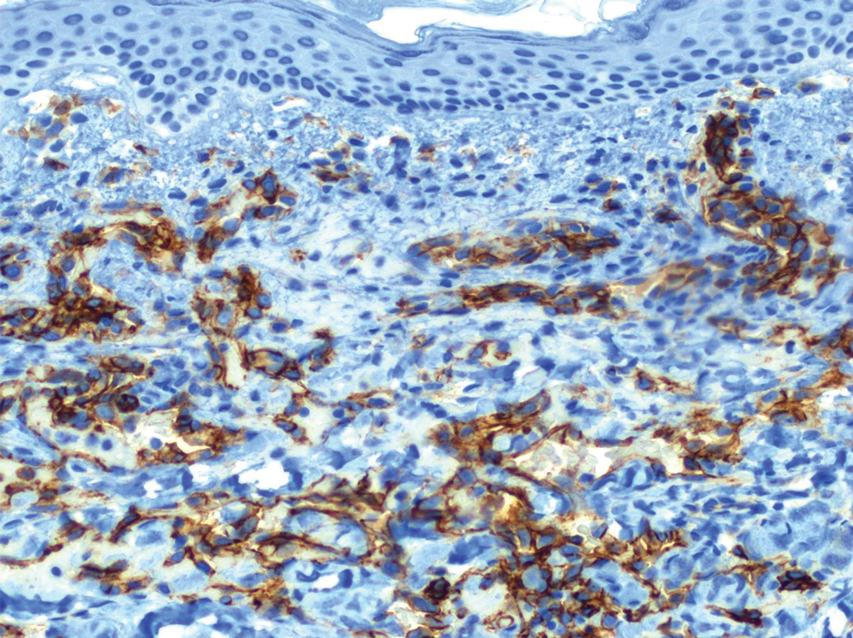
Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.
A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.
Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
Practice points
- Reactive angioendotheliomatosis (RAE) is a rare benign vascular proliferation of endothelial cells lining blood vessels that clinically appears similar to Kaposi sarcoma and must be differentiated by microscopic evaluation.
- An increasing number of reports link SARS-CoV-2 viral infection or vaccination against this virus with various cutaneous manifestations. Our case offers a link between RAE and Ad26.COV2.S vaccination.
Systemic Bias in AI Models May Undermine Diagnostic Accuracy
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
FROM JAMA
Cluster of Eye Syphilis Cases Prompts CDC Concern
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
FROM MMWR
What’s Eating You? Update on the Sticktight Flea (Echidnophaga gallinacea)
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8
Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Practice Points
- The sticktight flea (Echidnophaga gallinacea) attaches to its host by embedding its head in the skin for days at a time.
- Unlike other fleas that bite and run, the sticktight flea can be identified dermoscopically.
- The sticktight flea serves as a vector for plague as a carrier of Yersinia pestis, rickettsial infections, and other diseases.
Federal program offers free COVID, flu at-home tests, treatments
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
New KDIGO guideline encourages use of HCV-positive kidneys for HCV-negative recipients
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Why Are Prion Diseases on the Rise?
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New COVID variant JN.1 could disrupt holiday plans
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.