User login
Rapidly progressive pleural effusion
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
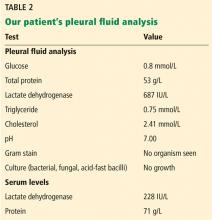
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
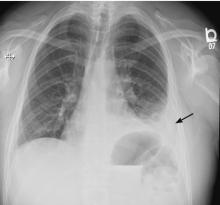
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
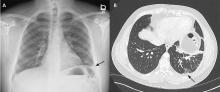
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
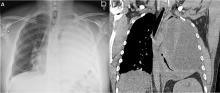
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
CDC: Flu activity ‘high’ in nine states
according to the Centers for Disease Control and Prevention.
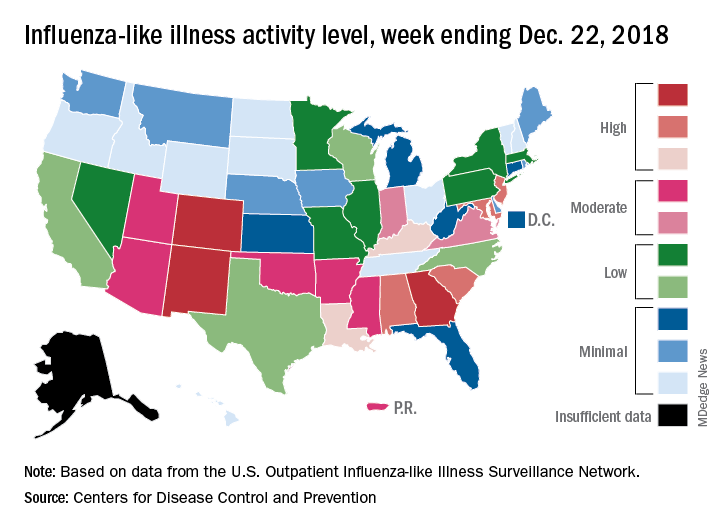
Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
2018-2019 flu season starts in earnest
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.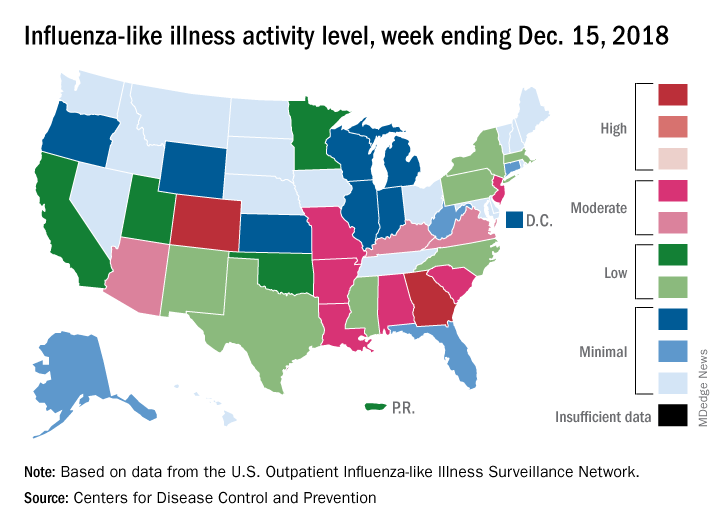
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
Playing by the Rules: Using Decision Rules Wisely Part 2, Nontraumatic Conditions
In this second part of “Playing by the Rules,” we will examine validated clinical decision rules that assist emergency physicians (EPs) in the diagnosis and treatment of nontraumatic conditions. Most trauma rules seek to answer a yes or no question regarding the utility of testing for specific disease states when the diagnosis is not clinically apparent.
For example, the Canadian CT Head Rule describes a number of conditions that, if met, can predict the absence of traumatic lesions requiring neurosurgical intervention in the alert patient with head injury, and thus obviate the need for imaging in those instances. In contrast, many medical rules are actually risk stratification scales for treatment and diagnosis, categorizing patients into low- to high-risk groups based on clinical factors. While traumatic conditions are linked to a specific inciting event or “trauma,” medical diseases may have multiple causative factors or may be delayed in presentation to the emergency department (ED), which subsequently increases the complexity of these decision instruments.
Rather than an exhaustive list of all clinical decision rules or risk stratification scales relevant to emergency medicine, this installment will provide EPs with a review of common instruments and the evidence behind them.
Central Nervous System
Ottawa Subarachnoid Hemorrhage Rule
The Ottawa Subarachnoid Hemorrhage Rule offers guidance for diagnosing atraumatic subarachnoid hemorrhage (SAH) in alert, neurologically intact adult patients presenting to the ED with a headache reaching maximal intensity within 1 hour of onset. The rule states that if none of the following conditions are present, then the diagnosis of SAH can be excluded without further testing:
Symptom of neck pain or stiffness
Age greater than 40 years old
Witnessed loss of consciousness
Onset during exertion
Thunderclap headache with peak pain instantly
Limited neck flexion on exam
The validation study prospectively enrolled 1153 adults of whom 67 had a positive workup for SAH (defined as subarachnoid blood visible on noncontrast CT scan of the head, xanthochromia of cerebrospinal fluid on visual inspection, or the presence of >1 million erythrocytes in the final tube of cerebrospinal fluid with an aneurysm or arteriovenous malformation confirmed on cerebral angiography).1 Of note, patients with prior history of cerebral aneurysm or SAH were excluded, as were patients with recurrent headaches similar to the presenting complaint, patients with focal neurologic deficits or papilledema, or patients with a history of brain neoplasm, ventricular shunt, or hydrocephalus. The authors found that the rule was 100% sensitive and 13% specific for detecting SAH, with a kappa of 0.82, which suggests good interrater reliability.1
Comment: It is important to note that the authors excluded patients with a history of cerebral aneurysm or prior SAH, and therefore the rule should not be applied to these patients in clinical practice. The utility of this rule is somewhat limited secondary to the age cutoff, as the incidence of aneurysmal SAH increases considerably after the fifth decade of life.2 Ultimately, this rule—combined with the authors’ previous work showing that CT performed within 6 hours of headache onset can rule out SAH—provides a powerful diagnostic tool for EPs considering SAH in the ED.3
ABCD2 Score
The ABCD2 score was developed to identify transient ischemic attack (TIA) patients at risk for early stroke, and thus inform decisions regarding admission and resource utilization in the ED and outpatient clinic setting.4 The score was created by combining elements of two previously existing rules, the California and the ABCD scales. Patients presenting with TIA symptoms are assigned points based on:
Age: 1 point if ≥ 60 years
Blood Pressure: 1 point if ≥ 140/90
Clinical Deficit: 2 points for unilateral weakness, 1 point for speech impairment without unilateral weakness
Duration: 2 points for ≥ 60 minutes, 1 point for 10 to 59 minutes
Diabetes: 1 point if diabetic
The greater the number of points, the higher the risk for imminent stroke, from low (0-3 points) to moderate (4-5 points) to high (6-7 points). The initial retrospective internal validation study found that the low, moderate, and high groups correlated to 7-day stroke risk of 1.2%, 5.9%, and 11.7%, respectively. Subsequently, the ABCD2 score was rapidly incorporated into institutional and national protocols for assessing risk for stroke and featured prominently in the 2009 American Heart Association guidelines on TIA, which recommend hospitalization for a score of 3 or greater.4,5
More recently, a multicenter prospective external validation study of more than 2000 TIA patients found that using the American Heart Association recommended cutoff of 3 or greater resulted in a sensitivity of 94.7% for detecting those patients who sustained a stroke within 7 days, but a specificity of only 12.5%.6 The investigators concluded that a specificity this low would require “almost all” of the TIA patients in their cohort (87.6%) to be admitted to the hospital—even though only 3.2% of their patients had a stroke within 90 days.6 Even when examined at other cutoff scores, the investigators found the ABCD2 score to have poor accuracy.6
Comment: Decreasing resource utilization is a laudable goal, but it does not appear that the ABCD2 score provides much guidance on which TIA patients can safely go home. Moreover, the increasing availability of advanced imaging and tele-neurology consultation in the ED have changed the landscape of TIA and stroke care. Many EPs have since argued that the ABCD2 score adds little to their evaluation.7
Abdomen
Alvarado Score
There are multiple clinical prediction rules for appendicitis. Among the most commonly utilized by EPs and surgical consultants are the Alvarado score and the Appendicitis Inflammatory Response Score. The Alvarado score was derived in 1986 based on a retrospective review of 305 abdominal pain patients of whom 227 (aged 4 to 80 years) had appendicitis.8 Factors were identified and weighted, which can be recalled through the mnemonic MANTRELS:
Migration of pain to the right lower quadrant: 1 point
Anorexia or acetone in urine: 1 point
Nausea or vomiting: 1 point
Tenderness in the right lower quadrant: 2 points
Rebound tenderness: 1 point
Elevation of the temperature > 37.3°C: 1 point
Leukocytosis >10K X 109/L: 2 points
Shift to the left of neutrophils (>75%): 1 point
The original article posited that a score of 5 or 6 was “compatible” with the diagnosis of acute appendicitis—necessitating further observation for possible appendicitis—and that higher scores indicated an increasing probability of disease.8 Of note, the rule has also been adapted for clinical settings where differentials are not easily obtainable with the left shift criterion removed; this is known as the modified Alvarado score and calculated out at a maximum of 9.9
Since the original Alvarado study was published, multiple small studies have attempted to validate or otherwise retrospectively assess the utility of this rule. A frequently cited systematic review of 42 prospective and retrospective studies by Ohle et al found that a score of <5 showed a sensitivity of 99% overall (96% in men, 99% in women, and 99% in children) for ruling out admission/observation of patient with suspected appendicitis, though the specificity for ruling in the diagnosis at scores 7 and higher was only 81% overall.10
However, a more recent prospective observational study of adult abdominal pain patients presenting to large American urban EDs found the modified Alvarado rule at cutoff levels of 3, 4, and 5 had sensitivities of only 72%, 55%, and 36%, respectively, of ruling out the diagnosis.11 In comparison, the study found that physicians’ clinical judgement of appendicitis being the first or second most likely diagnosis had a sensitivity of 93% for predicting appendicitis.11
Comment: The Alvarado score was developed to help rule out and rule in the diagnosis of appendicitis. However, with the increasing availability of CT scanning in EDs, the diagnostic pathway in unclear cases has shifted from admission/observation to CT scanning, which has the benefit of elucidating other pathology as well. The utility of the Alvarado rule has been called into question. Ultimately, there is data in support of the Alvarado rule from older articles and studies in resource-poor environments, and newer studies may reflect less rigorous application of the rule when CT scanning is the default clinical pathway. Further studies that focus specifically on the Alvarado score as a rule out test to decrease CT utilization may be instructive.
Appendicitis Inflammatory Response (AIR) score
The appendicitis inflammatory response (AIR) score was derived in a cohort of 316 patients and validated on a sample of 229 adults and children with suspected appendicitis.12 The authors specifically sought to create a rule that outperformed the Alvarado score; the criteria are:
Vomiting: 1 point
Right iliac fossa pain: 1 point
Rebound tenderness: 1 point for light, 2 for medium, 3 for strong
Temperature >38.5°C: 1 point
Polymorphonuclear leukocytes: 1 point for 70%-84%, 2 for 85% or greater
White blood cell count: 1 point for 10,000-14,900, 2 for 15,000 or greater
C-reactive protein level (mg/dL): 1 point for 10-49, 2 for 50 or greater
Patients with a score of 0-4 were classified as low risk, with recommendation for outpatient follow-up if general condition unchanged; a score of 5-8 as indeterminate risk, with recommendation for active observation with serial exams, imaging, or diagnostic laparoscopy; or a score of 9-12 as high risk, with recommendation for surgical exploration.12 In the validation cohort, the investigators found an AIR score or Alvarado score greater than 4 to have, respectively, 96% or 97% sensitivity and 73% or 61% specificity for detecting appendicitis.12 A high score of greater than 8 on either the AIR or Alvarado had respectively 37% or 28% sensitivity but specificity of 99% for detecting appendicitis with either instrument.12
In an external validation study, the AIR and Alvarado scores were calculated on a series of 941 patients (aged 1 to 97 years) being evaluated for possible appendicitis; 201 patients were younger than 18.13 At a cutoff of greater than 4, the sensitivity and specificity were found to be 93% and 85% for the AIR and 90% and 55% for Alvarado.13 In a cohort of 182 patients (aged 4 to 75 years), a score of 4 or greater on the AIR and Alvarado was found to have comparable sensitivity to that of a senior surgical consultant for detecting appendicitis—with sensitivities of 94%, 93%, and 90% respectively.14 Subsequently, the original investigators undertook a large multicenter implementation study of the AIR at 24 hospitals of patients (aged 5 to 96 years) with suspected appendicitis. As compared to the pre-implementation group, using AIR to categorize patients as low risk resulted in significantly fewer imaging studies, admissions, and surgical explorations.15
Comment: The AIR has the benefit of recent prospective studies that assess performance of the rule in settings that mirror the practice environments of most EPs today. The classification of rebound tenderness as light, medium, or strong may be difficult to ascertain. Ultimately, reductions in imaging, admissions, and surgical explorations are important goals and EPs might benefit from using this rule to guide imaging.
CHEST
HEART Score
The increasingly popular HEART score, first developed by physicians in the Netherlands in 2008, seeks to risk-stratify patients presenting to the ED with suspected cardiac chest pain without ST-elevation myocardial infarction (STEMI). It scores patients 0 to 2 on 5 different characteristics (with a total scored of 10 possible points):
History: 2 points for highly suspicious, 1 point for moderately suspicious
EKG: 2 points for significant ST deviation, 1 point for nonspecific repolarization disturbance
Age: 2 points for age 65 years or greater, 1 point for age 45-64 years
Risk Factors: 2 points for 3 or more risk factors or history of atherosclerotic disease, 1 point for 1 to 2 risk factors
Troponin: 2 points for troponin value >3 times the normal limit, 1 point for value 1-3 times the normal limit.
The authors developed these 5 categories “based on clinical experience and current medical literature,” and then applied the rule to 122 chest pain patients in the ED, finding a higher incidence of major adverse coronary events (MACE) with increasing score: 2.5% for low risk score of 0-3, 20.3% for intermediate risk score of 4-6, and 72.7% for score 7 or higher.16 The score has been retrospectively and prospectively validated.17,18 In a study of 2440 patients, the low risk group had a MACE of 1.7%, and the score had a c-statistic of 0.83, outperforming Thrombolysis in Myocardial Infarction (TIMI) and GRACE c-statistics of 0.75 and 0.70, respectively.18 In 2013, investigators calculated the HEART score on a multinational database of 2906 chest pain patients, finding a negative predictive value of 98.3% for MACE with HEART score less than or equal to 3.19
In the United States, Mahler et al have produced a series of 3 articles validating the HEART score and demonstrating its use in reducing cardiac testing and length of stay. In 1070 patients admitted to their observation unit, who were deemed low risk by physician assessment and TIMI <2, a score of less than or equal to 3 had a negative predictive value of 99.4% for MACE; the inclusion of serial troponins resulted in sensitivity of 100%, specificity of 83.1%, and negative predictive value of 100%.20 The team then conducted a secondary analysis of chest pain patients enrolled in a large multicenter trial (MIDAS) and compared HEART score, the North American Chest Pain Rule, and unstructured clinical assessment.21 Both rules had high sensitivities, but the HEART score identified 20% of patients suitable for early discharge, as compared to 4% for the North American Chest Pain Rule.21 Finally, Mahler’s team performed a randomized control trial of 282 patients investigating whether the HEART score with serial troponins compared with usual care could safely reduce cardiac testing.22 The HEART pathway resulted in an absolute reduction of 12.1% in cardiac testing, and median reduction in length of stay by 12 hours, with no missed MACE in discharged patients.22
Most recently, a stepped-wedge, cluster randomized trial across 9 hospitals published in 2017 investigated the utility of the HEART score. Despite enrolling only 3648 patients out of the statistically required sample size of 6600, they found that the HEART score was not inferior to usual care and there was no significant difference in median length of stay, but health care resources were typically lower in the HEART score group.23
Comment: While derived in a less conventional manner, the HEART score has held up in several validation studies and appears poised to safely decrease health care costs and increase ED efficiency and throughput. As more US EDs look to adopt high sensitivity troponin biomarkers, prospective studies will be needed to determine the role of the HEART score in this setting.
Thrombolysis in Myocardial Infarction (TIMI) score
The Thrombolysis in Myocardial Infarction (TIMI) score was developed in 2000 as a tool to risk-stratify patients with a diagnosis of unstable angina (UA) and non–ST-elevation myocardial infarction (NSTEMI). The score was derived from 1 arm (2047 patients) of a study comparing heparin with enoxaparin for treatment of NSTEMI, and validated in the other 3 arms of the study (5124 patients). Multivariate logistic regression was used to develop 7 variables of equal weight:
Age greater than or equal to 65yo
Three or more cardiac risk factors
Known coronary artery disease (with stenosis greater than or equal to 50%)
Aspirin use in the past 7 days
Severe angina (2 or more episodes in the past 24 hours)
EKG ST changes greater than or equal to 0.5 mm
Positive serum cardiac biomarkers
The investigators found that with a higher score, there was progressive increase in adverse cardiac outcomes, with a c-statistic of 0.65.24 This score was subsequently validated across several existing databases evaluating various therapeutic interventions for UA/NSTEMI and remained statistically significant, with increasing risk for MI and mortality with increasing score.25,26
Given the success in predicting patient outcomes and identifying patients who could benefit from more aggressive care, the TIMI risk score was then applied to unselected ED chest pain patients. In a secondary analysis of a prospective observational cohort of 3929 patient visits, the TIMI score correlated to the risk for adverse outcomes, with a risk of 2.1% at score 0.27
In a second prospective observational cohort of 1458 patient visits, a score of 0 correlated to a 1.7% incidence of adverse outcomes.28 In 2008, Body et al sought to increase the relative weight of EKG and biomarker factors to 5 (instead of 1) in a study of 796 patients, positing that these factors have more importance in the ED setting.29 Comparing the modified TIMI to the original, the modified instrument improved the area under curve (AUC) from 0.77 to 0.87.29 In follow-up validation studies, the modified score has an improved AUC, but the incidence of adverse outcomes at score 0 remains at about 2% for both modified and original score.30,31
In 2010, Hess et al performed a systematic review and meta-analysis of the studies that prospectively validated the TIMI score. They evaluated 10 validation studies, encompassing 17,265 patients across 5 countries, and found a strong linear relation between the TIMI score and adverse cardiac events.32 At TIMI score of 0, the incidence of cardiac events was 1.8%, with sensitivity of 97.2% and specificity of 25%. Subsequently, the ADAPT trial designed a diagnostic protocol consisting of TIMI risk assessment, EKG, and 0- and 2-hour troponin I biomarkers to find ED patients eligible for safe, early discharge.33 Of the 1975 patients, 20% were classified as low risk and eligible for early discharge, in that they had TIMI score of 0, a non-ischemic ECG, and negative troponins. Only one patient had a MACE at 30 days, giving the protocol a sensitivity of 99.7%, specificity of 23.4%, and negative predictive value of 99.7%.33
As the TIMI and HEART scores are both used to evaluate ED chest pain patients, several studies have sought to compare them. In 2015, Cartlon et al published a comparison of 5 established risk scores and 2 troponin assays in 963 patients: modified Goldman, TIMI, GRACE, HEART, and Vancouver Chest Pain Rule in combination with troponin T and I.34 The investigators found that a negative troponin T plus either TIMI score of 0 or a HEART score ≤3 gave a negative predictive value of greater than 99.5% with more than 30% of patients able to be discharged safely.34 In 2017, a comparison of the GRACE, HEART, and TIMI scores in 1833 chest pain patients found the HEART score identified more low risk patients than either of its comparators and had the highest AUC at 0.86.35 Other trials have similarly found HEART outperforming TIMI.36
Comment: The TIMI score was not specifically designed for ED use but has been adapted to serve this purpose. To the EP assessing the undifferentiated chest pain patient, the TIMI score uses clinical variables that may seem curious (eg, aspirin use) or impossible for EPs to ascertain (eg, presence or degree of stenosis). Even for patients with a score of 0, the risk for adverse outcomes remains stubbornly at the 2% level, similar to the original low risk HEART score findings.
Wells’ Criteria for Pulmonary Embolism
The diagnosis of pulmonary embolism (PE) is often challenging, requiring the use of multiple ED resources for timely diagnosis, and is therefore well suited for clinical decision instruments. The Wells’ Criteria were derived from a cohort of 1260 patients using logistic regression to identify 7 significant variables:
Clinical signs and symptoms of deep vein thrombosis (DVT): 3
PE is the most likely diagnosis: 3
Heart rate >100: 1.5
Immobilization or surgery in the previous 4 weeks: 1.5
Previously diagnosed DVT or PE: 1.5
Hemoptysis: 1
Malignancy with treatment within 6 months or palliative: 1
The investigators specifically linked the use of their instrument to the D-dimer assay, using their score to determine pretest probability and seeking to exclude the diagnosis in patients with low pretest probability and negative D-dimer result.37,38 They reported a three-tiered classification, with low risk at a score less than 2, moderate risk at scores from 2-6, and high risk at scores greater than 6. The risk for PE with a low risk score coupled with a negative D-dimer result were 1.5% and 2.7% in the derivation and validation cohorts. Using a two-tiered classification of PE unlikely at scores less than or equal to 4 and PE likely at scores 5 or greater, a PE unlikely score and a negative D-dimer had a 2.2% and 1.7% risk in the derivation and validation cohorts.
A subsequent study by Wells et al on 930 ED patients using the score plus D-dimer found a negative predictive value of 99.5% for a low risk score and a negative D-dimer.39 This allowed for reduced imaging in 53% of patients.39 Another external validation study found acceptable interrater agreement between physicians for the Wells’ score at kappa 0.62 for the three-tiered system and 0.7 for the two-tiered system.40 The Wells’ Criteria has been compared against the Geneva score with receiver operating characteristic curve analysis showing no difference between the two rules.41 In a large cohort of 3306 patients being evaluated for PE using the Wells’ score and D-dimer, for the 1028 patients with PE unlikely and a negative D-dimer, there was a 3-month incidence of venous thromboembolism (VTE) of 0.5%—none of which were fatal events.42
Comment: The Wells’ Criteria for pulmonary embolism combined with D-dimer is now the preferred approach for many EPs seeking to risk-stratify their patients for PE. Advances in age-adjusted cutoffs for D-dimer provide additional support for this powerful tool.
Pulmonary Embolism Rule-Out Criteria (PERC)
Given the low specificity of the D-dimer assay for VTE, researchers post–Wells’ Criteria have sought to further reduce unnecessary testing by reassessing the D-dimer’s role in the diagnostic pathway. The PERC rule was designed to reduce D-dimer use—and downstream CT scan testing—in low-risk patients. The investigators derived the rule from a population of patients for whom the pretest probability of PE was less than 15%, seeking a risk for PE less than 2% if the rule was satisfied. Using logistic regression in 3148 ED patients, 8 clinical criteria were obtained:
Age < 50 years Pulse <100
Pulse oximetry >94%
No unilateral leg swelling
No hemoptysis
No recent surgery
No prior PE/DVT
No hormone use
The variables were tested in 1427 low-risk and 382 very-low-risk patients (defined as being evaluated for dyspnea, but not part of the derivation or low-risk validation groups). In the low-risk group, the sensitivity, specificity, and false-negative rate was 96%, 27%, and 1.4% respectively. In the very-low-risk group, the sensitivity, specificity, and false-negative rate was 100%, 15%, and 0% respectively.43 The rule was further validated in a prospective multicenter study of 8138 patients; among patients with pretest probability less than 15% who were PERC negative, 1% had PE/DVT within 45 days.44 The large PERCEPIC trial on 1757 patients found low pretest probability patients who were PERC negative had a false-negative rate of 1.2% and estimated that the use of PERC could decrease the median length of stay in the ED by at least 2 hours.45 The PROPER study, a non-inferiority, crossover cluster-randomized trial in 14 EDs across France, found that use of the PERC rule was not inferior to conventional care and that it was associated with reduced ED length of stay and CT use.45,46
There has been criticism from some European studies that the PERC rule misses too many PEs. A provocatively titled multinational study from Hugli et al examined patients suspected to have PE in Switzerland, France, and Belgium. The investigators applied the PERC rule and then stratified the patients by pretest probability as defined by the Geneva score, which includes many of the same criteria as PERC. They found the PERC rule identified a small proportion of patients with suspected PE as very low risk (13.2%) and that the prevalence of PE among these patients was 5.4%. Critics of this study have noted that the PERC rule was designed to be applied in low-risk patients, not to define the low-risk population.47 Another study examined a retrospective cohort of patients in whom a D-dimer was ordered to exclude PE, and then calculated the Wells’ and PERC score from the medical record. The investigators found that the combination of Wells and PERC missed 2 PEs out of their population of 377 patients.48 However, a subsequent meta-analysis analyzed 11 studies—including the two negative studies—and found a pooled sensitivity of 97%, specificity of 23%, and negative likelihood ratio of 0.18, concluding that when the pretest probability is low, PERC is sensitive enough to exclude D-dimer testing.49
Comment: Given the number of disease states and sampling techniques that can cause nonspecific elevation in D-dimer assay, the PERC rule provides a useful tool in low-risk populations for excluding PE without laboratory testing. The key is applying the rule to the appropriate population, as stratified by gestalt or clinical score.
Infectious Disease
Mortality in Emergency Department Sepsis (MEDS) score
The Mortality in Emergency Department Sepsis (MEDS) score was developed as a risk stratification tool for patients presenting to the ED with concern for sepsis. This score was prospectively derived from a population of 3301 ED patient encounters during which a blood culture was ordered. Charts were reviewed and several data points extracted and analyzed to determine the following univariate predictors of 28-day mortality: terminal illness, tachypnea or hypoxia, septic shock, platelets <150,000/mm3, bands >5%, age >65 years, lower respiratory infection, nursing home residence, and altered mental status. These predictors were assigned point values based on their odds ratios, and points are added to generate a total score. Mortality risk was stratified into groups based on total score, with percentage mortality as follows: score 0-4: 0.9%; 5-7: 2.0%; 8-12: 7.8%; 13-15: 20.2%; >15: 50%. A separate validation cohort had the following mortality rates: score 0-4: 1.1%; 5-7: 4.4%; 8-12: 9.3%; 13-15: 16.1%; >15: 39%.50
The MEDS score was subsequently shown to also be predictive for 1-year mortality as well, with an area under receiver operating curve (AUROC) of 0.76 for 1-year mortality.51 A subsequent study showed similar mortality rates when expanding the patient population to include all patients with systemic inflammatory response syndrome (SIRS), potentially broadening the potential application of MEDS in ED risk stratification.52 However, the score was shown to be less predictive in patients with severe sepsis and septic shock, underestimating mortality in all MEDS score groups.53 Still, the MEDS score was demonstrated in multiple validation studies as a reliable risk stratification tool in patients with suspected infection or SIRS.54,55
Comment: The MEDS score is not as well studied in the literature as the SIRS criteria or QuickSOFA but is a validated risk stratification tool in patients with suspected infection and is ED specific. This tool, similar to Pneumonia Severity Index and CURB-65 (discussed below), can guide management of patients from the ED. Very-low-risk (score 0-4) patients can be treated as outpatients, low risk (score 5-7) patients warranting consideration of a short inpatient stay, and moderate to high risk (>8) requiring inpatient management. At present, there is insufficient evidence regarding the role of the MEDS score to guide inpatient disposition of floor vs. ICU in moderate to high-risk patients.
Pneumonia Severity Index
The Pneumonia Severity Index (PSI) was developed as a tool to predict mortality risk from pneumonia, allowing providers to appropriately manage care for these patients in the hospital or as outpatients. A derivation cohort of 14199 patients was utilized to create a prediction rule in two steps meant to parallel a clinician’s decision-making process. The first step identified a population of patients that were at low risk for death, which were assigned to class I. The second step quantified the risk for death in the remaining patients using weighted factors including demographics, comorbidities, exam findings, and clinical data. In all, 20 variables were used and assigned corresponding points, the sum of which would assign a patient to a particular risk for mortality (class II-V).56
Mortality risk was relatively low for patients in class I and II (0.4 and 0.7%, respectively). Class III carried a mortality risk of 2.8%. Mortality increased with class IV and class V classification: 8.5% and 31.1%, respectively. These data were replicated with a separate validation cohort of 38039 patients, with similar mortality rates in each class. This study concluded with the recommendation that patients diagnosed with pneumonia falling into class I and II mortality risk should be managed as outpatients, possible brief inpatient observation for class III, and class IV and V managed as inpatients.56
Subsequent trials evaluating the utility of the PSI score in the management of patients diagnosed with pneumonia randomized low-risk patients (class I-III PSI) to treatment as outpatients vs inpatients. There were no statistical differences in adverse outcomes (ICU admission, hospital readmission, mortality, complications), with notable improvements in hospital admission rates and patient satisfaction.57,58 A meta-analysis of 6 studies that used a clinical decision tools to identify low-risk patients to treat pneumonia as outpatients showed no significant difference in mortality, patient readmissions, or patient satisfaction. Low-risk patients that required admission often included comorbid illnesses not included in the PSI, inability to take oral medications, barriers to compliance, or hypoxemia.59
Though the PSI has been shown to successfully identify patients at low risk for mortality, it has been less accurate at predicting and stratifying classes of severe pneumonia. A meta-analysis by Loke et al showed that PSI class IV or V had pooled sensitivity of 0.90 and specificity 0.53 for 30-day mortality, which was significantly better than the CURB-65 rule (discussed below).60 However, a subsequent large meta-analysis showed that PSI class IV or V had a sensitivity of 75% and specificity 40% for requiring ICU intervention or admission, which are not sufficient to guide disposition decisions.61
CURB-65
One of the criticisms of PSI included its complexity, with inclusion of 20 factors making it impractical for use as a bedside tool. The CURB-65 score was developed with a similar goal of identifying low-risk patients with pneumonia who would be candidates for outpatient management, but also patients at high risk for mortality or ICU admission. Criteria for severe pneumonia published by the British Thoracic Society include: respiratory rate ≥ 30 breaths/min, diastolic blood pressure ≤60 mmHg, and blood urea nitrogen >7 mmol/L. The presence of 2 criteria was 88% sensitive and 72% specific for mortality or ICU admission.62 The CURB-65 tool was based on these criteria, with the addition of age ≥65 years, which was found to be a separate independent predictor of mortality. Thus, the 5 criteria making up the score are as follows (1 point each, 0-5 total):
Confusion, meaning Mental Test Score ≤8, or disorientation to person, place, or time
Urea >7 mmol/L (>19.6 mg/dL)
Respiratory rate ≥ 30 breaths/minute
Blood pressure (systolic < 90 mmHg or diastolic ≤ 60 mmHg)
Age ≥ 65 years
A score of 0-1 of these criteria characterized low mortality risk (<1.5%) in the test group, a score of 2 was intermediate mortality risk (9.2%), and a score of 3 or more associated with high mortality risk (22%). A score ≥ 2 was 93% sensitive and 49% specific for 30-day mortality.63
A subsequent prospective validation study by Aujesky et al that included 3181 patients with community-acquired pneumonia demonstrated slightly higher mortality rates for each CURB-65 score (0.6%, 3%, 6.1%, 13%, 17%, 43% mortality in scores of 0-5, respectively).64 In particular, the 3% mortality rate in CURB-65 scores of 1 is similar to PSI class III mortality rates, suggesting a lower threshold (CURB-65 ≥1) for consideration of admission for management. Another validation study by Capelastegui et al showed similar mortality rates to the derivation study for specific CURB-65 scores, but noted 53% of patients with a score of 1 also were found to have characteristics that were independent for a poor prognosis, and should be considered in the decision for outpatient or inpatient treatment.65 Furthermore, a recent study found that of patients in the ED with a CURB-65 score of 1, 8% still required critical care intervention.66 Thus, use of CURB-65 in screening for low-risk patients with community-acquired pneumonia is recommended to be limited to scores of 0. However, as with PSI, validation studies have yet to show predictive utility of scores suggesting severe pneumonia (CURB-65 ≥3) in predicting mortality or ICU requirement.60,61
As validation studies have suggested only patients with a CURB-65 score of 0 are screened low risk enough for outpatient treatment, greater weight may be placed on utility of CRB-65 as a tool. This rule, initially proposed in the same study as CURB-65, omits blood urea nitrogen as a factor to only rely on history and physical exam data with a score of 0 indicating low risk.63 In initial derivation and validation studies, this rule demonstrated <1.6% mortality risk with a score of 0, with risk increasing to 4-8.6% in scores of 1.63,65 Multiple studies have compared CRB-65 and CURB-65, with only marginal but not statistically significant improvement in prognostic utility of CURB-65.65,67 A meta-analysis of 1648 patients even showed only 0.5% mortality risk in CRB-65 ≤1; potentially including CRB-65 0-1 as low risk, though, would require further study.68 Although multiple validation studies have also successfully stratified low risk to similar mortality risk (<1.6%), accuracy wanes with higher CRB-65 scores.69
Several studies have directly compared CURB-65 and PSI both in terms of identifying low-risk patients and stratifying disease severity.60,61,64,68,70-72 Multiple studies have shown similar mortality risk in low-risk populations and have demonstrated sensitivities for mortality greater than 96% for CURB-65/CRB-65 = 0 and PSI class I-III, albeit with specificities ranging from 18-65%.64,68,70 In stratifying patients into different levels of severity (ward vs ICU patients), PSI has shown slightly better sensitivity/specificity for mortality and/or ICU intervention, though neither is strong enough to significantly stratify severe pneumonia to serve as tools for directing inpatient management.60,61
Comment: PSI, CRB-65, and CURB-65 have been well validated as screening tools for low-risk patients who should be treated as outpatients (CURB-65 or CRB-65 = 0, PSI class I and II). A moderate-risk population (CURB-65 = 1 or 2, PSI class III) may benefit from treatment as inpatient or outpatient at clinician judgement. Use of these tools for determining disease severity and possible ICU requirement is not as reliable, and other clinical factors should be considered.
Conclusion
This article provides an overview of several common clinical decision instruments and the evidence behind them. Ultimately, many institutions have incorporated clinical decision rules directly into the electronic medical record, and this strategy will not only increase their use, but hopefully collect further data on whether the instruments truly perform better than unstructured clinical judgement.
1. Perry JJ, Sivilotti MLA, Sutherland J, et al. Validation of the Ottawa Subarachnoid Hemorrhage Rule in patients with acute headache. CMAJ. 2017;189(45):E1379-E1385.
2. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365-1372.
3. Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
4. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
5. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293
6. Perry JJ, Sharma M, Sivilotti ML, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ. 2011;183(10):1137-1145.
7. Stead LG, Suravaram S, Bellolio MF, et al. An assessment of the incremental value of the ABCD2 score in the emergeny department evaluation of transient ischemic attack. Ann Emerg Med. 2011;57(1):46-51.
8. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15(5):557-564.
9. Kalan M, Talbot D, Cunliffe WJ, Rich AJ. Evaluation of the modified Alvarado score in the diagnosis of acute appendicitis: a prospective study. Ann R Coll Surg Engl. 1994;76(6):418-419.
10. Ohle R, O'Reilly F, O'Brien KK, Fahey T, Dimitrov BD. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
11. Meltzer AC, Baumann BM, Chen EH, Shofer FS, Mills AM. Poor sensitivity of a modified Alvarado score in adults with suspected appendicitis. Ann Emerg Med. 2013;62(2):126-31.
12. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32(8):1843-1849.
13. de Castro SM, Ünlü C, Steller EP, van Wagensveld BA, Vrouenraets BC. Evaluation of the appendicitis inflammatory response score for patients with acute appendicitis. World J Surg. 2012;36(7):1540-1545.
14. Kollár D, McCartan DP, Bourke M, Cross KS, Dowdall J. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39(1):104-109.
15. Andersson M, Kolodziej B, Andersson RE; STRAPPSCORE Study Group. Randomized clinical trial of Appendicitis Inflammatory Response score-based management of patients with suspected appendicitis. Br J Surg. 2017;104(11):1451-1461.
16. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Netherlands Heart J. 2008;16(6):191-196.
17. Backus BE, Six AJ, Kelder JC, et al. Chest Pain in the Emergency Room. A Multicenter Validation of the HEART Score. Crit Pathways Cardiol. 2010;9:164-169.
18. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. In J Cardiol. 2013;168:2153-2158.
19. Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department. Crit Pathways Cardiol. 2013;12:121-126.
20. Mahler SA, Hiestand BC, Goff DC, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for acute coronary syndrome? Crit Pathw Cardiol. 2011:10(3):128-133.
21. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795-802.
22. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway Randomized Trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
23. Poldervaart JM, Reitsma JB, Backus BE, et al. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med. 2017;166:687-697.
24. Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST eevation MI. JAMA. 2000;284:835-842.
25. Scirica BM, Cannon CP, Antman EM, et al. Validation of the Thrombolysis In Myocardial Infarction (TIMI) risk score for unstable angina pectoris and non-ST-elevation myocardial infarction in the TIMI III registry. Am J Cardiol. 2002;90:303-305.
26. Morrow DA, Antman EM, Snapinn SM, McCabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non-ST elevation acute coronary syndromes. Eur Heart J. 2002;23:223-229.
27. Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non–ST-elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006:13(1):13-18.
28. Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction risk score in the emergency department chest pain population. Ann Emerg Med. 2006;48(3):252-259.
29. Body R, Carley S, McDowell G, Ferguson J, Mackway-Jones K. Can a modified thrombolysis in myocardial infarction risk score outperform the original for risk stratifying emergency department patients with chest pain? Emerg Med J. 2009;26:95-99.
30. Hess EP, Perry JJ, Calder LA, et al. Prospective validation of a modified Thrombolysis In Myocardial Infarction risk score in emergency department patients with chest pain and possible acute coronary syndrome. Acad Emerg Med. 2010;17(4):368-375.
31. Macdonald SPJ, Nagree Y, Fatovich DM, ad Brown SGA. Modified TIMI risk score cannot be used to identify low-risk chest pain in the emergency department: a multicenter validation study. Emerg Med J. 2014;31:281-285.
32. Hess EP, Agarwal D, Chandra S, et al. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: a meta-analysis. CMAJ. 2010;182(10):1039-1044.
33. Than, M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. JACC. 2012;59(23):2091-2098.
34. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity Troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66(6):635-645.
35. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661.
36. Nieuwets A, Poldervaart JM, Reitsma JB, et al. Medical consumption compared for TIMI and HEART score in chest pain patients at the emergency department: a retrospective cost analysis. BMJ Open. 2016;6:e010694.
37. Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997-1005.
38. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
39. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107.
40. Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells’ criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004;44:503-510.
41. Chagnon I, Bounameaux H, Aujesky D, et al. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269-275.
42. Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172-179.
43. Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255.
44. Kline JA, Courtney DM, Kabrhel C, et al. Propsective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772-780.
45. Penaloza A, Soulie C, Moumneh T, et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): a multicenter, prospective, observational study. Lancet Haematol. 2017;4:e615-e621.
46. Freund Y, Cachanado M, Aubry A, et al. Effect of the Pulmonary Embolism Rule-Out Criteria on subsequent thromboembolic events among low-risk emergency department patients. The PROPER randomized clinical trial. JAMA. 2018;319(6):559-566.
47. Hugli O, Righini M, Le Gal G, et al. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost. 2011;9:300-4.
48. Theunissen JMG, Scholing C, van Hasselt WE, van der Maten J, ter Avest E. A retrospective analysis of the combined use of PERC rule and Wells score to exclude pulmonary embolism in the Emergency Department. Emerg Med J. 2016;33:696-701.
49. Singh B, Parsaik AK, Aharwal D, Surana A, Mascarenhas SS, Chandra S. Diagnostic accuracy of Pulmonary Embolism Rule-Out Criteria: a systematic review and meta-analysis. Ann Emerg Med. 2012;59(6):517-520.
50. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670-675.
51. Shapiro NI, Howell MD, Talmor D, Donnino M, Ngo L, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score predicts 1-year mortality. Crit Care Med. 2007;35(1):192-198.
52. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med. 2008;36(2):421-26.
53. Jones AE, Saak K, Kline JA. Performance of the Mortality in Emergency Department Sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26(6):689-692.
54. Carpenter CR., Keim SM, Upadhye S, Nguyen HB. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med. 2009;37(3):319-327.
55. Hermans MAW, Leffers P, Jansen LM, Keulemans YC, Stassen PM. The value of the Mortality in Emergency Department Sepsis (MEDS) score, C reactive protein and lactate in predicting 28-day mortality of sepsis in a Dutch emergency department. Emerg Med J. 2012;29(4):295–300.
56. Fine MJ, Auble TE, Yealy DM, et al. A Prediction Rule to Identify Low-Risk Patients with Community Acquired Pneumonia. N Engl J Med. 1997;326(4):243-250.
57. Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA. 2000;283(6):749-755. doi:10.1001/jama.283.6.749.
58. Carratalà J, Fernandez-Sabe N. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients . Ann Intern Med. 2005;142:165-172. doi:10.7326/0003-4819-142-3-200502010-00006.
59. Chalmers JD, Akram AR, Hill AT. Increasing outpatient treatment of mild community-acquired pneumonia: Systematic review and meta-analysis. Eur Respir J. 2011;37(4):858-864. doi:10.1183/09031936.00065610.
60. Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax. 2010;65(10):884-890. doi:10.1136/thx.2009.134072.
61. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit Care. 2012;16(4):R141. doi:10.1186/cc11447.
62. Neill AM, Martin IR, Weir R, et al. Community-acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010-1016. doi:10.1136/thx.51.10.1010.
63. Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58(5):377-382. doi:10.1136/thorax.58.5.377.
64. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384-392. doi:10.1016/j.amjmed.2005.01.006.
65. Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27(1):151-157. doi:10.1183/09031936.06.00062505.
66. Ilg A, Moskowitz A, Konanki V, et al. Performance of the CURB-65 score in predicting critical care interventions in patients admitted with community-acquired pneumonia. Ann Emerg Med. 2018. doi:10.1016/j.annemergmed.2018.06.017.
67. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93-101. doi:10.1111/j.1365-2796.2006.01657.x.
68. Akram AR, Chalmers JD, Hill AT. Predicting mortality with severity assessment tools in out-patients with community-acquired pneumonia. QJM. 2011;104(10):871-879. doi:10.1093/qjmed/hcr088.
69. McNally M, Curtain J, O’Brien KK, Dimitrov BD, Fahey T. Validity of British Thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: Systematic review and meta-analysis. Br J Gen Pract. 2010;60(579):423-433. doi:10.3399/bjgp10X532422.
70. Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of Pneumonia Severity Index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian Setting. Indian J Chest Dis Allied Sci. 2010;52(1):9-17.
71. Noguchi S, Yatera K, Kawanami T, et al. Pneumonia severity assessment tools for predicting mortality in patients with cealthcare-associated pneumonia: a systematic review and meta-analysis. Respiration. 2017;93(6):441-450. doi:10.1159/000470915.
72. Kolditz M, Braeken D, Ewig S, Rohde G. Severity assessment and the immediate and long-term prognosis in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37(6):886-896. doi:http://dx.doi.org/10.1055/s-0036-1592127.
In this second part of “Playing by the Rules,” we will examine validated clinical decision rules that assist emergency physicians (EPs) in the diagnosis and treatment of nontraumatic conditions. Most trauma rules seek to answer a yes or no question regarding the utility of testing for specific disease states when the diagnosis is not clinically apparent.
For example, the Canadian CT Head Rule describes a number of conditions that, if met, can predict the absence of traumatic lesions requiring neurosurgical intervention in the alert patient with head injury, and thus obviate the need for imaging in those instances. In contrast, many medical rules are actually risk stratification scales for treatment and diagnosis, categorizing patients into low- to high-risk groups based on clinical factors. While traumatic conditions are linked to a specific inciting event or “trauma,” medical diseases may have multiple causative factors or may be delayed in presentation to the emergency department (ED), which subsequently increases the complexity of these decision instruments.
Rather than an exhaustive list of all clinical decision rules or risk stratification scales relevant to emergency medicine, this installment will provide EPs with a review of common instruments and the evidence behind them.
Central Nervous System
Ottawa Subarachnoid Hemorrhage Rule
The Ottawa Subarachnoid Hemorrhage Rule offers guidance for diagnosing atraumatic subarachnoid hemorrhage (SAH) in alert, neurologically intact adult patients presenting to the ED with a headache reaching maximal intensity within 1 hour of onset. The rule states that if none of the following conditions are present, then the diagnosis of SAH can be excluded without further testing:
Symptom of neck pain or stiffness
Age greater than 40 years old
Witnessed loss of consciousness
Onset during exertion
Thunderclap headache with peak pain instantly
Limited neck flexion on exam
The validation study prospectively enrolled 1153 adults of whom 67 had a positive workup for SAH (defined as subarachnoid blood visible on noncontrast CT scan of the head, xanthochromia of cerebrospinal fluid on visual inspection, or the presence of >1 million erythrocytes in the final tube of cerebrospinal fluid with an aneurysm or arteriovenous malformation confirmed on cerebral angiography).1 Of note, patients with prior history of cerebral aneurysm or SAH were excluded, as were patients with recurrent headaches similar to the presenting complaint, patients with focal neurologic deficits or papilledema, or patients with a history of brain neoplasm, ventricular shunt, or hydrocephalus. The authors found that the rule was 100% sensitive and 13% specific for detecting SAH, with a kappa of 0.82, which suggests good interrater reliability.1
Comment: It is important to note that the authors excluded patients with a history of cerebral aneurysm or prior SAH, and therefore the rule should not be applied to these patients in clinical practice. The utility of this rule is somewhat limited secondary to the age cutoff, as the incidence of aneurysmal SAH increases considerably after the fifth decade of life.2 Ultimately, this rule—combined with the authors’ previous work showing that CT performed within 6 hours of headache onset can rule out SAH—provides a powerful diagnostic tool for EPs considering SAH in the ED.3
ABCD2 Score
The ABCD2 score was developed to identify transient ischemic attack (TIA) patients at risk for early stroke, and thus inform decisions regarding admission and resource utilization in the ED and outpatient clinic setting.4 The score was created by combining elements of two previously existing rules, the California and the ABCD scales. Patients presenting with TIA symptoms are assigned points based on:
Age: 1 point if ≥ 60 years
Blood Pressure: 1 point if ≥ 140/90
Clinical Deficit: 2 points for unilateral weakness, 1 point for speech impairment without unilateral weakness
Duration: 2 points for ≥ 60 minutes, 1 point for 10 to 59 minutes
Diabetes: 1 point if diabetic
The greater the number of points, the higher the risk for imminent stroke, from low (0-3 points) to moderate (4-5 points) to high (6-7 points). The initial retrospective internal validation study found that the low, moderate, and high groups correlated to 7-day stroke risk of 1.2%, 5.9%, and 11.7%, respectively. Subsequently, the ABCD2 score was rapidly incorporated into institutional and national protocols for assessing risk for stroke and featured prominently in the 2009 American Heart Association guidelines on TIA, which recommend hospitalization for a score of 3 or greater.4,5
More recently, a multicenter prospective external validation study of more than 2000 TIA patients found that using the American Heart Association recommended cutoff of 3 or greater resulted in a sensitivity of 94.7% for detecting those patients who sustained a stroke within 7 days, but a specificity of only 12.5%.6 The investigators concluded that a specificity this low would require “almost all” of the TIA patients in their cohort (87.6%) to be admitted to the hospital—even though only 3.2% of their patients had a stroke within 90 days.6 Even when examined at other cutoff scores, the investigators found the ABCD2 score to have poor accuracy.6
Comment: Decreasing resource utilization is a laudable goal, but it does not appear that the ABCD2 score provides much guidance on which TIA patients can safely go home. Moreover, the increasing availability of advanced imaging and tele-neurology consultation in the ED have changed the landscape of TIA and stroke care. Many EPs have since argued that the ABCD2 score adds little to their evaluation.7
Abdomen
Alvarado Score
There are multiple clinical prediction rules for appendicitis. Among the most commonly utilized by EPs and surgical consultants are the Alvarado score and the Appendicitis Inflammatory Response Score. The Alvarado score was derived in 1986 based on a retrospective review of 305 abdominal pain patients of whom 227 (aged 4 to 80 years) had appendicitis.8 Factors were identified and weighted, which can be recalled through the mnemonic MANTRELS:
Migration of pain to the right lower quadrant: 1 point
Anorexia or acetone in urine: 1 point
Nausea or vomiting: 1 point
Tenderness in the right lower quadrant: 2 points
Rebound tenderness: 1 point
Elevation of the temperature > 37.3°C: 1 point
Leukocytosis >10K X 109/L: 2 points
Shift to the left of neutrophils (>75%): 1 point
The original article posited that a score of 5 or 6 was “compatible” with the diagnosis of acute appendicitis—necessitating further observation for possible appendicitis—and that higher scores indicated an increasing probability of disease.8 Of note, the rule has also been adapted for clinical settings where differentials are not easily obtainable with the left shift criterion removed; this is known as the modified Alvarado score and calculated out at a maximum of 9.9
Since the original Alvarado study was published, multiple small studies have attempted to validate or otherwise retrospectively assess the utility of this rule. A frequently cited systematic review of 42 prospective and retrospective studies by Ohle et al found that a score of <5 showed a sensitivity of 99% overall (96% in men, 99% in women, and 99% in children) for ruling out admission/observation of patient with suspected appendicitis, though the specificity for ruling in the diagnosis at scores 7 and higher was only 81% overall.10
However, a more recent prospective observational study of adult abdominal pain patients presenting to large American urban EDs found the modified Alvarado rule at cutoff levels of 3, 4, and 5 had sensitivities of only 72%, 55%, and 36%, respectively, of ruling out the diagnosis.11 In comparison, the study found that physicians’ clinical judgement of appendicitis being the first or second most likely diagnosis had a sensitivity of 93% for predicting appendicitis.11
Comment: The Alvarado score was developed to help rule out and rule in the diagnosis of appendicitis. However, with the increasing availability of CT scanning in EDs, the diagnostic pathway in unclear cases has shifted from admission/observation to CT scanning, which has the benefit of elucidating other pathology as well. The utility of the Alvarado rule has been called into question. Ultimately, there is data in support of the Alvarado rule from older articles and studies in resource-poor environments, and newer studies may reflect less rigorous application of the rule when CT scanning is the default clinical pathway. Further studies that focus specifically on the Alvarado score as a rule out test to decrease CT utilization may be instructive.
Appendicitis Inflammatory Response (AIR) score
The appendicitis inflammatory response (AIR) score was derived in a cohort of 316 patients and validated on a sample of 229 adults and children with suspected appendicitis.12 The authors specifically sought to create a rule that outperformed the Alvarado score; the criteria are:
Vomiting: 1 point
Right iliac fossa pain: 1 point
Rebound tenderness: 1 point for light, 2 for medium, 3 for strong
Temperature >38.5°C: 1 point
Polymorphonuclear leukocytes: 1 point for 70%-84%, 2 for 85% or greater
White blood cell count: 1 point for 10,000-14,900, 2 for 15,000 or greater
C-reactive protein level (mg/dL): 1 point for 10-49, 2 for 50 or greater
Patients with a score of 0-4 were classified as low risk, with recommendation for outpatient follow-up if general condition unchanged; a score of 5-8 as indeterminate risk, with recommendation for active observation with serial exams, imaging, or diagnostic laparoscopy; or a score of 9-12 as high risk, with recommendation for surgical exploration.12 In the validation cohort, the investigators found an AIR score or Alvarado score greater than 4 to have, respectively, 96% or 97% sensitivity and 73% or 61% specificity for detecting appendicitis.12 A high score of greater than 8 on either the AIR or Alvarado had respectively 37% or 28% sensitivity but specificity of 99% for detecting appendicitis with either instrument.12
In an external validation study, the AIR and Alvarado scores were calculated on a series of 941 patients (aged 1 to 97 years) being evaluated for possible appendicitis; 201 patients were younger than 18.13 At a cutoff of greater than 4, the sensitivity and specificity were found to be 93% and 85% for the AIR and 90% and 55% for Alvarado.13 In a cohort of 182 patients (aged 4 to 75 years), a score of 4 or greater on the AIR and Alvarado was found to have comparable sensitivity to that of a senior surgical consultant for detecting appendicitis—with sensitivities of 94%, 93%, and 90% respectively.14 Subsequently, the original investigators undertook a large multicenter implementation study of the AIR at 24 hospitals of patients (aged 5 to 96 years) with suspected appendicitis. As compared to the pre-implementation group, using AIR to categorize patients as low risk resulted in significantly fewer imaging studies, admissions, and surgical explorations.15
Comment: The AIR has the benefit of recent prospective studies that assess performance of the rule in settings that mirror the practice environments of most EPs today. The classification of rebound tenderness as light, medium, or strong may be difficult to ascertain. Ultimately, reductions in imaging, admissions, and surgical explorations are important goals and EPs might benefit from using this rule to guide imaging.
CHEST
HEART Score
The increasingly popular HEART score, first developed by physicians in the Netherlands in 2008, seeks to risk-stratify patients presenting to the ED with suspected cardiac chest pain without ST-elevation myocardial infarction (STEMI). It scores patients 0 to 2 on 5 different characteristics (with a total scored of 10 possible points):
History: 2 points for highly suspicious, 1 point for moderately suspicious
EKG: 2 points for significant ST deviation, 1 point for nonspecific repolarization disturbance
Age: 2 points for age 65 years or greater, 1 point for age 45-64 years
Risk Factors: 2 points for 3 or more risk factors or history of atherosclerotic disease, 1 point for 1 to 2 risk factors
Troponin: 2 points for troponin value >3 times the normal limit, 1 point for value 1-3 times the normal limit.
The authors developed these 5 categories “based on clinical experience and current medical literature,” and then applied the rule to 122 chest pain patients in the ED, finding a higher incidence of major adverse coronary events (MACE) with increasing score: 2.5% for low risk score of 0-3, 20.3% for intermediate risk score of 4-6, and 72.7% for score 7 or higher.16 The score has been retrospectively and prospectively validated.17,18 In a study of 2440 patients, the low risk group had a MACE of 1.7%, and the score had a c-statistic of 0.83, outperforming Thrombolysis in Myocardial Infarction (TIMI) and GRACE c-statistics of 0.75 and 0.70, respectively.18 In 2013, investigators calculated the HEART score on a multinational database of 2906 chest pain patients, finding a negative predictive value of 98.3% for MACE with HEART score less than or equal to 3.19
In the United States, Mahler et al have produced a series of 3 articles validating the HEART score and demonstrating its use in reducing cardiac testing and length of stay. In 1070 patients admitted to their observation unit, who were deemed low risk by physician assessment and TIMI <2, a score of less than or equal to 3 had a negative predictive value of 99.4% for MACE; the inclusion of serial troponins resulted in sensitivity of 100%, specificity of 83.1%, and negative predictive value of 100%.20 The team then conducted a secondary analysis of chest pain patients enrolled in a large multicenter trial (MIDAS) and compared HEART score, the North American Chest Pain Rule, and unstructured clinical assessment.21 Both rules had high sensitivities, but the HEART score identified 20% of patients suitable for early discharge, as compared to 4% for the North American Chest Pain Rule.21 Finally, Mahler’s team performed a randomized control trial of 282 patients investigating whether the HEART score with serial troponins compared with usual care could safely reduce cardiac testing.22 The HEART pathway resulted in an absolute reduction of 12.1% in cardiac testing, and median reduction in length of stay by 12 hours, with no missed MACE in discharged patients.22
Most recently, a stepped-wedge, cluster randomized trial across 9 hospitals published in 2017 investigated the utility of the HEART score. Despite enrolling only 3648 patients out of the statistically required sample size of 6600, they found that the HEART score was not inferior to usual care and there was no significant difference in median length of stay, but health care resources were typically lower in the HEART score group.23
Comment: While derived in a less conventional manner, the HEART score has held up in several validation studies and appears poised to safely decrease health care costs and increase ED efficiency and throughput. As more US EDs look to adopt high sensitivity troponin biomarkers, prospective studies will be needed to determine the role of the HEART score in this setting.
Thrombolysis in Myocardial Infarction (TIMI) score
The Thrombolysis in Myocardial Infarction (TIMI) score was developed in 2000 as a tool to risk-stratify patients with a diagnosis of unstable angina (UA) and non–ST-elevation myocardial infarction (NSTEMI). The score was derived from 1 arm (2047 patients) of a study comparing heparin with enoxaparin for treatment of NSTEMI, and validated in the other 3 arms of the study (5124 patients). Multivariate logistic regression was used to develop 7 variables of equal weight:
Age greater than or equal to 65yo
Three or more cardiac risk factors
Known coronary artery disease (with stenosis greater than or equal to 50%)
Aspirin use in the past 7 days
Severe angina (2 or more episodes in the past 24 hours)
EKG ST changes greater than or equal to 0.5 mm
Positive serum cardiac biomarkers
The investigators found that with a higher score, there was progressive increase in adverse cardiac outcomes, with a c-statistic of 0.65.24 This score was subsequently validated across several existing databases evaluating various therapeutic interventions for UA/NSTEMI and remained statistically significant, with increasing risk for MI and mortality with increasing score.25,26
Given the success in predicting patient outcomes and identifying patients who could benefit from more aggressive care, the TIMI risk score was then applied to unselected ED chest pain patients. In a secondary analysis of a prospective observational cohort of 3929 patient visits, the TIMI score correlated to the risk for adverse outcomes, with a risk of 2.1% at score 0.27
In a second prospective observational cohort of 1458 patient visits, a score of 0 correlated to a 1.7% incidence of adverse outcomes.28 In 2008, Body et al sought to increase the relative weight of EKG and biomarker factors to 5 (instead of 1) in a study of 796 patients, positing that these factors have more importance in the ED setting.29 Comparing the modified TIMI to the original, the modified instrument improved the area under curve (AUC) from 0.77 to 0.87.29 In follow-up validation studies, the modified score has an improved AUC, but the incidence of adverse outcomes at score 0 remains at about 2% for both modified and original score.30,31
In 2010, Hess et al performed a systematic review and meta-analysis of the studies that prospectively validated the TIMI score. They evaluated 10 validation studies, encompassing 17,265 patients across 5 countries, and found a strong linear relation between the TIMI score and adverse cardiac events.32 At TIMI score of 0, the incidence of cardiac events was 1.8%, with sensitivity of 97.2% and specificity of 25%. Subsequently, the ADAPT trial designed a diagnostic protocol consisting of TIMI risk assessment, EKG, and 0- and 2-hour troponin I biomarkers to find ED patients eligible for safe, early discharge.33 Of the 1975 patients, 20% were classified as low risk and eligible for early discharge, in that they had TIMI score of 0, a non-ischemic ECG, and negative troponins. Only one patient had a MACE at 30 days, giving the protocol a sensitivity of 99.7%, specificity of 23.4%, and negative predictive value of 99.7%.33
As the TIMI and HEART scores are both used to evaluate ED chest pain patients, several studies have sought to compare them. In 2015, Cartlon et al published a comparison of 5 established risk scores and 2 troponin assays in 963 patients: modified Goldman, TIMI, GRACE, HEART, and Vancouver Chest Pain Rule in combination with troponin T and I.34 The investigators found that a negative troponin T plus either TIMI score of 0 or a HEART score ≤3 gave a negative predictive value of greater than 99.5% with more than 30% of patients able to be discharged safely.34 In 2017, a comparison of the GRACE, HEART, and TIMI scores in 1833 chest pain patients found the HEART score identified more low risk patients than either of its comparators and had the highest AUC at 0.86.35 Other trials have similarly found HEART outperforming TIMI.36
Comment: The TIMI score was not specifically designed for ED use but has been adapted to serve this purpose. To the EP assessing the undifferentiated chest pain patient, the TIMI score uses clinical variables that may seem curious (eg, aspirin use) or impossible for EPs to ascertain (eg, presence or degree of stenosis). Even for patients with a score of 0, the risk for adverse outcomes remains stubbornly at the 2% level, similar to the original low risk HEART score findings.
Wells’ Criteria for Pulmonary Embolism
The diagnosis of pulmonary embolism (PE) is often challenging, requiring the use of multiple ED resources for timely diagnosis, and is therefore well suited for clinical decision instruments. The Wells’ Criteria were derived from a cohort of 1260 patients using logistic regression to identify 7 significant variables:
Clinical signs and symptoms of deep vein thrombosis (DVT): 3
PE is the most likely diagnosis: 3
Heart rate >100: 1.5
Immobilization or surgery in the previous 4 weeks: 1.5
Previously diagnosed DVT or PE: 1.5
Hemoptysis: 1
Malignancy with treatment within 6 months or palliative: 1
The investigators specifically linked the use of their instrument to the D-dimer assay, using their score to determine pretest probability and seeking to exclude the diagnosis in patients with low pretest probability and negative D-dimer result.37,38 They reported a three-tiered classification, with low risk at a score less than 2, moderate risk at scores from 2-6, and high risk at scores greater than 6. The risk for PE with a low risk score coupled with a negative D-dimer result were 1.5% and 2.7% in the derivation and validation cohorts. Using a two-tiered classification of PE unlikely at scores less than or equal to 4 and PE likely at scores 5 or greater, a PE unlikely score and a negative D-dimer had a 2.2% and 1.7% risk in the derivation and validation cohorts.
A subsequent study by Wells et al on 930 ED patients using the score plus D-dimer found a negative predictive value of 99.5% for a low risk score and a negative D-dimer.39 This allowed for reduced imaging in 53% of patients.39 Another external validation study found acceptable interrater agreement between physicians for the Wells’ score at kappa 0.62 for the three-tiered system and 0.7 for the two-tiered system.40 The Wells’ Criteria has been compared against the Geneva score with receiver operating characteristic curve analysis showing no difference between the two rules.41 In a large cohort of 3306 patients being evaluated for PE using the Wells’ score and D-dimer, for the 1028 patients with PE unlikely and a negative D-dimer, there was a 3-month incidence of venous thromboembolism (VTE) of 0.5%—none of which were fatal events.42
Comment: The Wells’ Criteria for pulmonary embolism combined with D-dimer is now the preferred approach for many EPs seeking to risk-stratify their patients for PE. Advances in age-adjusted cutoffs for D-dimer provide additional support for this powerful tool.
Pulmonary Embolism Rule-Out Criteria (PERC)
Given the low specificity of the D-dimer assay for VTE, researchers post–Wells’ Criteria have sought to further reduce unnecessary testing by reassessing the D-dimer’s role in the diagnostic pathway. The PERC rule was designed to reduce D-dimer use—and downstream CT scan testing—in low-risk patients. The investigators derived the rule from a population of patients for whom the pretest probability of PE was less than 15%, seeking a risk for PE less than 2% if the rule was satisfied. Using logistic regression in 3148 ED patients, 8 clinical criteria were obtained:
Age < 50 years Pulse <100
Pulse oximetry >94%
No unilateral leg swelling
No hemoptysis
No recent surgery
No prior PE/DVT
No hormone use
The variables were tested in 1427 low-risk and 382 very-low-risk patients (defined as being evaluated for dyspnea, but not part of the derivation or low-risk validation groups). In the low-risk group, the sensitivity, specificity, and false-negative rate was 96%, 27%, and 1.4% respectively. In the very-low-risk group, the sensitivity, specificity, and false-negative rate was 100%, 15%, and 0% respectively.43 The rule was further validated in a prospective multicenter study of 8138 patients; among patients with pretest probability less than 15% who were PERC negative, 1% had PE/DVT within 45 days.44 The large PERCEPIC trial on 1757 patients found low pretest probability patients who were PERC negative had a false-negative rate of 1.2% and estimated that the use of PERC could decrease the median length of stay in the ED by at least 2 hours.45 The PROPER study, a non-inferiority, crossover cluster-randomized trial in 14 EDs across France, found that use of the PERC rule was not inferior to conventional care and that it was associated with reduced ED length of stay and CT use.45,46
There has been criticism from some European studies that the PERC rule misses too many PEs. A provocatively titled multinational study from Hugli et al examined patients suspected to have PE in Switzerland, France, and Belgium. The investigators applied the PERC rule and then stratified the patients by pretest probability as defined by the Geneva score, which includes many of the same criteria as PERC. They found the PERC rule identified a small proportion of patients with suspected PE as very low risk (13.2%) and that the prevalence of PE among these patients was 5.4%. Critics of this study have noted that the PERC rule was designed to be applied in low-risk patients, not to define the low-risk population.47 Another study examined a retrospective cohort of patients in whom a D-dimer was ordered to exclude PE, and then calculated the Wells’ and PERC score from the medical record. The investigators found that the combination of Wells and PERC missed 2 PEs out of their population of 377 patients.48 However, a subsequent meta-analysis analyzed 11 studies—including the two negative studies—and found a pooled sensitivity of 97%, specificity of 23%, and negative likelihood ratio of 0.18, concluding that when the pretest probability is low, PERC is sensitive enough to exclude D-dimer testing.49
Comment: Given the number of disease states and sampling techniques that can cause nonspecific elevation in D-dimer assay, the PERC rule provides a useful tool in low-risk populations for excluding PE without laboratory testing. The key is applying the rule to the appropriate population, as stratified by gestalt or clinical score.
Infectious Disease
Mortality in Emergency Department Sepsis (MEDS) score
The Mortality in Emergency Department Sepsis (MEDS) score was developed as a risk stratification tool for patients presenting to the ED with concern for sepsis. This score was prospectively derived from a population of 3301 ED patient encounters during which a blood culture was ordered. Charts were reviewed and several data points extracted and analyzed to determine the following univariate predictors of 28-day mortality: terminal illness, tachypnea or hypoxia, septic shock, platelets <150,000/mm3, bands >5%, age >65 years, lower respiratory infection, nursing home residence, and altered mental status. These predictors were assigned point values based on their odds ratios, and points are added to generate a total score. Mortality risk was stratified into groups based on total score, with percentage mortality as follows: score 0-4: 0.9%; 5-7: 2.0%; 8-12: 7.8%; 13-15: 20.2%; >15: 50%. A separate validation cohort had the following mortality rates: score 0-4: 1.1%; 5-7: 4.4%; 8-12: 9.3%; 13-15: 16.1%; >15: 39%.50
The MEDS score was subsequently shown to also be predictive for 1-year mortality as well, with an area under receiver operating curve (AUROC) of 0.76 for 1-year mortality.51 A subsequent study showed similar mortality rates when expanding the patient population to include all patients with systemic inflammatory response syndrome (SIRS), potentially broadening the potential application of MEDS in ED risk stratification.52 However, the score was shown to be less predictive in patients with severe sepsis and septic shock, underestimating mortality in all MEDS score groups.53 Still, the MEDS score was demonstrated in multiple validation studies as a reliable risk stratification tool in patients with suspected infection or SIRS.54,55
Comment: The MEDS score is not as well studied in the literature as the SIRS criteria or QuickSOFA but is a validated risk stratification tool in patients with suspected infection and is ED specific. This tool, similar to Pneumonia Severity Index and CURB-65 (discussed below), can guide management of patients from the ED. Very-low-risk (score 0-4) patients can be treated as outpatients, low risk (score 5-7) patients warranting consideration of a short inpatient stay, and moderate to high risk (>8) requiring inpatient management. At present, there is insufficient evidence regarding the role of the MEDS score to guide inpatient disposition of floor vs. ICU in moderate to high-risk patients.
Pneumonia Severity Index
The Pneumonia Severity Index (PSI) was developed as a tool to predict mortality risk from pneumonia, allowing providers to appropriately manage care for these patients in the hospital or as outpatients. A derivation cohort of 14199 patients was utilized to create a prediction rule in two steps meant to parallel a clinician’s decision-making process. The first step identified a population of patients that were at low risk for death, which were assigned to class I. The second step quantified the risk for death in the remaining patients using weighted factors including demographics, comorbidities, exam findings, and clinical data. In all, 20 variables were used and assigned corresponding points, the sum of which would assign a patient to a particular risk for mortality (class II-V).56
Mortality risk was relatively low for patients in class I and II (0.4 and 0.7%, respectively). Class III carried a mortality risk of 2.8%. Mortality increased with class IV and class V classification: 8.5% and 31.1%, respectively. These data were replicated with a separate validation cohort of 38039 patients, with similar mortality rates in each class. This study concluded with the recommendation that patients diagnosed with pneumonia falling into class I and II mortality risk should be managed as outpatients, possible brief inpatient observation for class III, and class IV and V managed as inpatients.56
Subsequent trials evaluating the utility of the PSI score in the management of patients diagnosed with pneumonia randomized low-risk patients (class I-III PSI) to treatment as outpatients vs inpatients. There were no statistical differences in adverse outcomes (ICU admission, hospital readmission, mortality, complications), with notable improvements in hospital admission rates and patient satisfaction.57,58 A meta-analysis of 6 studies that used a clinical decision tools to identify low-risk patients to treat pneumonia as outpatients showed no significant difference in mortality, patient readmissions, or patient satisfaction. Low-risk patients that required admission often included comorbid illnesses not included in the PSI, inability to take oral medications, barriers to compliance, or hypoxemia.59
Though the PSI has been shown to successfully identify patients at low risk for mortality, it has been less accurate at predicting and stratifying classes of severe pneumonia. A meta-analysis by Loke et al showed that PSI class IV or V had pooled sensitivity of 0.90 and specificity 0.53 for 30-day mortality, which was significantly better than the CURB-65 rule (discussed below).60 However, a subsequent large meta-analysis showed that PSI class IV or V had a sensitivity of 75% and specificity 40% for requiring ICU intervention or admission, which are not sufficient to guide disposition decisions.61
CURB-65
One of the criticisms of PSI included its complexity, with inclusion of 20 factors making it impractical for use as a bedside tool. The CURB-65 score was developed with a similar goal of identifying low-risk patients with pneumonia who would be candidates for outpatient management, but also patients at high risk for mortality or ICU admission. Criteria for severe pneumonia published by the British Thoracic Society include: respiratory rate ≥ 30 breaths/min, diastolic blood pressure ≤60 mmHg, and blood urea nitrogen >7 mmol/L. The presence of 2 criteria was 88% sensitive and 72% specific for mortality or ICU admission.62 The CURB-65 tool was based on these criteria, with the addition of age ≥65 years, which was found to be a separate independent predictor of mortality. Thus, the 5 criteria making up the score are as follows (1 point each, 0-5 total):
Confusion, meaning Mental Test Score ≤8, or disorientation to person, place, or time
Urea >7 mmol/L (>19.6 mg/dL)
Respiratory rate ≥ 30 breaths/minute
Blood pressure (systolic < 90 mmHg or diastolic ≤ 60 mmHg)
Age ≥ 65 years
A score of 0-1 of these criteria characterized low mortality risk (<1.5%) in the test group, a score of 2 was intermediate mortality risk (9.2%), and a score of 3 or more associated with high mortality risk (22%). A score ≥ 2 was 93% sensitive and 49% specific for 30-day mortality.63
A subsequent prospective validation study by Aujesky et al that included 3181 patients with community-acquired pneumonia demonstrated slightly higher mortality rates for each CURB-65 score (0.6%, 3%, 6.1%, 13%, 17%, 43% mortality in scores of 0-5, respectively).64 In particular, the 3% mortality rate in CURB-65 scores of 1 is similar to PSI class III mortality rates, suggesting a lower threshold (CURB-65 ≥1) for consideration of admission for management. Another validation study by Capelastegui et al showed similar mortality rates to the derivation study for specific CURB-65 scores, but noted 53% of patients with a score of 1 also were found to have characteristics that were independent for a poor prognosis, and should be considered in the decision for outpatient or inpatient treatment.65 Furthermore, a recent study found that of patients in the ED with a CURB-65 score of 1, 8% still required critical care intervention.66 Thus, use of CURB-65 in screening for low-risk patients with community-acquired pneumonia is recommended to be limited to scores of 0. However, as with PSI, validation studies have yet to show predictive utility of scores suggesting severe pneumonia (CURB-65 ≥3) in predicting mortality or ICU requirement.60,61
As validation studies have suggested only patients with a CURB-65 score of 0 are screened low risk enough for outpatient treatment, greater weight may be placed on utility of CRB-65 as a tool. This rule, initially proposed in the same study as CURB-65, omits blood urea nitrogen as a factor to only rely on history and physical exam data with a score of 0 indicating low risk.63 In initial derivation and validation studies, this rule demonstrated <1.6% mortality risk with a score of 0, with risk increasing to 4-8.6% in scores of 1.63,65 Multiple studies have compared CRB-65 and CURB-65, with only marginal but not statistically significant improvement in prognostic utility of CURB-65.65,67 A meta-analysis of 1648 patients even showed only 0.5% mortality risk in CRB-65 ≤1; potentially including CRB-65 0-1 as low risk, though, would require further study.68 Although multiple validation studies have also successfully stratified low risk to similar mortality risk (<1.6%), accuracy wanes with higher CRB-65 scores.69
Several studies have directly compared CURB-65 and PSI both in terms of identifying low-risk patients and stratifying disease severity.60,61,64,68,70-72 Multiple studies have shown similar mortality risk in low-risk populations and have demonstrated sensitivities for mortality greater than 96% for CURB-65/CRB-65 = 0 and PSI class I-III, albeit with specificities ranging from 18-65%.64,68,70 In stratifying patients into different levels of severity (ward vs ICU patients), PSI has shown slightly better sensitivity/specificity for mortality and/or ICU intervention, though neither is strong enough to significantly stratify severe pneumonia to serve as tools for directing inpatient management.60,61
Comment: PSI, CRB-65, and CURB-65 have been well validated as screening tools for low-risk patients who should be treated as outpatients (CURB-65 or CRB-65 = 0, PSI class I and II). A moderate-risk population (CURB-65 = 1 or 2, PSI class III) may benefit from treatment as inpatient or outpatient at clinician judgement. Use of these tools for determining disease severity and possible ICU requirement is not as reliable, and other clinical factors should be considered.
Conclusion
This article provides an overview of several common clinical decision instruments and the evidence behind them. Ultimately, many institutions have incorporated clinical decision rules directly into the electronic medical record, and this strategy will not only increase their use, but hopefully collect further data on whether the instruments truly perform better than unstructured clinical judgement.
In this second part of “Playing by the Rules,” we will examine validated clinical decision rules that assist emergency physicians (EPs) in the diagnosis and treatment of nontraumatic conditions. Most trauma rules seek to answer a yes or no question regarding the utility of testing for specific disease states when the diagnosis is not clinically apparent.
For example, the Canadian CT Head Rule describes a number of conditions that, if met, can predict the absence of traumatic lesions requiring neurosurgical intervention in the alert patient with head injury, and thus obviate the need for imaging in those instances. In contrast, many medical rules are actually risk stratification scales for treatment and diagnosis, categorizing patients into low- to high-risk groups based on clinical factors. While traumatic conditions are linked to a specific inciting event or “trauma,” medical diseases may have multiple causative factors or may be delayed in presentation to the emergency department (ED), which subsequently increases the complexity of these decision instruments.
Rather than an exhaustive list of all clinical decision rules or risk stratification scales relevant to emergency medicine, this installment will provide EPs with a review of common instruments and the evidence behind them.
Central Nervous System
Ottawa Subarachnoid Hemorrhage Rule
The Ottawa Subarachnoid Hemorrhage Rule offers guidance for diagnosing atraumatic subarachnoid hemorrhage (SAH) in alert, neurologically intact adult patients presenting to the ED with a headache reaching maximal intensity within 1 hour of onset. The rule states that if none of the following conditions are present, then the diagnosis of SAH can be excluded without further testing:
Symptom of neck pain or stiffness
Age greater than 40 years old
Witnessed loss of consciousness
Onset during exertion
Thunderclap headache with peak pain instantly
Limited neck flexion on exam
The validation study prospectively enrolled 1153 adults of whom 67 had a positive workup for SAH (defined as subarachnoid blood visible on noncontrast CT scan of the head, xanthochromia of cerebrospinal fluid on visual inspection, or the presence of >1 million erythrocytes in the final tube of cerebrospinal fluid with an aneurysm or arteriovenous malformation confirmed on cerebral angiography).1 Of note, patients with prior history of cerebral aneurysm or SAH were excluded, as were patients with recurrent headaches similar to the presenting complaint, patients with focal neurologic deficits or papilledema, or patients with a history of brain neoplasm, ventricular shunt, or hydrocephalus. The authors found that the rule was 100% sensitive and 13% specific for detecting SAH, with a kappa of 0.82, which suggests good interrater reliability.1
Comment: It is important to note that the authors excluded patients with a history of cerebral aneurysm or prior SAH, and therefore the rule should not be applied to these patients in clinical practice. The utility of this rule is somewhat limited secondary to the age cutoff, as the incidence of aneurysmal SAH increases considerably after the fifth decade of life.2 Ultimately, this rule—combined with the authors’ previous work showing that CT performed within 6 hours of headache onset can rule out SAH—provides a powerful diagnostic tool for EPs considering SAH in the ED.3
ABCD2 Score
The ABCD2 score was developed to identify transient ischemic attack (TIA) patients at risk for early stroke, and thus inform decisions regarding admission and resource utilization in the ED and outpatient clinic setting.4 The score was created by combining elements of two previously existing rules, the California and the ABCD scales. Patients presenting with TIA symptoms are assigned points based on:
Age: 1 point if ≥ 60 years
Blood Pressure: 1 point if ≥ 140/90
Clinical Deficit: 2 points for unilateral weakness, 1 point for speech impairment without unilateral weakness
Duration: 2 points for ≥ 60 minutes, 1 point for 10 to 59 minutes
Diabetes: 1 point if diabetic
The greater the number of points, the higher the risk for imminent stroke, from low (0-3 points) to moderate (4-5 points) to high (6-7 points). The initial retrospective internal validation study found that the low, moderate, and high groups correlated to 7-day stroke risk of 1.2%, 5.9%, and 11.7%, respectively. Subsequently, the ABCD2 score was rapidly incorporated into institutional and national protocols for assessing risk for stroke and featured prominently in the 2009 American Heart Association guidelines on TIA, which recommend hospitalization for a score of 3 or greater.4,5
More recently, a multicenter prospective external validation study of more than 2000 TIA patients found that using the American Heart Association recommended cutoff of 3 or greater resulted in a sensitivity of 94.7% for detecting those patients who sustained a stroke within 7 days, but a specificity of only 12.5%.6 The investigators concluded that a specificity this low would require “almost all” of the TIA patients in their cohort (87.6%) to be admitted to the hospital—even though only 3.2% of their patients had a stroke within 90 days.6 Even when examined at other cutoff scores, the investigators found the ABCD2 score to have poor accuracy.6
Comment: Decreasing resource utilization is a laudable goal, but it does not appear that the ABCD2 score provides much guidance on which TIA patients can safely go home. Moreover, the increasing availability of advanced imaging and tele-neurology consultation in the ED have changed the landscape of TIA and stroke care. Many EPs have since argued that the ABCD2 score adds little to their evaluation.7
Abdomen
Alvarado Score
There are multiple clinical prediction rules for appendicitis. Among the most commonly utilized by EPs and surgical consultants are the Alvarado score and the Appendicitis Inflammatory Response Score. The Alvarado score was derived in 1986 based on a retrospective review of 305 abdominal pain patients of whom 227 (aged 4 to 80 years) had appendicitis.8 Factors were identified and weighted, which can be recalled through the mnemonic MANTRELS:
Migration of pain to the right lower quadrant: 1 point
Anorexia or acetone in urine: 1 point
Nausea or vomiting: 1 point
Tenderness in the right lower quadrant: 2 points
Rebound tenderness: 1 point
Elevation of the temperature > 37.3°C: 1 point
Leukocytosis >10K X 109/L: 2 points
Shift to the left of neutrophils (>75%): 1 point
The original article posited that a score of 5 or 6 was “compatible” with the diagnosis of acute appendicitis—necessitating further observation for possible appendicitis—and that higher scores indicated an increasing probability of disease.8 Of note, the rule has also been adapted for clinical settings where differentials are not easily obtainable with the left shift criterion removed; this is known as the modified Alvarado score and calculated out at a maximum of 9.9
Since the original Alvarado study was published, multiple small studies have attempted to validate or otherwise retrospectively assess the utility of this rule. A frequently cited systematic review of 42 prospective and retrospective studies by Ohle et al found that a score of <5 showed a sensitivity of 99% overall (96% in men, 99% in women, and 99% in children) for ruling out admission/observation of patient with suspected appendicitis, though the specificity for ruling in the diagnosis at scores 7 and higher was only 81% overall.10
However, a more recent prospective observational study of adult abdominal pain patients presenting to large American urban EDs found the modified Alvarado rule at cutoff levels of 3, 4, and 5 had sensitivities of only 72%, 55%, and 36%, respectively, of ruling out the diagnosis.11 In comparison, the study found that physicians’ clinical judgement of appendicitis being the first or second most likely diagnosis had a sensitivity of 93% for predicting appendicitis.11
Comment: The Alvarado score was developed to help rule out and rule in the diagnosis of appendicitis. However, with the increasing availability of CT scanning in EDs, the diagnostic pathway in unclear cases has shifted from admission/observation to CT scanning, which has the benefit of elucidating other pathology as well. The utility of the Alvarado rule has been called into question. Ultimately, there is data in support of the Alvarado rule from older articles and studies in resource-poor environments, and newer studies may reflect less rigorous application of the rule when CT scanning is the default clinical pathway. Further studies that focus specifically on the Alvarado score as a rule out test to decrease CT utilization may be instructive.
Appendicitis Inflammatory Response (AIR) score
The appendicitis inflammatory response (AIR) score was derived in a cohort of 316 patients and validated on a sample of 229 adults and children with suspected appendicitis.12 The authors specifically sought to create a rule that outperformed the Alvarado score; the criteria are:
Vomiting: 1 point
Right iliac fossa pain: 1 point
Rebound tenderness: 1 point for light, 2 for medium, 3 for strong
Temperature >38.5°C: 1 point
Polymorphonuclear leukocytes: 1 point for 70%-84%, 2 for 85% or greater
White blood cell count: 1 point for 10,000-14,900, 2 for 15,000 or greater
C-reactive protein level (mg/dL): 1 point for 10-49, 2 for 50 or greater
Patients with a score of 0-4 were classified as low risk, with recommendation for outpatient follow-up if general condition unchanged; a score of 5-8 as indeterminate risk, with recommendation for active observation with serial exams, imaging, or diagnostic laparoscopy; or a score of 9-12 as high risk, with recommendation for surgical exploration.12 In the validation cohort, the investigators found an AIR score or Alvarado score greater than 4 to have, respectively, 96% or 97% sensitivity and 73% or 61% specificity for detecting appendicitis.12 A high score of greater than 8 on either the AIR or Alvarado had respectively 37% or 28% sensitivity but specificity of 99% for detecting appendicitis with either instrument.12
In an external validation study, the AIR and Alvarado scores were calculated on a series of 941 patients (aged 1 to 97 years) being evaluated for possible appendicitis; 201 patients were younger than 18.13 At a cutoff of greater than 4, the sensitivity and specificity were found to be 93% and 85% for the AIR and 90% and 55% for Alvarado.13 In a cohort of 182 patients (aged 4 to 75 years), a score of 4 or greater on the AIR and Alvarado was found to have comparable sensitivity to that of a senior surgical consultant for detecting appendicitis—with sensitivities of 94%, 93%, and 90% respectively.14 Subsequently, the original investigators undertook a large multicenter implementation study of the AIR at 24 hospitals of patients (aged 5 to 96 years) with suspected appendicitis. As compared to the pre-implementation group, using AIR to categorize patients as low risk resulted in significantly fewer imaging studies, admissions, and surgical explorations.15
Comment: The AIR has the benefit of recent prospective studies that assess performance of the rule in settings that mirror the practice environments of most EPs today. The classification of rebound tenderness as light, medium, or strong may be difficult to ascertain. Ultimately, reductions in imaging, admissions, and surgical explorations are important goals and EPs might benefit from using this rule to guide imaging.
CHEST
HEART Score
The increasingly popular HEART score, first developed by physicians in the Netherlands in 2008, seeks to risk-stratify patients presenting to the ED with suspected cardiac chest pain without ST-elevation myocardial infarction (STEMI). It scores patients 0 to 2 on 5 different characteristics (with a total scored of 10 possible points):
History: 2 points for highly suspicious, 1 point for moderately suspicious
EKG: 2 points for significant ST deviation, 1 point for nonspecific repolarization disturbance
Age: 2 points for age 65 years or greater, 1 point for age 45-64 years
Risk Factors: 2 points for 3 or more risk factors or history of atherosclerotic disease, 1 point for 1 to 2 risk factors
Troponin: 2 points for troponin value >3 times the normal limit, 1 point for value 1-3 times the normal limit.
The authors developed these 5 categories “based on clinical experience and current medical literature,” and then applied the rule to 122 chest pain patients in the ED, finding a higher incidence of major adverse coronary events (MACE) with increasing score: 2.5% for low risk score of 0-3, 20.3% for intermediate risk score of 4-6, and 72.7% for score 7 or higher.16 The score has been retrospectively and prospectively validated.17,18 In a study of 2440 patients, the low risk group had a MACE of 1.7%, and the score had a c-statistic of 0.83, outperforming Thrombolysis in Myocardial Infarction (TIMI) and GRACE c-statistics of 0.75 and 0.70, respectively.18 In 2013, investigators calculated the HEART score on a multinational database of 2906 chest pain patients, finding a negative predictive value of 98.3% for MACE with HEART score less than or equal to 3.19
In the United States, Mahler et al have produced a series of 3 articles validating the HEART score and demonstrating its use in reducing cardiac testing and length of stay. In 1070 patients admitted to their observation unit, who were deemed low risk by physician assessment and TIMI <2, a score of less than or equal to 3 had a negative predictive value of 99.4% for MACE; the inclusion of serial troponins resulted in sensitivity of 100%, specificity of 83.1%, and negative predictive value of 100%.20 The team then conducted a secondary analysis of chest pain patients enrolled in a large multicenter trial (MIDAS) and compared HEART score, the North American Chest Pain Rule, and unstructured clinical assessment.21 Both rules had high sensitivities, but the HEART score identified 20% of patients suitable for early discharge, as compared to 4% for the North American Chest Pain Rule.21 Finally, Mahler’s team performed a randomized control trial of 282 patients investigating whether the HEART score with serial troponins compared with usual care could safely reduce cardiac testing.22 The HEART pathway resulted in an absolute reduction of 12.1% in cardiac testing, and median reduction in length of stay by 12 hours, with no missed MACE in discharged patients.22
Most recently, a stepped-wedge, cluster randomized trial across 9 hospitals published in 2017 investigated the utility of the HEART score. Despite enrolling only 3648 patients out of the statistically required sample size of 6600, they found that the HEART score was not inferior to usual care and there was no significant difference in median length of stay, but health care resources were typically lower in the HEART score group.23
Comment: While derived in a less conventional manner, the HEART score has held up in several validation studies and appears poised to safely decrease health care costs and increase ED efficiency and throughput. As more US EDs look to adopt high sensitivity troponin biomarkers, prospective studies will be needed to determine the role of the HEART score in this setting.
Thrombolysis in Myocardial Infarction (TIMI) score
The Thrombolysis in Myocardial Infarction (TIMI) score was developed in 2000 as a tool to risk-stratify patients with a diagnosis of unstable angina (UA) and non–ST-elevation myocardial infarction (NSTEMI). The score was derived from 1 arm (2047 patients) of a study comparing heparin with enoxaparin for treatment of NSTEMI, and validated in the other 3 arms of the study (5124 patients). Multivariate logistic regression was used to develop 7 variables of equal weight:
Age greater than or equal to 65yo
Three or more cardiac risk factors
Known coronary artery disease (with stenosis greater than or equal to 50%)
Aspirin use in the past 7 days
Severe angina (2 or more episodes in the past 24 hours)
EKG ST changes greater than or equal to 0.5 mm
Positive serum cardiac biomarkers
The investigators found that with a higher score, there was progressive increase in adverse cardiac outcomes, with a c-statistic of 0.65.24 This score was subsequently validated across several existing databases evaluating various therapeutic interventions for UA/NSTEMI and remained statistically significant, with increasing risk for MI and mortality with increasing score.25,26
Given the success in predicting patient outcomes and identifying patients who could benefit from more aggressive care, the TIMI risk score was then applied to unselected ED chest pain patients. In a secondary analysis of a prospective observational cohort of 3929 patient visits, the TIMI score correlated to the risk for adverse outcomes, with a risk of 2.1% at score 0.27
In a second prospective observational cohort of 1458 patient visits, a score of 0 correlated to a 1.7% incidence of adverse outcomes.28 In 2008, Body et al sought to increase the relative weight of EKG and biomarker factors to 5 (instead of 1) in a study of 796 patients, positing that these factors have more importance in the ED setting.29 Comparing the modified TIMI to the original, the modified instrument improved the area under curve (AUC) from 0.77 to 0.87.29 In follow-up validation studies, the modified score has an improved AUC, but the incidence of adverse outcomes at score 0 remains at about 2% for both modified and original score.30,31
In 2010, Hess et al performed a systematic review and meta-analysis of the studies that prospectively validated the TIMI score. They evaluated 10 validation studies, encompassing 17,265 patients across 5 countries, and found a strong linear relation between the TIMI score and adverse cardiac events.32 At TIMI score of 0, the incidence of cardiac events was 1.8%, with sensitivity of 97.2% and specificity of 25%. Subsequently, the ADAPT trial designed a diagnostic protocol consisting of TIMI risk assessment, EKG, and 0- and 2-hour troponin I biomarkers to find ED patients eligible for safe, early discharge.33 Of the 1975 patients, 20% were classified as low risk and eligible for early discharge, in that they had TIMI score of 0, a non-ischemic ECG, and negative troponins. Only one patient had a MACE at 30 days, giving the protocol a sensitivity of 99.7%, specificity of 23.4%, and negative predictive value of 99.7%.33
As the TIMI and HEART scores are both used to evaluate ED chest pain patients, several studies have sought to compare them. In 2015, Cartlon et al published a comparison of 5 established risk scores and 2 troponin assays in 963 patients: modified Goldman, TIMI, GRACE, HEART, and Vancouver Chest Pain Rule in combination with troponin T and I.34 The investigators found that a negative troponin T plus either TIMI score of 0 or a HEART score ≤3 gave a negative predictive value of greater than 99.5% with more than 30% of patients able to be discharged safely.34 In 2017, a comparison of the GRACE, HEART, and TIMI scores in 1833 chest pain patients found the HEART score identified more low risk patients than either of its comparators and had the highest AUC at 0.86.35 Other trials have similarly found HEART outperforming TIMI.36
Comment: The TIMI score was not specifically designed for ED use but has been adapted to serve this purpose. To the EP assessing the undifferentiated chest pain patient, the TIMI score uses clinical variables that may seem curious (eg, aspirin use) or impossible for EPs to ascertain (eg, presence or degree of stenosis). Even for patients with a score of 0, the risk for adverse outcomes remains stubbornly at the 2% level, similar to the original low risk HEART score findings.
Wells’ Criteria for Pulmonary Embolism
The diagnosis of pulmonary embolism (PE) is often challenging, requiring the use of multiple ED resources for timely diagnosis, and is therefore well suited for clinical decision instruments. The Wells’ Criteria were derived from a cohort of 1260 patients using logistic regression to identify 7 significant variables:
Clinical signs and symptoms of deep vein thrombosis (DVT): 3
PE is the most likely diagnosis: 3
Heart rate >100: 1.5
Immobilization or surgery in the previous 4 weeks: 1.5
Previously diagnosed DVT or PE: 1.5
Hemoptysis: 1
Malignancy with treatment within 6 months or palliative: 1
The investigators specifically linked the use of their instrument to the D-dimer assay, using their score to determine pretest probability and seeking to exclude the diagnosis in patients with low pretest probability and negative D-dimer result.37,38 They reported a three-tiered classification, with low risk at a score less than 2, moderate risk at scores from 2-6, and high risk at scores greater than 6. The risk for PE with a low risk score coupled with a negative D-dimer result were 1.5% and 2.7% in the derivation and validation cohorts. Using a two-tiered classification of PE unlikely at scores less than or equal to 4 and PE likely at scores 5 or greater, a PE unlikely score and a negative D-dimer had a 2.2% and 1.7% risk in the derivation and validation cohorts.
A subsequent study by Wells et al on 930 ED patients using the score plus D-dimer found a negative predictive value of 99.5% for a low risk score and a negative D-dimer.39 This allowed for reduced imaging in 53% of patients.39 Another external validation study found acceptable interrater agreement between physicians for the Wells’ score at kappa 0.62 for the three-tiered system and 0.7 for the two-tiered system.40 The Wells’ Criteria has been compared against the Geneva score with receiver operating characteristic curve analysis showing no difference between the two rules.41 In a large cohort of 3306 patients being evaluated for PE using the Wells’ score and D-dimer, for the 1028 patients with PE unlikely and a negative D-dimer, there was a 3-month incidence of venous thromboembolism (VTE) of 0.5%—none of which were fatal events.42
Comment: The Wells’ Criteria for pulmonary embolism combined with D-dimer is now the preferred approach for many EPs seeking to risk-stratify their patients for PE. Advances in age-adjusted cutoffs for D-dimer provide additional support for this powerful tool.
Pulmonary Embolism Rule-Out Criteria (PERC)
Given the low specificity of the D-dimer assay for VTE, researchers post–Wells’ Criteria have sought to further reduce unnecessary testing by reassessing the D-dimer’s role in the diagnostic pathway. The PERC rule was designed to reduce D-dimer use—and downstream CT scan testing—in low-risk patients. The investigators derived the rule from a population of patients for whom the pretest probability of PE was less than 15%, seeking a risk for PE less than 2% if the rule was satisfied. Using logistic regression in 3148 ED patients, 8 clinical criteria were obtained:
Age < 50 years Pulse <100
Pulse oximetry >94%
No unilateral leg swelling
No hemoptysis
No recent surgery
No prior PE/DVT
No hormone use
The variables were tested in 1427 low-risk and 382 very-low-risk patients (defined as being evaluated for dyspnea, but not part of the derivation or low-risk validation groups). In the low-risk group, the sensitivity, specificity, and false-negative rate was 96%, 27%, and 1.4% respectively. In the very-low-risk group, the sensitivity, specificity, and false-negative rate was 100%, 15%, and 0% respectively.43 The rule was further validated in a prospective multicenter study of 8138 patients; among patients with pretest probability less than 15% who were PERC negative, 1% had PE/DVT within 45 days.44 The large PERCEPIC trial on 1757 patients found low pretest probability patients who were PERC negative had a false-negative rate of 1.2% and estimated that the use of PERC could decrease the median length of stay in the ED by at least 2 hours.45 The PROPER study, a non-inferiority, crossover cluster-randomized trial in 14 EDs across France, found that use of the PERC rule was not inferior to conventional care and that it was associated with reduced ED length of stay and CT use.45,46
There has been criticism from some European studies that the PERC rule misses too many PEs. A provocatively titled multinational study from Hugli et al examined patients suspected to have PE in Switzerland, France, and Belgium. The investigators applied the PERC rule and then stratified the patients by pretest probability as defined by the Geneva score, which includes many of the same criteria as PERC. They found the PERC rule identified a small proportion of patients with suspected PE as very low risk (13.2%) and that the prevalence of PE among these patients was 5.4%. Critics of this study have noted that the PERC rule was designed to be applied in low-risk patients, not to define the low-risk population.47 Another study examined a retrospective cohort of patients in whom a D-dimer was ordered to exclude PE, and then calculated the Wells’ and PERC score from the medical record. The investigators found that the combination of Wells and PERC missed 2 PEs out of their population of 377 patients.48 However, a subsequent meta-analysis analyzed 11 studies—including the two negative studies—and found a pooled sensitivity of 97%, specificity of 23%, and negative likelihood ratio of 0.18, concluding that when the pretest probability is low, PERC is sensitive enough to exclude D-dimer testing.49
Comment: Given the number of disease states and sampling techniques that can cause nonspecific elevation in D-dimer assay, the PERC rule provides a useful tool in low-risk populations for excluding PE without laboratory testing. The key is applying the rule to the appropriate population, as stratified by gestalt or clinical score.
Infectious Disease
Mortality in Emergency Department Sepsis (MEDS) score
The Mortality in Emergency Department Sepsis (MEDS) score was developed as a risk stratification tool for patients presenting to the ED with concern for sepsis. This score was prospectively derived from a population of 3301 ED patient encounters during which a blood culture was ordered. Charts were reviewed and several data points extracted and analyzed to determine the following univariate predictors of 28-day mortality: terminal illness, tachypnea or hypoxia, septic shock, platelets <150,000/mm3, bands >5%, age >65 years, lower respiratory infection, nursing home residence, and altered mental status. These predictors were assigned point values based on their odds ratios, and points are added to generate a total score. Mortality risk was stratified into groups based on total score, with percentage mortality as follows: score 0-4: 0.9%; 5-7: 2.0%; 8-12: 7.8%; 13-15: 20.2%; >15: 50%. A separate validation cohort had the following mortality rates: score 0-4: 1.1%; 5-7: 4.4%; 8-12: 9.3%; 13-15: 16.1%; >15: 39%.50
The MEDS score was subsequently shown to also be predictive for 1-year mortality as well, with an area under receiver operating curve (AUROC) of 0.76 for 1-year mortality.51 A subsequent study showed similar mortality rates when expanding the patient population to include all patients with systemic inflammatory response syndrome (SIRS), potentially broadening the potential application of MEDS in ED risk stratification.52 However, the score was shown to be less predictive in patients with severe sepsis and septic shock, underestimating mortality in all MEDS score groups.53 Still, the MEDS score was demonstrated in multiple validation studies as a reliable risk stratification tool in patients with suspected infection or SIRS.54,55
Comment: The MEDS score is not as well studied in the literature as the SIRS criteria or QuickSOFA but is a validated risk stratification tool in patients with suspected infection and is ED specific. This tool, similar to Pneumonia Severity Index and CURB-65 (discussed below), can guide management of patients from the ED. Very-low-risk (score 0-4) patients can be treated as outpatients, low risk (score 5-7) patients warranting consideration of a short inpatient stay, and moderate to high risk (>8) requiring inpatient management. At present, there is insufficient evidence regarding the role of the MEDS score to guide inpatient disposition of floor vs. ICU in moderate to high-risk patients.
Pneumonia Severity Index
The Pneumonia Severity Index (PSI) was developed as a tool to predict mortality risk from pneumonia, allowing providers to appropriately manage care for these patients in the hospital or as outpatients. A derivation cohort of 14199 patients was utilized to create a prediction rule in two steps meant to parallel a clinician’s decision-making process. The first step identified a population of patients that were at low risk for death, which were assigned to class I. The second step quantified the risk for death in the remaining patients using weighted factors including demographics, comorbidities, exam findings, and clinical data. In all, 20 variables were used and assigned corresponding points, the sum of which would assign a patient to a particular risk for mortality (class II-V).56
Mortality risk was relatively low for patients in class I and II (0.4 and 0.7%, respectively). Class III carried a mortality risk of 2.8%. Mortality increased with class IV and class V classification: 8.5% and 31.1%, respectively. These data were replicated with a separate validation cohort of 38039 patients, with similar mortality rates in each class. This study concluded with the recommendation that patients diagnosed with pneumonia falling into class I and II mortality risk should be managed as outpatients, possible brief inpatient observation for class III, and class IV and V managed as inpatients.56
Subsequent trials evaluating the utility of the PSI score in the management of patients diagnosed with pneumonia randomized low-risk patients (class I-III PSI) to treatment as outpatients vs inpatients. There were no statistical differences in adverse outcomes (ICU admission, hospital readmission, mortality, complications), with notable improvements in hospital admission rates and patient satisfaction.57,58 A meta-analysis of 6 studies that used a clinical decision tools to identify low-risk patients to treat pneumonia as outpatients showed no significant difference in mortality, patient readmissions, or patient satisfaction. Low-risk patients that required admission often included comorbid illnesses not included in the PSI, inability to take oral medications, barriers to compliance, or hypoxemia.59
Though the PSI has been shown to successfully identify patients at low risk for mortality, it has been less accurate at predicting and stratifying classes of severe pneumonia. A meta-analysis by Loke et al showed that PSI class IV or V had pooled sensitivity of 0.90 and specificity 0.53 for 30-day mortality, which was significantly better than the CURB-65 rule (discussed below).60 However, a subsequent large meta-analysis showed that PSI class IV or V had a sensitivity of 75% and specificity 40% for requiring ICU intervention or admission, which are not sufficient to guide disposition decisions.61
CURB-65
One of the criticisms of PSI included its complexity, with inclusion of 20 factors making it impractical for use as a bedside tool. The CURB-65 score was developed with a similar goal of identifying low-risk patients with pneumonia who would be candidates for outpatient management, but also patients at high risk for mortality or ICU admission. Criteria for severe pneumonia published by the British Thoracic Society include: respiratory rate ≥ 30 breaths/min, diastolic blood pressure ≤60 mmHg, and blood urea nitrogen >7 mmol/L. The presence of 2 criteria was 88% sensitive and 72% specific for mortality or ICU admission.62 The CURB-65 tool was based on these criteria, with the addition of age ≥65 years, which was found to be a separate independent predictor of mortality. Thus, the 5 criteria making up the score are as follows (1 point each, 0-5 total):
Confusion, meaning Mental Test Score ≤8, or disorientation to person, place, or time
Urea >7 mmol/L (>19.6 mg/dL)
Respiratory rate ≥ 30 breaths/minute
Blood pressure (systolic < 90 mmHg or diastolic ≤ 60 mmHg)
Age ≥ 65 years
A score of 0-1 of these criteria characterized low mortality risk (<1.5%) in the test group, a score of 2 was intermediate mortality risk (9.2%), and a score of 3 or more associated with high mortality risk (22%). A score ≥ 2 was 93% sensitive and 49% specific for 30-day mortality.63
A subsequent prospective validation study by Aujesky et al that included 3181 patients with community-acquired pneumonia demonstrated slightly higher mortality rates for each CURB-65 score (0.6%, 3%, 6.1%, 13%, 17%, 43% mortality in scores of 0-5, respectively).64 In particular, the 3% mortality rate in CURB-65 scores of 1 is similar to PSI class III mortality rates, suggesting a lower threshold (CURB-65 ≥1) for consideration of admission for management. Another validation study by Capelastegui et al showed similar mortality rates to the derivation study for specific CURB-65 scores, but noted 53% of patients with a score of 1 also were found to have characteristics that were independent for a poor prognosis, and should be considered in the decision for outpatient or inpatient treatment.65 Furthermore, a recent study found that of patients in the ED with a CURB-65 score of 1, 8% still required critical care intervention.66 Thus, use of CURB-65 in screening for low-risk patients with community-acquired pneumonia is recommended to be limited to scores of 0. However, as with PSI, validation studies have yet to show predictive utility of scores suggesting severe pneumonia (CURB-65 ≥3) in predicting mortality or ICU requirement.60,61
As validation studies have suggested only patients with a CURB-65 score of 0 are screened low risk enough for outpatient treatment, greater weight may be placed on utility of CRB-65 as a tool. This rule, initially proposed in the same study as CURB-65, omits blood urea nitrogen as a factor to only rely on history and physical exam data with a score of 0 indicating low risk.63 In initial derivation and validation studies, this rule demonstrated <1.6% mortality risk with a score of 0, with risk increasing to 4-8.6% in scores of 1.63,65 Multiple studies have compared CRB-65 and CURB-65, with only marginal but not statistically significant improvement in prognostic utility of CURB-65.65,67 A meta-analysis of 1648 patients even showed only 0.5% mortality risk in CRB-65 ≤1; potentially including CRB-65 0-1 as low risk, though, would require further study.68 Although multiple validation studies have also successfully stratified low risk to similar mortality risk (<1.6%), accuracy wanes with higher CRB-65 scores.69
Several studies have directly compared CURB-65 and PSI both in terms of identifying low-risk patients and stratifying disease severity.60,61,64,68,70-72 Multiple studies have shown similar mortality risk in low-risk populations and have demonstrated sensitivities for mortality greater than 96% for CURB-65/CRB-65 = 0 and PSI class I-III, albeit with specificities ranging from 18-65%.64,68,70 In stratifying patients into different levels of severity (ward vs ICU patients), PSI has shown slightly better sensitivity/specificity for mortality and/or ICU intervention, though neither is strong enough to significantly stratify severe pneumonia to serve as tools for directing inpatient management.60,61
Comment: PSI, CRB-65, and CURB-65 have been well validated as screening tools for low-risk patients who should be treated as outpatients (CURB-65 or CRB-65 = 0, PSI class I and II). A moderate-risk population (CURB-65 = 1 or 2, PSI class III) may benefit from treatment as inpatient or outpatient at clinician judgement. Use of these tools for determining disease severity and possible ICU requirement is not as reliable, and other clinical factors should be considered.
Conclusion
This article provides an overview of several common clinical decision instruments and the evidence behind them. Ultimately, many institutions have incorporated clinical decision rules directly into the electronic medical record, and this strategy will not only increase their use, but hopefully collect further data on whether the instruments truly perform better than unstructured clinical judgement.
1. Perry JJ, Sivilotti MLA, Sutherland J, et al. Validation of the Ottawa Subarachnoid Hemorrhage Rule in patients with acute headache. CMAJ. 2017;189(45):E1379-E1385.
2. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365-1372.
3. Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
4. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
5. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293
6. Perry JJ, Sharma M, Sivilotti ML, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ. 2011;183(10):1137-1145.
7. Stead LG, Suravaram S, Bellolio MF, et al. An assessment of the incremental value of the ABCD2 score in the emergeny department evaluation of transient ischemic attack. Ann Emerg Med. 2011;57(1):46-51.
8. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15(5):557-564.
9. Kalan M, Talbot D, Cunliffe WJ, Rich AJ. Evaluation of the modified Alvarado score in the diagnosis of acute appendicitis: a prospective study. Ann R Coll Surg Engl. 1994;76(6):418-419.
10. Ohle R, O'Reilly F, O'Brien KK, Fahey T, Dimitrov BD. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
11. Meltzer AC, Baumann BM, Chen EH, Shofer FS, Mills AM. Poor sensitivity of a modified Alvarado score in adults with suspected appendicitis. Ann Emerg Med. 2013;62(2):126-31.
12. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32(8):1843-1849.
13. de Castro SM, Ünlü C, Steller EP, van Wagensveld BA, Vrouenraets BC. Evaluation of the appendicitis inflammatory response score for patients with acute appendicitis. World J Surg. 2012;36(7):1540-1545.
14. Kollár D, McCartan DP, Bourke M, Cross KS, Dowdall J. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39(1):104-109.
15. Andersson M, Kolodziej B, Andersson RE; STRAPPSCORE Study Group. Randomized clinical trial of Appendicitis Inflammatory Response score-based management of patients with suspected appendicitis. Br J Surg. 2017;104(11):1451-1461.
16. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Netherlands Heart J. 2008;16(6):191-196.
17. Backus BE, Six AJ, Kelder JC, et al. Chest Pain in the Emergency Room. A Multicenter Validation of the HEART Score. Crit Pathways Cardiol. 2010;9:164-169.
18. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. In J Cardiol. 2013;168:2153-2158.
19. Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department. Crit Pathways Cardiol. 2013;12:121-126.
20. Mahler SA, Hiestand BC, Goff DC, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for acute coronary syndrome? Crit Pathw Cardiol. 2011:10(3):128-133.
21. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795-802.
22. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway Randomized Trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
23. Poldervaart JM, Reitsma JB, Backus BE, et al. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med. 2017;166:687-697.
24. Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST eevation MI. JAMA. 2000;284:835-842.
25. Scirica BM, Cannon CP, Antman EM, et al. Validation of the Thrombolysis In Myocardial Infarction (TIMI) risk score for unstable angina pectoris and non-ST-elevation myocardial infarction in the TIMI III registry. Am J Cardiol. 2002;90:303-305.
26. Morrow DA, Antman EM, Snapinn SM, McCabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non-ST elevation acute coronary syndromes. Eur Heart J. 2002;23:223-229.
27. Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non–ST-elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006:13(1):13-18.
28. Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction risk score in the emergency department chest pain population. Ann Emerg Med. 2006;48(3):252-259.
29. Body R, Carley S, McDowell G, Ferguson J, Mackway-Jones K. Can a modified thrombolysis in myocardial infarction risk score outperform the original for risk stratifying emergency department patients with chest pain? Emerg Med J. 2009;26:95-99.
30. Hess EP, Perry JJ, Calder LA, et al. Prospective validation of a modified Thrombolysis In Myocardial Infarction risk score in emergency department patients with chest pain and possible acute coronary syndrome. Acad Emerg Med. 2010;17(4):368-375.
31. Macdonald SPJ, Nagree Y, Fatovich DM, ad Brown SGA. Modified TIMI risk score cannot be used to identify low-risk chest pain in the emergency department: a multicenter validation study. Emerg Med J. 2014;31:281-285.
32. Hess EP, Agarwal D, Chandra S, et al. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: a meta-analysis. CMAJ. 2010;182(10):1039-1044.
33. Than, M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. JACC. 2012;59(23):2091-2098.
34. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity Troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66(6):635-645.
35. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661.
36. Nieuwets A, Poldervaart JM, Reitsma JB, et al. Medical consumption compared for TIMI and HEART score in chest pain patients at the emergency department: a retrospective cost analysis. BMJ Open. 2016;6:e010694.
37. Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997-1005.
38. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
39. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107.
40. Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells’ criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004;44:503-510.
41. Chagnon I, Bounameaux H, Aujesky D, et al. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269-275.
42. Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172-179.
43. Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255.
44. Kline JA, Courtney DM, Kabrhel C, et al. Propsective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772-780.
45. Penaloza A, Soulie C, Moumneh T, et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): a multicenter, prospective, observational study. Lancet Haematol. 2017;4:e615-e621.
46. Freund Y, Cachanado M, Aubry A, et al. Effect of the Pulmonary Embolism Rule-Out Criteria on subsequent thromboembolic events among low-risk emergency department patients. The PROPER randomized clinical trial. JAMA. 2018;319(6):559-566.
47. Hugli O, Righini M, Le Gal G, et al. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost. 2011;9:300-4.
48. Theunissen JMG, Scholing C, van Hasselt WE, van der Maten J, ter Avest E. A retrospective analysis of the combined use of PERC rule and Wells score to exclude pulmonary embolism in the Emergency Department. Emerg Med J. 2016;33:696-701.
49. Singh B, Parsaik AK, Aharwal D, Surana A, Mascarenhas SS, Chandra S. Diagnostic accuracy of Pulmonary Embolism Rule-Out Criteria: a systematic review and meta-analysis. Ann Emerg Med. 2012;59(6):517-520.
50. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670-675.
51. Shapiro NI, Howell MD, Talmor D, Donnino M, Ngo L, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score predicts 1-year mortality. Crit Care Med. 2007;35(1):192-198.
52. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med. 2008;36(2):421-26.
53. Jones AE, Saak K, Kline JA. Performance of the Mortality in Emergency Department Sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26(6):689-692.
54. Carpenter CR., Keim SM, Upadhye S, Nguyen HB. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med. 2009;37(3):319-327.
55. Hermans MAW, Leffers P, Jansen LM, Keulemans YC, Stassen PM. The value of the Mortality in Emergency Department Sepsis (MEDS) score, C reactive protein and lactate in predicting 28-day mortality of sepsis in a Dutch emergency department. Emerg Med J. 2012;29(4):295–300.
56. Fine MJ, Auble TE, Yealy DM, et al. A Prediction Rule to Identify Low-Risk Patients with Community Acquired Pneumonia. N Engl J Med. 1997;326(4):243-250.
57. Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA. 2000;283(6):749-755. doi:10.1001/jama.283.6.749.
58. Carratalà J, Fernandez-Sabe N. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients . Ann Intern Med. 2005;142:165-172. doi:10.7326/0003-4819-142-3-200502010-00006.
59. Chalmers JD, Akram AR, Hill AT. Increasing outpatient treatment of mild community-acquired pneumonia: Systematic review and meta-analysis. Eur Respir J. 2011;37(4):858-864. doi:10.1183/09031936.00065610.
60. Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax. 2010;65(10):884-890. doi:10.1136/thx.2009.134072.
61. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit Care. 2012;16(4):R141. doi:10.1186/cc11447.
62. Neill AM, Martin IR, Weir R, et al. Community-acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010-1016. doi:10.1136/thx.51.10.1010.
63. Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58(5):377-382. doi:10.1136/thorax.58.5.377.
64. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384-392. doi:10.1016/j.amjmed.2005.01.006.
65. Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27(1):151-157. doi:10.1183/09031936.06.00062505.
66. Ilg A, Moskowitz A, Konanki V, et al. Performance of the CURB-65 score in predicting critical care interventions in patients admitted with community-acquired pneumonia. Ann Emerg Med. 2018. doi:10.1016/j.annemergmed.2018.06.017.
67. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93-101. doi:10.1111/j.1365-2796.2006.01657.x.
68. Akram AR, Chalmers JD, Hill AT. Predicting mortality with severity assessment tools in out-patients with community-acquired pneumonia. QJM. 2011;104(10):871-879. doi:10.1093/qjmed/hcr088.
69. McNally M, Curtain J, O’Brien KK, Dimitrov BD, Fahey T. Validity of British Thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: Systematic review and meta-analysis. Br J Gen Pract. 2010;60(579):423-433. doi:10.3399/bjgp10X532422.
70. Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of Pneumonia Severity Index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian Setting. Indian J Chest Dis Allied Sci. 2010;52(1):9-17.
71. Noguchi S, Yatera K, Kawanami T, et al. Pneumonia severity assessment tools for predicting mortality in patients with cealthcare-associated pneumonia: a systematic review and meta-analysis. Respiration. 2017;93(6):441-450. doi:10.1159/000470915.
72. Kolditz M, Braeken D, Ewig S, Rohde G. Severity assessment and the immediate and long-term prognosis in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37(6):886-896. doi:http://dx.doi.org/10.1055/s-0036-1592127.
1. Perry JJ, Sivilotti MLA, Sutherland J, et al. Validation of the Ottawa Subarachnoid Hemorrhage Rule in patients with acute headache. CMAJ. 2017;189(45):E1379-E1385.
2. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365-1372.
3. Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
4. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
5. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293
6. Perry JJ, Sharma M, Sivilotti ML, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ. 2011;183(10):1137-1145.
7. Stead LG, Suravaram S, Bellolio MF, et al. An assessment of the incremental value of the ABCD2 score in the emergeny department evaluation of transient ischemic attack. Ann Emerg Med. 2011;57(1):46-51.
8. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15(5):557-564.
9. Kalan M, Talbot D, Cunliffe WJ, Rich AJ. Evaluation of the modified Alvarado score in the diagnosis of acute appendicitis: a prospective study. Ann R Coll Surg Engl. 1994;76(6):418-419.
10. Ohle R, O'Reilly F, O'Brien KK, Fahey T, Dimitrov BD. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
11. Meltzer AC, Baumann BM, Chen EH, Shofer FS, Mills AM. Poor sensitivity of a modified Alvarado score in adults with suspected appendicitis. Ann Emerg Med. 2013;62(2):126-31.
12. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32(8):1843-1849.
13. de Castro SM, Ünlü C, Steller EP, van Wagensveld BA, Vrouenraets BC. Evaluation of the appendicitis inflammatory response score for patients with acute appendicitis. World J Surg. 2012;36(7):1540-1545.
14. Kollár D, McCartan DP, Bourke M, Cross KS, Dowdall J. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39(1):104-109.
15. Andersson M, Kolodziej B, Andersson RE; STRAPPSCORE Study Group. Randomized clinical trial of Appendicitis Inflammatory Response score-based management of patients with suspected appendicitis. Br J Surg. 2017;104(11):1451-1461.
16. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Netherlands Heart J. 2008;16(6):191-196.
17. Backus BE, Six AJ, Kelder JC, et al. Chest Pain in the Emergency Room. A Multicenter Validation of the HEART Score. Crit Pathways Cardiol. 2010;9:164-169.
18. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. In J Cardiol. 2013;168:2153-2158.
19. Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department. Crit Pathways Cardiol. 2013;12:121-126.
20. Mahler SA, Hiestand BC, Goff DC, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for acute coronary syndrome? Crit Pathw Cardiol. 2011:10(3):128-133.
21. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795-802.
22. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway Randomized Trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
23. Poldervaart JM, Reitsma JB, Backus BE, et al. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med. 2017;166:687-697.
24. Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST eevation MI. JAMA. 2000;284:835-842.
25. Scirica BM, Cannon CP, Antman EM, et al. Validation of the Thrombolysis In Myocardial Infarction (TIMI) risk score for unstable angina pectoris and non-ST-elevation myocardial infarction in the TIMI III registry. Am J Cardiol. 2002;90:303-305.
26. Morrow DA, Antman EM, Snapinn SM, McCabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non-ST elevation acute coronary syndromes. Eur Heart J. 2002;23:223-229.
27. Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non–ST-elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006:13(1):13-18.
28. Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction risk score in the emergency department chest pain population. Ann Emerg Med. 2006;48(3):252-259.
29. Body R, Carley S, McDowell G, Ferguson J, Mackway-Jones K. Can a modified thrombolysis in myocardial infarction risk score outperform the original for risk stratifying emergency department patients with chest pain? Emerg Med J. 2009;26:95-99.
30. Hess EP, Perry JJ, Calder LA, et al. Prospective validation of a modified Thrombolysis In Myocardial Infarction risk score in emergency department patients with chest pain and possible acute coronary syndrome. Acad Emerg Med. 2010;17(4):368-375.
31. Macdonald SPJ, Nagree Y, Fatovich DM, ad Brown SGA. Modified TIMI risk score cannot be used to identify low-risk chest pain in the emergency department: a multicenter validation study. Emerg Med J. 2014;31:281-285.
32. Hess EP, Agarwal D, Chandra S, et al. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: a meta-analysis. CMAJ. 2010;182(10):1039-1044.
33. Than, M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. JACC. 2012;59(23):2091-2098.
34. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity Troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66(6):635-645.
35. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661.
36. Nieuwets A, Poldervaart JM, Reitsma JB, et al. Medical consumption compared for TIMI and HEART score in chest pain patients at the emergency department: a retrospective cost analysis. BMJ Open. 2016;6:e010694.
37. Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997-1005.
38. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
39. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107.
40. Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells’ criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004;44:503-510.
41. Chagnon I, Bounameaux H, Aujesky D, et al. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269-275.
42. Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172-179.
43. Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255.
44. Kline JA, Courtney DM, Kabrhel C, et al. Propsective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772-780.
45. Penaloza A, Soulie C, Moumneh T, et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): a multicenter, prospective, observational study. Lancet Haematol. 2017;4:e615-e621.
46. Freund Y, Cachanado M, Aubry A, et al. Effect of the Pulmonary Embolism Rule-Out Criteria on subsequent thromboembolic events among low-risk emergency department patients. The PROPER randomized clinical trial. JAMA. 2018;319(6):559-566.
47. Hugli O, Righini M, Le Gal G, et al. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost. 2011;9:300-4.
48. Theunissen JMG, Scholing C, van Hasselt WE, van der Maten J, ter Avest E. A retrospective analysis of the combined use of PERC rule and Wells score to exclude pulmonary embolism in the Emergency Department. Emerg Med J. 2016;33:696-701.
49. Singh B, Parsaik AK, Aharwal D, Surana A, Mascarenhas SS, Chandra S. Diagnostic accuracy of Pulmonary Embolism Rule-Out Criteria: a systematic review and meta-analysis. Ann Emerg Med. 2012;59(6):517-520.
50. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670-675.
51. Shapiro NI, Howell MD, Talmor D, Donnino M, Ngo L, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score predicts 1-year mortality. Crit Care Med. 2007;35(1):192-198.
52. Sankoff JD, Goyal M, Gaieski DF, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS). Crit Care Med. 2008;36(2):421-26.
53. Jones AE, Saak K, Kline JA. Performance of the Mortality in Emergency Department Sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26(6):689-692.
54. Carpenter CR., Keim SM, Upadhye S, Nguyen HB. Risk stratification of the potentially septic patient in the emergency department: the Mortality in the Emergency Department Sepsis (MEDS) score. J Emerg Med. 2009;37(3):319-327.
55. Hermans MAW, Leffers P, Jansen LM, Keulemans YC, Stassen PM. The value of the Mortality in Emergency Department Sepsis (MEDS) score, C reactive protein and lactate in predicting 28-day mortality of sepsis in a Dutch emergency department. Emerg Med J. 2012;29(4):295–300.
56. Fine MJ, Auble TE, Yealy DM, et al. A Prediction Rule to Identify Low-Risk Patients with Community Acquired Pneumonia. N Engl J Med. 1997;326(4):243-250.
57. Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. JAMA. 2000;283(6):749-755. doi:10.1001/jama.283.6.749.
58. Carratalà J, Fernandez-Sabe N. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients . Ann Intern Med. 2005;142:165-172. doi:10.7326/0003-4819-142-3-200502010-00006.
59. Chalmers JD, Akram AR, Hill AT. Increasing outpatient treatment of mild community-acquired pneumonia: Systematic review and meta-analysis. Eur Respir J. 2011;37(4):858-864. doi:10.1183/09031936.00065610.
60. Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax. 2010;65(10):884-890. doi:10.1136/thx.2009.134072.
61. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit Care. 2012;16(4):R141. doi:10.1186/cc11447.
62. Neill AM, Martin IR, Weir R, et al. Community-acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010-1016. doi:10.1136/thx.51.10.1010.
63. Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax. 2003;58(5):377-382. doi:10.1136/thorax.58.5.377.
64. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384-392. doi:10.1016/j.amjmed.2005.01.006.
65. Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27(1):151-157. doi:10.1183/09031936.06.00062505.
66. Ilg A, Moskowitz A, Konanki V, et al. Performance of the CURB-65 score in predicting critical care interventions in patients admitted with community-acquired pneumonia. Ann Emerg Med. 2018. doi:10.1016/j.annemergmed.2018.06.017.
67. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93-101. doi:10.1111/j.1365-2796.2006.01657.x.
68. Akram AR, Chalmers JD, Hill AT. Predicting mortality with severity assessment tools in out-patients with community-acquired pneumonia. QJM. 2011;104(10):871-879. doi:10.1093/qjmed/hcr088.
69. McNally M, Curtain J, O’Brien KK, Dimitrov BD, Fahey T. Validity of British Thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: Systematic review and meta-analysis. Br J Gen Pract. 2010;60(579):423-433. doi:10.3399/bjgp10X532422.
70. Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of Pneumonia Severity Index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian Setting. Indian J Chest Dis Allied Sci. 2010;52(1):9-17.
71. Noguchi S, Yatera K, Kawanami T, et al. Pneumonia severity assessment tools for predicting mortality in patients with cealthcare-associated pneumonia: a systematic review and meta-analysis. Respiration. 2017;93(6):441-450. doi:10.1159/000470915.
72. Kolditz M, Braeken D, Ewig S, Rohde G. Severity assessment and the immediate and long-term prognosis in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37(6):886-896. doi:http://dx.doi.org/10.1055/s-0036-1592127.
Deadly Marburg virus found in West Africa
Marburg virus has been found in fruit bats in Sierra Leone, marking the first appearance of the deadly, Ebola-like virus in West Africa, the Centers for Disease Control and Prevention (CDC) is reporting.
Five Egyptian rousette fruit bats found in three different districts tested positive for infection with Marburg virus, a cousin to Ebola that can cause a hemorrhagic fever with case fatality rates up to 90%, according to CDC.
While no confirmed cases of Marburg infection have been reported in Sierra Leone, the presence of virus in these bats indicates that people nearby may be at risk, according to scientists.
“We have known for a long time that rousette bats, which carry Marburg virus in other parts of Africa, also live in West Africa, so it’s not surprising that we’d find the virus in bats there,” CDC ecologist Jonathan S. Towner, PhD, said in a news release.
The Egyptian rousette bat (Rousettus aegyptiacus) is the natural reservoir for Marburg, shedding the virus in saliva, urine, and feces while feeding on fruit. People and are exposed to the virus when they eat contaminated fruit or capture bats for food, according to the CDC.
The most recent Marburg virus outbreak, which occurred in Uganda in 2017, was the 12th reported outbreak linked to Africa, according to the agency. The largest and deadliest outbreak occurred in 2005 in Angola, infecting 252 people, of whom 90% died.
Testing of the Marburg-positive bats revealed genetically diverse strains, suggesting the virus has been present in Sierra Leone bat colonies for many years, the agency said. Two of the four Marburg virus strains identified in the Sierra Leone bats were genetically similar to the strain implicated in the Angola outbreak.
Egyptian fruit bats are in fact common throughout Africa, living in caves or underground mines. Marburg-positive bats have been found in sub-Saharan Africa, according to researchers, mainly in Uganda and the Democratic Republic of Congo.
Colonies of Egyptian fruit bats can number more than 100,000 animals in eastern and central Africa, while in Sierra Leone, colonies are much smaller, which may explain the lack of Marburg virus disease outbreaks in that country, CDC said.
Discovery of Marburg virus in Sierra Leone was the result of two projects, one led by the CDC and Njala University in Freetown, Sierra Leone, and the other by the University of California, Davis, and the University of Makeni, Sierra Leone, which was funded by the United States Agency for International Development (USAID).
“This discovery is an excellent example of how our work can identify a threat and help us warn people of the risk before they get sick.” Dr. Towner said in the news release.
The two projects began in 2016 after the large Ebola outbreak in West Africa with the aim of identifying the reservoir of Ebola, according to CDC.
SOURCES: U.S. Department of Health and Human Services CDC Newsroom and Centers for Disease Control and Prevention (Marburg Virus).
Marburg virus has been found in fruit bats in Sierra Leone, marking the first appearance of the deadly, Ebola-like virus in West Africa, the Centers for Disease Control and Prevention (CDC) is reporting.
Five Egyptian rousette fruit bats found in three different districts tested positive for infection with Marburg virus, a cousin to Ebola that can cause a hemorrhagic fever with case fatality rates up to 90%, according to CDC.
While no confirmed cases of Marburg infection have been reported in Sierra Leone, the presence of virus in these bats indicates that people nearby may be at risk, according to scientists.
“We have known for a long time that rousette bats, which carry Marburg virus in other parts of Africa, also live in West Africa, so it’s not surprising that we’d find the virus in bats there,” CDC ecologist Jonathan S. Towner, PhD, said in a news release.
The Egyptian rousette bat (Rousettus aegyptiacus) is the natural reservoir for Marburg, shedding the virus in saliva, urine, and feces while feeding on fruit. People and are exposed to the virus when they eat contaminated fruit or capture bats for food, according to the CDC.
The most recent Marburg virus outbreak, which occurred in Uganda in 2017, was the 12th reported outbreak linked to Africa, according to the agency. The largest and deadliest outbreak occurred in 2005 in Angola, infecting 252 people, of whom 90% died.
Testing of the Marburg-positive bats revealed genetically diverse strains, suggesting the virus has been present in Sierra Leone bat colonies for many years, the agency said. Two of the four Marburg virus strains identified in the Sierra Leone bats were genetically similar to the strain implicated in the Angola outbreak.
Egyptian fruit bats are in fact common throughout Africa, living in caves or underground mines. Marburg-positive bats have been found in sub-Saharan Africa, according to researchers, mainly in Uganda and the Democratic Republic of Congo.
Colonies of Egyptian fruit bats can number more than 100,000 animals in eastern and central Africa, while in Sierra Leone, colonies are much smaller, which may explain the lack of Marburg virus disease outbreaks in that country, CDC said.
Discovery of Marburg virus in Sierra Leone was the result of two projects, one led by the CDC and Njala University in Freetown, Sierra Leone, and the other by the University of California, Davis, and the University of Makeni, Sierra Leone, which was funded by the United States Agency for International Development (USAID).
“This discovery is an excellent example of how our work can identify a threat and help us warn people of the risk before they get sick.” Dr. Towner said in the news release.
The two projects began in 2016 after the large Ebola outbreak in West Africa with the aim of identifying the reservoir of Ebola, according to CDC.
SOURCES: U.S. Department of Health and Human Services CDC Newsroom and Centers for Disease Control and Prevention (Marburg Virus).
Marburg virus has been found in fruit bats in Sierra Leone, marking the first appearance of the deadly, Ebola-like virus in West Africa, the Centers for Disease Control and Prevention (CDC) is reporting.
Five Egyptian rousette fruit bats found in three different districts tested positive for infection with Marburg virus, a cousin to Ebola that can cause a hemorrhagic fever with case fatality rates up to 90%, according to CDC.
While no confirmed cases of Marburg infection have been reported in Sierra Leone, the presence of virus in these bats indicates that people nearby may be at risk, according to scientists.
“We have known for a long time that rousette bats, which carry Marburg virus in other parts of Africa, also live in West Africa, so it’s not surprising that we’d find the virus in bats there,” CDC ecologist Jonathan S. Towner, PhD, said in a news release.
The Egyptian rousette bat (Rousettus aegyptiacus) is the natural reservoir for Marburg, shedding the virus in saliva, urine, and feces while feeding on fruit. People and are exposed to the virus when they eat contaminated fruit or capture bats for food, according to the CDC.
The most recent Marburg virus outbreak, which occurred in Uganda in 2017, was the 12th reported outbreak linked to Africa, according to the agency. The largest and deadliest outbreak occurred in 2005 in Angola, infecting 252 people, of whom 90% died.
Testing of the Marburg-positive bats revealed genetically diverse strains, suggesting the virus has been present in Sierra Leone bat colonies for many years, the agency said. Two of the four Marburg virus strains identified in the Sierra Leone bats were genetically similar to the strain implicated in the Angola outbreak.
Egyptian fruit bats are in fact common throughout Africa, living in caves or underground mines. Marburg-positive bats have been found in sub-Saharan Africa, according to researchers, mainly in Uganda and the Democratic Republic of Congo.
Colonies of Egyptian fruit bats can number more than 100,000 animals in eastern and central Africa, while in Sierra Leone, colonies are much smaller, which may explain the lack of Marburg virus disease outbreaks in that country, CDC said.
Discovery of Marburg virus in Sierra Leone was the result of two projects, one led by the CDC and Njala University in Freetown, Sierra Leone, and the other by the University of California, Davis, and the University of Makeni, Sierra Leone, which was funded by the United States Agency for International Development (USAID).
“This discovery is an excellent example of how our work can identify a threat and help us warn people of the risk before they get sick.” Dr. Towner said in the news release.
The two projects began in 2016 after the large Ebola outbreak in West Africa with the aim of identifying the reservoir of Ebola, according to CDC.
SOURCES: U.S. Department of Health and Human Services CDC Newsroom and Centers for Disease Control and Prevention (Marburg Virus).
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Guideline-concordant treatment still unlikely in nonchildren’s hospitals for pediatric CAP
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
according to new research.
“This gap is concerning because approximately 70% of children hospitalized with pneumonia receive care in nonchildren’s hospitals,” wrote Alison C. Tribble, MD, of C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor, and her associates. The report is in JAMA Pediatrics.
Data were collected from the Pediatric Health Information System (children’s hospitals) and Premier Perspectives (all hospitals) databases and included a total of 120,238 children aged 1-17 years diagnosed with CAP between Jan. 1, 2009, and Sept. 30, 2015. Before the publication of the new guideline in October 2011, the probability of receiving what would become guideline-concordant antibiotics was 0.25 in children’s hospitals and 0.06 in nonchildren’s hospitals.
By the end of the study period, the probability of receiving guideline-concordant antibiotics for pediatric CAP was 0.61 in children’s hospitals and 0.27 in nonchildren’s hospitals. Without the interventions, the probabilities would have been 0.31 and 0.08, respectively. The rate of growth over the 4-year postintervention period was similar in both children’s and nonchildren’s hospitals.
“Studies in children’s hospitals have suggested that local implementation efforts may be important in facilitating guideline uptake. Nonchildren’s hospitals likely have fewer resources to lead pediatric-specific efforts, and care may be influenced by adult CAP guidelines,” the authors noted.
No conflicts of interest were reported.
SOURCE: Tribble AC et al. JAMA Pediatr. 2018 Dec 10. doi: 10.1001/jamapediatrics.2018.4270.
FROM JAMA PEDIATRICS
Pregnant women commonly refuse the influenza vaccine
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Although almost all ob.gyns. recommend the influenza and Tdap vaccines for pregnant women, both commonly are refused.
Major finding: A total of 62% of ob.gyns. reported that 10% or greater of their pregnant patients refused the influenza vaccine; 32% reported this for Tdap vaccine.
Study details: An email and mail survey sent to a national network of ob.gyns. between March and June 2016.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
Source: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Risk-based testing missed 35% of HCV-positive prison inmates
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
Routine testing for hepatitis C virus at inmate entry should be considered by U.S. state prisons, according to Sabrina A. Assoumou, MD, of the Boston Medical Center and her colleagues.
The researchers performed a retrospective analysis of individuals entering the Washington state prison system, which routinely offers hepatitis C virus (HCV) testing, in order to compare routine opt-out testing with current risk-based and one-time testing for individuals born between 1945 and 1965. Additionally, liver fibrosis stage was characterized in blood samples from HCV-positive individuals, the investigators wrote in the American Journal of Preventative Medicine.
Between 2012 and 2016, 24,567 (83%) individuals were tested for HCV antibody, and of these, 4,921 (20%) tested positive. A total of 2,403 (49%) of those testing positive had subsequent hepatitis HCV RNA testing, with 1,727 (72%) of these showing chronic infection.
As expected, Dr. Assoumou and her colleagues found that reactive antibodies was more prevalent in individuals born between 1945 and 1965, compared with other years (44% vs. 17%). However, in actual case numbers, most (72%) were outside of this age bracket. Overall, they calculated that up to 35% of positive HCV tests would be missed using testing targeted by birth cohort and risk behavior alone. Among the chronically infected individuals, 23% had showed at least moderate liver fibrosis.
“Routine opt-out testing identified a substantial number of HCV cases that would have been missed by targeted testing. Almost one-quarter of individuals with chronic HCV had significant liver fibrosis and thus a more urgent need for treatment to prevent complications,” Dr Assoumou and her colleagues concluded.
The researchers reported that they had no conflicts of interest.
SOURCE: Assoumou SA et al, Am J Prev Med. 2019;56:8-16.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Young opioid users in NYC savvy about HCV
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
Assessing patient knowledge about hepatitis C virus (HCV) transmission knowledge is difficult given the lack of psychometrically tested measures available, according to researchers who developed and validated an HCV injection–risk knowledge scale.
The 5-item, validated HCV Injection-Risk Knowledge Scale may provide educators, care providers, and researchers with critical information for reducing HCV among people who inject drugs (PWID), according to their report.
The researchers analyzed data from 539 New York City opioid users aged 18-29 years who were recruited via respondent-driven sampling in 2014-2016, according to the study published in Drug and Alcohol Dependence.
Principal components analysis (PCA) of nine knowledge items answered true, false, or don’t know identified useful scale items. These were then evaluated for internal consistency and assessed by comparing knowledge levels with those from a previously validated general HCV knowledge scale and by comparing key subgroup knowledge levels.
PCA identified one component with five items that explained 45% of the total variance and had high internal consistency (alpha, 0.91). All of the component items referred to transmission through drug-injection equipment and practices: sharing materials such as cookers, cottons, diluting water, water containers, and cleaning syringes with water.
The mean percent correct was 75%, and was moderately correlated with general HCV knowledge. Knowledge levels were highest for those previously tested for HCV, those with HCV antibody–positive status, PWID, and those who had received harm reduction information in various settings.
“The low percentage correct among those who had never injected [52% vs. 83% correct among injectors] is concerning given the possibility of their experimenting with drug injection as route of administration,” they added.
“These results suggest that primary care providers, drug treatment programs, and syringe exchange programs are important sources of HCV risk knowledge among at-risk populations, similar to other studies’ findings,” the researchers concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest.
SOURCE: Quinn K et al. Drug Alcohol Depend. 2019;194:453-9.
FROM DRUG AND ALCOHOL DEPENDENCE
Avoiding the Pitfalls of “Half-visits”
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.
A 3-and-a-half-year-old girl presented to a pediatrician’s office with complaints of vomiting and high fever (103.3°). She was seen by a nurse practitioner, who diagnosed gastroenteritis, prescribed fluid replacement and acetaminophen, and sent the child home.
The NP did not chart the child’s blood pressure, pulse, or respiratory rate. She did note swollen lymph nodes and absence of diarrhea. The NP performed a flu screen but did not order a rapid strep test or urinalysis.
Several hours later, the child was taken to the emergency department with shortness of breath, cough, congestion, tachycardia, hypoxia, dehydration, and lethargy. She was admitted to the pediatric ICU with diagnoses of pneumonia, acute respiratory distress, hypoxemia, neutropenia, and sepsis. She was given IV antibiotics.
Several hours later, the decision was made to transfer the patient to a regional medical center. During transfer, she suffered cardiopulmonary arrest while being placed on a ventilator for transport. Upon arrival at the hospital, she arrested again and required resuscitation for several hours until spontaneous circulation could not be restored.
An autopsy concluded the child died of sepsis and shock from Group A beta-hemolytic streptococcal infection.
It was argued that the NP failed to diagnose and treat streptococcal toxic shock syndrome at the time of the child’s presentation. In support of this contention, it was argued that the NP had failed to perform basic follow-up when the child’s flu test came back negative and that the child’s swollen lymph nodes and lack of diarrhea both mitigated against the NP’s diagnosis of gastroenteritis.
VERDICT
The parties in this case reached a $950,000 settlement.
Continue to: DISCUSSION
DISCUSSION
Every headache and fever could be an early meningitis, every vague abdominal pain an early appendicitis. So how do we handle innocuous-appearing cases with early, nonspecific symptoms of a very serious illness about to unfold?
We must start by following the Miyagi rule. In The Karate Kid, Mr. Miyagi advised that walking on the left or the right side of the road was safe, but walking in the middle would result, sooner or later, in “squish, just like grape.” Although he related this premise to karate, we can also apply it to medicine: See a patient or do not see a patient; but if you see a patient “so-so,” you will be squished—by the patient, by a plaintiff’s attorney, and/or by your state’s medical board.
A case such as this one strikes fear in the heart of anyone who has seen patients in an ambulatory setting. The initial presentation was modest: a toddler with vomiting and fever. We do not know what the other vital signs were, and we do not know whether the child appeared toxic. The lack of vital signs or recorded vital signs represent half-measures. The patient’s vitals could have been normal, and the NP’s actions could have been fully defensible. The problem is, we don’t know—and the clinician is on the hook.
All patients require vital signs. They must be done; they must be complete, and they must be recorded. At a minimum, temperature, blood pressure, respiratory rate, and (generally) O2 saturation are required. Some specialties may have other requirements (eg, fingerstick glucose for patients with diabetes, visual acuity testing for those with eye complaints). A full list of data you should be obtaining is practice specific and beyond the scope of this article; the point is, decide on the relevant set of vitals and intake data and be sure it is recorded at every visit.
Failure to obtain and record vital signs—as seen in this case—is sloppy practice, difficult to defend, and sets up an inference of negligence. Even when the care is perfect (and without bad outcome), if the medical board reviews the record for any reason, you will be sanctioned for “failure to keep adequate and accurate medical records” and your license burdened. Here, we are told the defendant NP “did not chart the child’s blood pressure, pulse, or respiratory rate.” I am willing to bet the NP was not responsible for charting the values in the normal course of practice, but see how responsibility is parked with the clinician? If intake staff do not record vital signs, politely (yet firmly) insist they do so.
Continue to: Furthermore, the disposition of many child visits...
Furthermore, the disposition of many child visits turns on whether the patient “appeared toxic.” Any child’s condition could worsen after evaluation—and in litigation, parents, friends, and family will testify the patient was extremely ill, they “knew something was wrong,” and the clinician ignored their loved one. Thus, the jury will be invited to reconstruct how the child appeared.
When assessing children and the question of “toxic appearance” arises, don’t state a conclusion—paint a picture. Don’t merely state “child appeared nontoxic.” Use your powers of observation to record why they appear nontoxic: “Child sitting up, watching Moana on parent’s phone, smiling and laughing appropriately.” Get interactive; some pediatric providers carry a small vial of bubbles with them and record the child’s response to bubble-making (“Child appropriately reaching for bubbles, smiling, holding one on finger”). The cost is less than $1 for the bubbles, plus the documentation time. The benefit is that it paints a clear picture for the jury of a child responding appropriately. And if your observations suggest a child who is at least unwell—if the movie is poorly received or the bubbles prompt the child to scream or bury her face in her mom’s shoulder—you can consider oral antipyretics/analgesics, fluid, and re-observation.
Another way to create a strong and defensible record is to use patient quotations. These can be extremely helpful to your defense in a malpractice action; as an attorney, I have searched 8,000 pages of records in a medical malpractice case, hoping to find a clear description from a human (not a template) of how a patient looked. Make it clear by adding patient remarks to the chart—just remember that “the only thing that belongs in quotes is what comes out of the patient’s mouth.” Words from an 8-year-old boy— such as “My brother found a legendary scar [a reference to Fortnite] and almost won”—may seem silly, but this documentation itself could win your case.
With teenagers, you may have to ask more questions to glean something suitable; you could ask a 13-year-old her favorite sport and when her one-word answer is “Lacrosse,” ask why. Even if the response is “Because, I don’t know, it’s exciting. There are a lot of goals,” write that down exactly (along with any other observations, such as Teen texting on her phone). These notations tell the plaintiff’s attorney, the judge, and the jury that the patient was behaving normally and interacting with the environment. Should this teen later deteriorate with meningitis, the plaintiff will claim she was toxic in the office. The medical record, however, will show that the patient’s condition changed, and it was a departure from how she looked in your office.
Also, it never hurts to get backup. In any close call, ask the nurse to reevaluate the patient as to whether he or she is “toxic appearing” or is interacting normally with the environment. Have the nurse or medical assistant record facts, such as “patient trying to make a plane out of two tongue depressors, pretending to land it on sister’s leg.” This will create a strong and defensible record: two clinicians relaying two sets of detailed observations.
Continue to: Likewise, encourage intake staff to document...
Likewise, encourage intake staff to document what they see rather than what they conclude from it. Buzzwords (eg, listless, lethargic) should be avoided. If such characterizations find their way into the record, you must take active steps to address them. Either agree with the characterization and perform appropriate work-up, or establish why you do not agree using the methods described (detailed description, verification by another clinician).
Taking these steps will help to protect you in the event of a changing clinical course. But also be wary of those predictable circumstances that lead you into Mr. Miyagi’s middle of the road (what I call “half-visits”): a quick look at a sibling in the room during a patient’s appointment; a “curbside consult” on the medical assistant’s child; the neighborhood acquaintance who asks you to “just take a look.” Why are these dangerous? Because they remove the clinician from his or her usual routine: proper examination on a properly undressed patient, formal assessment of vital signs, and review of relevant history in the chart, among other things. (In this way, phone and email communications with patients require similar caution.) Skipping the routine leads to shortcuts, and shortcuts lead to bad medicine. And if that doesn’t worry you, remember: All these scenarios create a full legal duty and clinician/patient relationship—making them potential pathways to misdiagnosis and eventual loss of license.
IN SUMMARY
Don’t be party to a “half-visit”; insist on full vital signs and a complete visit following your usual routine. Use observational powers and patient quotations to paint a picture of how a patient looked, get backup from another clinician with similar observations. If you can’t document a reassuring record, protect the patient and make the required intervention.