User login
Two new Novel Coronavirus cases confirmed among quarantined U.S. patients
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
The Centers for Disease Control and Prevention announced two new patients now have the 2019 Novel Coronavirus (2019-nCoV), bringing the case total in the United States to 15.

The 14th case was discovered in California among a group of people under federal quarantine after returning from the Hubei Province in China. That patient was on a U.S. State Department–chartered flight that arrived in the United States on Feb. 7.
The 15th case was discovered in Texas among a group of people who also are under federal quarantine. That patient arrived on a State Department–chartered flight that arrived on Feb. 7. It is the first person in Texas that has tested positive for 2019-nCoV.
CDC said in a statement announcing the Texas case that there “will likely be additional cases in the coming days and weeks, including among other people recently returned from Wuhan.” Officials noted that more than 600 people who have returned as part of State Department–chartered flights are currently under that 14-day quarantine.
The agency is preparing for more widespread cases of 2019-nCoV.
Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, said that containment has been the early focus for the agency.
“The goal of the measures we have taken to date are to slow the introduction and impact of this disease in the United States, but at some point, we are likely to see community spread in the U.S.,” Dr. Messonnier said during a Feb. 12 teleconference with reporters. She added that the federal response will change over time as the virus spreads.
Dr. Messonnier noted that public health officials are planning for the increased demands that a wider outbreak of 2019-nCov would place on the health care delivery system, including ensuring an adequate supply of medical equipment.
Pathways to new therapeutic agents for human coronaviruses
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
FROM DRUG DISCOVERY TODAY
2019-nCoV outbreak: A few lessons learned for pediatric practices
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
CDC confirms 13th case of coronavirus in U.S.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
The Centers for Disease Control and Prevention announced the number of confirmed cases of the 2019 Novel Coronavirus (2019-nCoV) in the United States has reached 13.
The latest case, announced Feb. 11, 2020, by the CDC, was in a person in California who was previously under federal quarantine because the patient had traveled to Wuhan, China.
The CDC is currently looking into who the patient may have come in contact with to understand the potential for further spread of the coronavirus.
“The contact investigation is ongoing,” CDC principal deputy director Anne Schuchat, MD, said during a Feb. 11 press conference to provide an update on coronavirus containment activities being taken by the CDC.
Dr. Schuchat also addressed issues related to the laboratory test, as the patient in California was initially thought to be negative for the coronavirus.
“With other cases around the country that we are evaluating, we have been doing serial tests to understand whether they are still infectious” and to gather other information about how results change over time, Dr. Schuchat said.
She noted that the CDC does not “have as much information as we would like on the severity of the virus,” noting that there are many cases in China with severe reactions, while the 13 cases in the United States represent a much more mild reaction to the virus so far.
With the latest case in California, she noted that there was “probably a mix-up and the original test wasn’t negative,” although she did not elaborate on what the nature of the mix-up was, stating that was all the information that she had.
In general, Dr. Schuchat touted the actions taken by the CDC and the federal government focused primarily on containing the spread of the virus in the United States, including the implementation of travel advisories, quarantining passengers returning from China, as well as the new test kits that are being distributed by the agency across the nation and around the world. She also mentioned CDC staff are being deployed around the world to monitor the spreading of the disease and highlighted the outreach efforts to keep the public informed.
Dr. Schuchat highlighted the fact that, of the 13 cases in the United States, 11 were with patients that were in Wuhan, and only 2 were because of close contact with a patient, something that she attributed to the actions being taken.
She also noted that cases in the United States have not been as severe as they have been in China, where deaths have been attributed to the coronavirus outbreak. She added that there have been only two deaths outside of mainland China attributed to the coronavirus.
“Some of the steps the CDC has taken have really put us in better shape should widespread transmission occur in the United States,” she said.
Dr. Schuchat also highlighted that the first charter flight of people quarantined after returning from Wuhan have reached the 14-day milestone and should be on their way home beginning today.
Consider PET/CT when infectious source is a puzzler
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
REPORTING FROM RSNA 2019
Coronavirus outbreak: Putting it into perspective
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
Flu activity increases for third straight week
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.
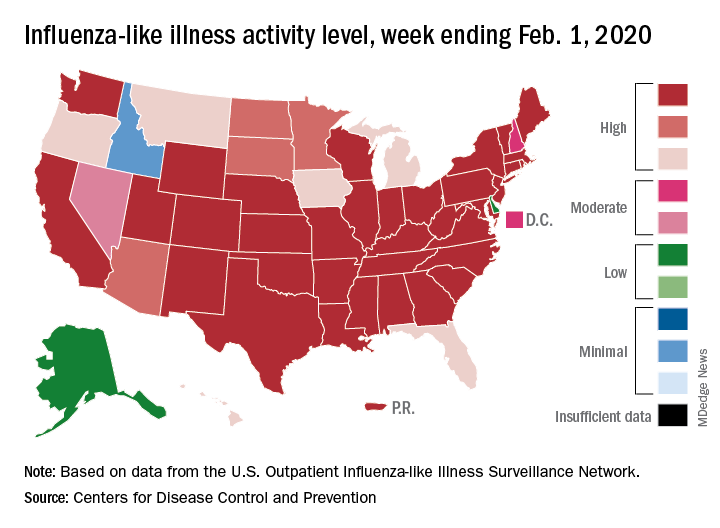
The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.

The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
For the second time during the 2019-2020 flu season, activity measures have climbed into noteworthy territory.

The proportion of outpatient visits for influenza-like illness (ILI) reached its highest December level, 7.1%, since 2003 and then dropped for 2 weeks. Three weeks of increases since then, however, have the outpatient-visit rate at 6.7% for the week ending Feb. 1, 2020, the Centers for Disease Control and Prevention reported. The baseline rate for the United States is 2.4%.
That rate of 6.7% is already above the highest rates recorded in eight of the last nine flu seasons, and another increase could mean a second, separate trip above 7.0% in the 2019-2020 season – something that has not occurred since national tracking began in 1997, CDC data show.
Those same data also show that,
Another important measure on the rise, the proportion of respiratory specimens testing positive for influenza, reached a new high for the season, 29.8%, during the week of Feb. 1, the CDC’s influenza division said.
Tests at clinical laboratories also show that predominance is continuing to switch from type B (45.6%) to type A (54.4%), the influenza division noted. Overall predominance for the season, however, continues to favor type B, 59.3% to 40.7%.
The percentage of deaths caused by pneumonia and influenza, which passed the threshold for epidemic of 7.2% back in early January, has been trending downward for the last 3 weeks and was 7.1% as of Feb. 1, according to the influenza division.
ILI-related deaths among children continue to remain high, with a total count of 78 for the season after another 10 deaths were reported during the week ending Feb. 1, the CDC reported. Comparable numbers for the last three seasons are 44 (2018-2019), 97 (2017-2018), and 35 (2016-2017).
The CDC estimates put the total number of ILIs at around 22 million for the season so far, leading to 210,000 hospitalizations. The agency said that it expects to release estimates of vaccine effectiveness later this month.
Remdesivir under study as treatment for novel coronavirus

“What they’re looking at is the effect of this drug -- either the drug plus standard of care versus standard of care alone,” Anthony S. Fauci, MD, reported Feb. 7 during a press briefing held by members of President Trump’s Coronavirus Task Force. “I think pretty soon we are going to get a definitive answer, whether one of these among several drugs works.”
Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases, added that several organizations and individual investigators are developing vaccines for 2019-nCoV. In one such effort, the National Institutes of Health is working with Moderna Inc. to develop a vaccine built on a messenger RNA platform. “One of the first steps is to successfully get that [novel coronavirus] gene and insert it into the messenger RNA platform successfully and allow it to express proteins,” Dr. Fauci explained. “We’ve succeeded in that. The next [step] is to put it in a mouse animal model to induce immunogenicity, and to get the company to make [gold nanoparticle] products. All of those have been successfully implemented. There have been no glitches so far. If that continues, we will be in Phase 1 trials in people within the next two-and-a-half months.”
In another development on the same day, Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, announced that Heath & Human Services issued an interim final rule to amend foreign quarantine regulations in the wake of the public health threat posed by the 2019-nCoV. “This will enable CDC to collect certain contact information data regarding airline passengers and crew when they arrive from other countries. . .and may be exposed to communicable disease,” Dr. Redfield said. “This action is part of our multi-layered approach to the U.S. response and demonstrates our commitment to take all necessary actions to protect the American people.”
According to Alex Azar, secretary of Health and Human Services, and chair of President Trump’s Coronavirus Task Force, there are 12 confirmed cases of the novel coronavirus in the United States, including two cases of transmission to people who had not recently been in China. “Although the virus represents a potentially very serious public health threat, and we expect to continue seeing more cases here, the immediate risk to the American public is low at this time,” Mr. Azar said. “We are working as quickly as possible on the many unanswered questions about this virus. That includes exactly how it spreads, how deadly it is, whether it’s commonly transmitted by patients who are not yet displaying symptoms, and other issues.”

“What they’re looking at is the effect of this drug -- either the drug plus standard of care versus standard of care alone,” Anthony S. Fauci, MD, reported Feb. 7 during a press briefing held by members of President Trump’s Coronavirus Task Force. “I think pretty soon we are going to get a definitive answer, whether one of these among several drugs works.”
Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases, added that several organizations and individual investigators are developing vaccines for 2019-nCoV. In one such effort, the National Institutes of Health is working with Moderna Inc. to develop a vaccine built on a messenger RNA platform. “One of the first steps is to successfully get that [novel coronavirus] gene and insert it into the messenger RNA platform successfully and allow it to express proteins,” Dr. Fauci explained. “We’ve succeeded in that. The next [step] is to put it in a mouse animal model to induce immunogenicity, and to get the company to make [gold nanoparticle] products. All of those have been successfully implemented. There have been no glitches so far. If that continues, we will be in Phase 1 trials in people within the next two-and-a-half months.”
In another development on the same day, Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, announced that Heath & Human Services issued an interim final rule to amend foreign quarantine regulations in the wake of the public health threat posed by the 2019-nCoV. “This will enable CDC to collect certain contact information data regarding airline passengers and crew when they arrive from other countries. . .and may be exposed to communicable disease,” Dr. Redfield said. “This action is part of our multi-layered approach to the U.S. response and demonstrates our commitment to take all necessary actions to protect the American people.”
According to Alex Azar, secretary of Health and Human Services, and chair of President Trump’s Coronavirus Task Force, there are 12 confirmed cases of the novel coronavirus in the United States, including two cases of transmission to people who had not recently been in China. “Although the virus represents a potentially very serious public health threat, and we expect to continue seeing more cases here, the immediate risk to the American public is low at this time,” Mr. Azar said. “We are working as quickly as possible on the many unanswered questions about this virus. That includes exactly how it spreads, how deadly it is, whether it’s commonly transmitted by patients who are not yet displaying symptoms, and other issues.”

“What they’re looking at is the effect of this drug -- either the drug plus standard of care versus standard of care alone,” Anthony S. Fauci, MD, reported Feb. 7 during a press briefing held by members of President Trump’s Coronavirus Task Force. “I think pretty soon we are going to get a definitive answer, whether one of these among several drugs works.”
Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases, added that several organizations and individual investigators are developing vaccines for 2019-nCoV. In one such effort, the National Institutes of Health is working with Moderna Inc. to develop a vaccine built on a messenger RNA platform. “One of the first steps is to successfully get that [novel coronavirus] gene and insert it into the messenger RNA platform successfully and allow it to express proteins,” Dr. Fauci explained. “We’ve succeeded in that. The next [step] is to put it in a mouse animal model to induce immunogenicity, and to get the company to make [gold nanoparticle] products. All of those have been successfully implemented. There have been no glitches so far. If that continues, we will be in Phase 1 trials in people within the next two-and-a-half months.”
In another development on the same day, Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, announced that Heath & Human Services issued an interim final rule to amend foreign quarantine regulations in the wake of the public health threat posed by the 2019-nCoV. “This will enable CDC to collect certain contact information data regarding airline passengers and crew when they arrive from other countries. . .and may be exposed to communicable disease,” Dr. Redfield said. “This action is part of our multi-layered approach to the U.S. response and demonstrates our commitment to take all necessary actions to protect the American people.”
According to Alex Azar, secretary of Health and Human Services, and chair of President Trump’s Coronavirus Task Force, there are 12 confirmed cases of the novel coronavirus in the United States, including two cases of transmission to people who had not recently been in China. “Although the virus represents a potentially very serious public health threat, and we expect to continue seeing more cases here, the immediate risk to the American public is low at this time,” Mr. Azar said. “We are working as quickly as possible on the many unanswered questions about this virus. That includes exactly how it spreads, how deadly it is, whether it’s commonly transmitted by patients who are not yet displaying symptoms, and other issues.”
Fewer complications, better outcomes with outpatient UKA
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
FROM ORTHOPEDIC CLINICS OF NORTH AMERICA
‘Antibacterial’ soap labels still list banned ingredients
The website of retail pharmacy giant Walgreens, for example, lists Dial Complete antibacterial soap with the active ingredient triclosan, a chemical the Food and Drug Administration banned along with others in 2017. The agency cited a lack of evidence that the ingredients were more effective than plain soap and water and that they were safe for long-term daily use.
A Dial Complete soap product page on Walgreens’ website lists, as of Feb. 4, 2020, an ingredient that was banned by the FDA.
Yet banned substances such as the triclosan in this Dial soap still commonly appear on online product descriptions, researchers found after searching the National Drug Code Directory and the websites of major online retailers, including Amazon, Walmart, and Target. The health effects of antibacterial ingredients “are very poorly defined,” said Chandler Rundle, MD, first author of the study, which was published in Dermatitis. Dr. Rundle is with the department of dermatology at the University of Colorado at Denver, Aurora.
The label on the back of the Dial soap bottle sold on Walgreens.com states that it “[k]ills more bacteria than ordinary liquid hand soap.” The website displays a close-up graphic of a hand that has been washed with Dial soap and that has fewer bacteria than a hand washed with “Others.” The graphic includes a dramatization disclaimer.
When asked about the product, a Walgreens corporate relations spokesperson checked the soap’s ingredients list they had on file from Dial’s parent company, Henkel North American Consumer Goods.
“I did not see that particular ingredient,” the representative said. Their ingredients list reflected a version of the soap that was updated after the ban. That label differs from Walgreens.com’s product information. The updated, ban-compliant version of the soap contains an alternative antibacterial compound, benzalkonium chloride. The spokesperson wasn’t sure of the source of the incorrect information on the website. Dial did not respond to a request for comment.
The ingredients list for Dial Complete soap on Walgreens.com shows FDA-banned triclosan as the active ingredient.
The 2017 FDA ban restricted the marketing of triclosan and triclocarban along with 17 other ingredients in consumer antibacterial soaps because manufacturers did not provide sufficient data to demonstrate that the ingredients were safe and effective, according to the FDA’s announcement. Independent research also showed that some ingredients worked no better than traditional soap and could create antibacterial-resistant microbes. Regular hand soap “still kills bacteria,” Dr. Rundle said. “The inclusion of an antibacterial substance does not make it better.”
Retailers (such as Walgreens) aren’t required to update their products’ online ingredient lists, which can pose a challenge for people who suffer from skin allergies, said Dr. Rundle. People at risk of having a reaction must read labels to verify the ingredients that are included.
Consumer antibacterial soap products that contain the banned compounds have largely been replaced with stand-ins, such as benzalkonium chloride and chloroxylenol, according to Dr. Rundle’s study. He and other researchers are trying to determine whether those compounds have the same shortcomings. “We’re talking 10-20 years down the line, and we’re worried about things like antibacterial resistance and systemic effects,” Dr. Rundle said.
The FDA has considered a ban on benzalkonium chloride and additional antibacterial ingredients, but in 2016, it granted ban deferrals, pending more research. The agency exchanged letters with the American Cleaning Institute (ACI), a trade association that represents companies, including Henkel. The FDA required that its companies fund research to show that the new antibacterial ingredients are safe and effective. The FDA granted subsequent annual extensions in 2017, 2018, and, most recently, in August 2019 to allow continued research into whether several ingredients are effective in soaps. In its most recent letter to the ACI, the FDA gave a checklist of research tasks to be submitted by July 2020.
The letter from August 2019 stated that the ACI, in its March 2019 progress report, failed to address milestones in studies of health care personnel handwashing for two of the substances. It also referenced the ACI’s lack of funding for the studies and reminded the organization that further deferrals would not be granted unless the ACI can show ongoing progress.
The ACI plans to meet with the FDA to have an in-depth discussion, Brian Sansoni, a spokesperson for the ACI, told Medscape Medical News. The ACI plans to give the FDA data that show the effectiveness of these ingredients over the course of several years, “due to the complexity of what FDA is asking for,” Mr. Sansoni said. “We’re working as diligently as possible to meet FDA requests.”
This article first appeared on Medscape.com.
The website of retail pharmacy giant Walgreens, for example, lists Dial Complete antibacterial soap with the active ingredient triclosan, a chemical the Food and Drug Administration banned along with others in 2017. The agency cited a lack of evidence that the ingredients were more effective than plain soap and water and that they were safe for long-term daily use.
A Dial Complete soap product page on Walgreens’ website lists, as of Feb. 4, 2020, an ingredient that was banned by the FDA.
Yet banned substances such as the triclosan in this Dial soap still commonly appear on online product descriptions, researchers found after searching the National Drug Code Directory and the websites of major online retailers, including Amazon, Walmart, and Target. The health effects of antibacterial ingredients “are very poorly defined,” said Chandler Rundle, MD, first author of the study, which was published in Dermatitis. Dr. Rundle is with the department of dermatology at the University of Colorado at Denver, Aurora.
The label on the back of the Dial soap bottle sold on Walgreens.com states that it “[k]ills more bacteria than ordinary liquid hand soap.” The website displays a close-up graphic of a hand that has been washed with Dial soap and that has fewer bacteria than a hand washed with “Others.” The graphic includes a dramatization disclaimer.
When asked about the product, a Walgreens corporate relations spokesperson checked the soap’s ingredients list they had on file from Dial’s parent company, Henkel North American Consumer Goods.
“I did not see that particular ingredient,” the representative said. Their ingredients list reflected a version of the soap that was updated after the ban. That label differs from Walgreens.com’s product information. The updated, ban-compliant version of the soap contains an alternative antibacterial compound, benzalkonium chloride. The spokesperson wasn’t sure of the source of the incorrect information on the website. Dial did not respond to a request for comment.
The ingredients list for Dial Complete soap on Walgreens.com shows FDA-banned triclosan as the active ingredient.
The 2017 FDA ban restricted the marketing of triclosan and triclocarban along with 17 other ingredients in consumer antibacterial soaps because manufacturers did not provide sufficient data to demonstrate that the ingredients were safe and effective, according to the FDA’s announcement. Independent research also showed that some ingredients worked no better than traditional soap and could create antibacterial-resistant microbes. Regular hand soap “still kills bacteria,” Dr. Rundle said. “The inclusion of an antibacterial substance does not make it better.”
Retailers (such as Walgreens) aren’t required to update their products’ online ingredient lists, which can pose a challenge for people who suffer from skin allergies, said Dr. Rundle. People at risk of having a reaction must read labels to verify the ingredients that are included.
Consumer antibacterial soap products that contain the banned compounds have largely been replaced with stand-ins, such as benzalkonium chloride and chloroxylenol, according to Dr. Rundle’s study. He and other researchers are trying to determine whether those compounds have the same shortcomings. “We’re talking 10-20 years down the line, and we’re worried about things like antibacterial resistance and systemic effects,” Dr. Rundle said.
The FDA has considered a ban on benzalkonium chloride and additional antibacterial ingredients, but in 2016, it granted ban deferrals, pending more research. The agency exchanged letters with the American Cleaning Institute (ACI), a trade association that represents companies, including Henkel. The FDA required that its companies fund research to show that the new antibacterial ingredients are safe and effective. The FDA granted subsequent annual extensions in 2017, 2018, and, most recently, in August 2019 to allow continued research into whether several ingredients are effective in soaps. In its most recent letter to the ACI, the FDA gave a checklist of research tasks to be submitted by July 2020.
The letter from August 2019 stated that the ACI, in its March 2019 progress report, failed to address milestones in studies of health care personnel handwashing for two of the substances. It also referenced the ACI’s lack of funding for the studies and reminded the organization that further deferrals would not be granted unless the ACI can show ongoing progress.
The ACI plans to meet with the FDA to have an in-depth discussion, Brian Sansoni, a spokesperson for the ACI, told Medscape Medical News. The ACI plans to give the FDA data that show the effectiveness of these ingredients over the course of several years, “due to the complexity of what FDA is asking for,” Mr. Sansoni said. “We’re working as diligently as possible to meet FDA requests.”
This article first appeared on Medscape.com.
The website of retail pharmacy giant Walgreens, for example, lists Dial Complete antibacterial soap with the active ingredient triclosan, a chemical the Food and Drug Administration banned along with others in 2017. The agency cited a lack of evidence that the ingredients were more effective than plain soap and water and that they were safe for long-term daily use.
A Dial Complete soap product page on Walgreens’ website lists, as of Feb. 4, 2020, an ingredient that was banned by the FDA.
Yet banned substances such as the triclosan in this Dial soap still commonly appear on online product descriptions, researchers found after searching the National Drug Code Directory and the websites of major online retailers, including Amazon, Walmart, and Target. The health effects of antibacterial ingredients “are very poorly defined,” said Chandler Rundle, MD, first author of the study, which was published in Dermatitis. Dr. Rundle is with the department of dermatology at the University of Colorado at Denver, Aurora.
The label on the back of the Dial soap bottle sold on Walgreens.com states that it “[k]ills more bacteria than ordinary liquid hand soap.” The website displays a close-up graphic of a hand that has been washed with Dial soap and that has fewer bacteria than a hand washed with “Others.” The graphic includes a dramatization disclaimer.
When asked about the product, a Walgreens corporate relations spokesperson checked the soap’s ingredients list they had on file from Dial’s parent company, Henkel North American Consumer Goods.
“I did not see that particular ingredient,” the representative said. Their ingredients list reflected a version of the soap that was updated after the ban. That label differs from Walgreens.com’s product information. The updated, ban-compliant version of the soap contains an alternative antibacterial compound, benzalkonium chloride. The spokesperson wasn’t sure of the source of the incorrect information on the website. Dial did not respond to a request for comment.
The ingredients list for Dial Complete soap on Walgreens.com shows FDA-banned triclosan as the active ingredient.
The 2017 FDA ban restricted the marketing of triclosan and triclocarban along with 17 other ingredients in consumer antibacterial soaps because manufacturers did not provide sufficient data to demonstrate that the ingredients were safe and effective, according to the FDA’s announcement. Independent research also showed that some ingredients worked no better than traditional soap and could create antibacterial-resistant microbes. Regular hand soap “still kills bacteria,” Dr. Rundle said. “The inclusion of an antibacterial substance does not make it better.”
Retailers (such as Walgreens) aren’t required to update their products’ online ingredient lists, which can pose a challenge for people who suffer from skin allergies, said Dr. Rundle. People at risk of having a reaction must read labels to verify the ingredients that are included.
Consumer antibacterial soap products that contain the banned compounds have largely been replaced with stand-ins, such as benzalkonium chloride and chloroxylenol, according to Dr. Rundle’s study. He and other researchers are trying to determine whether those compounds have the same shortcomings. “We’re talking 10-20 years down the line, and we’re worried about things like antibacterial resistance and systemic effects,” Dr. Rundle said.
The FDA has considered a ban on benzalkonium chloride and additional antibacterial ingredients, but in 2016, it granted ban deferrals, pending more research. The agency exchanged letters with the American Cleaning Institute (ACI), a trade association that represents companies, including Henkel. The FDA required that its companies fund research to show that the new antibacterial ingredients are safe and effective. The FDA granted subsequent annual extensions in 2017, 2018, and, most recently, in August 2019 to allow continued research into whether several ingredients are effective in soaps. In its most recent letter to the ACI, the FDA gave a checklist of research tasks to be submitted by July 2020.
The letter from August 2019 stated that the ACI, in its March 2019 progress report, failed to address milestones in studies of health care personnel handwashing for two of the substances. It also referenced the ACI’s lack of funding for the studies and reminded the organization that further deferrals would not be granted unless the ACI can show ongoing progress.
The ACI plans to meet with the FDA to have an in-depth discussion, Brian Sansoni, a spokesperson for the ACI, told Medscape Medical News. The ACI plans to give the FDA data that show the effectiveness of these ingredients over the course of several years, “due to the complexity of what FDA is asking for,” Mr. Sansoni said. “We’re working as diligently as possible to meet FDA requests.”
This article first appeared on Medscape.com.