User login
Drop in flu activity suggests season may have peaked
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.
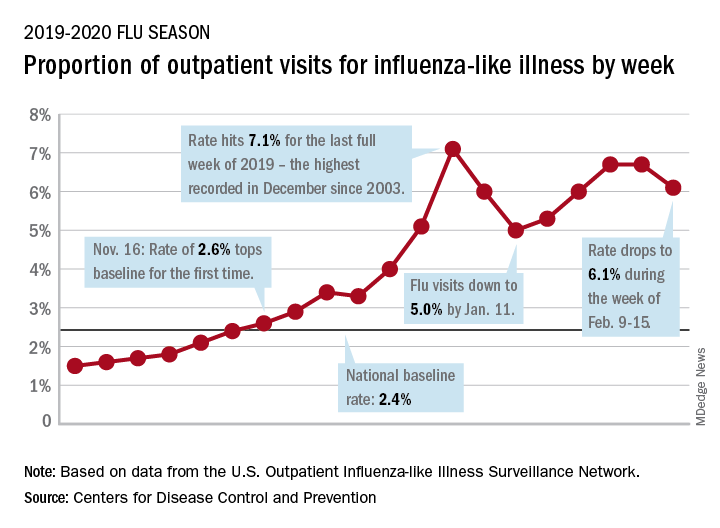
The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.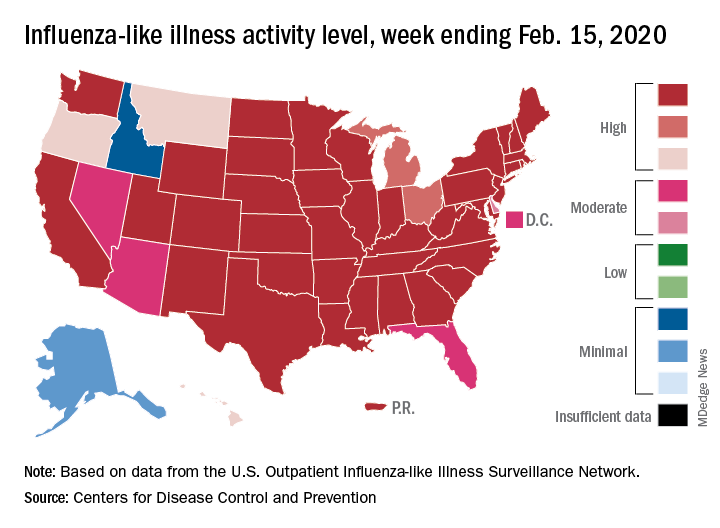
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
FROM THE CDC
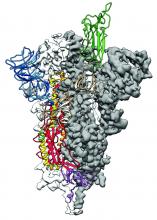
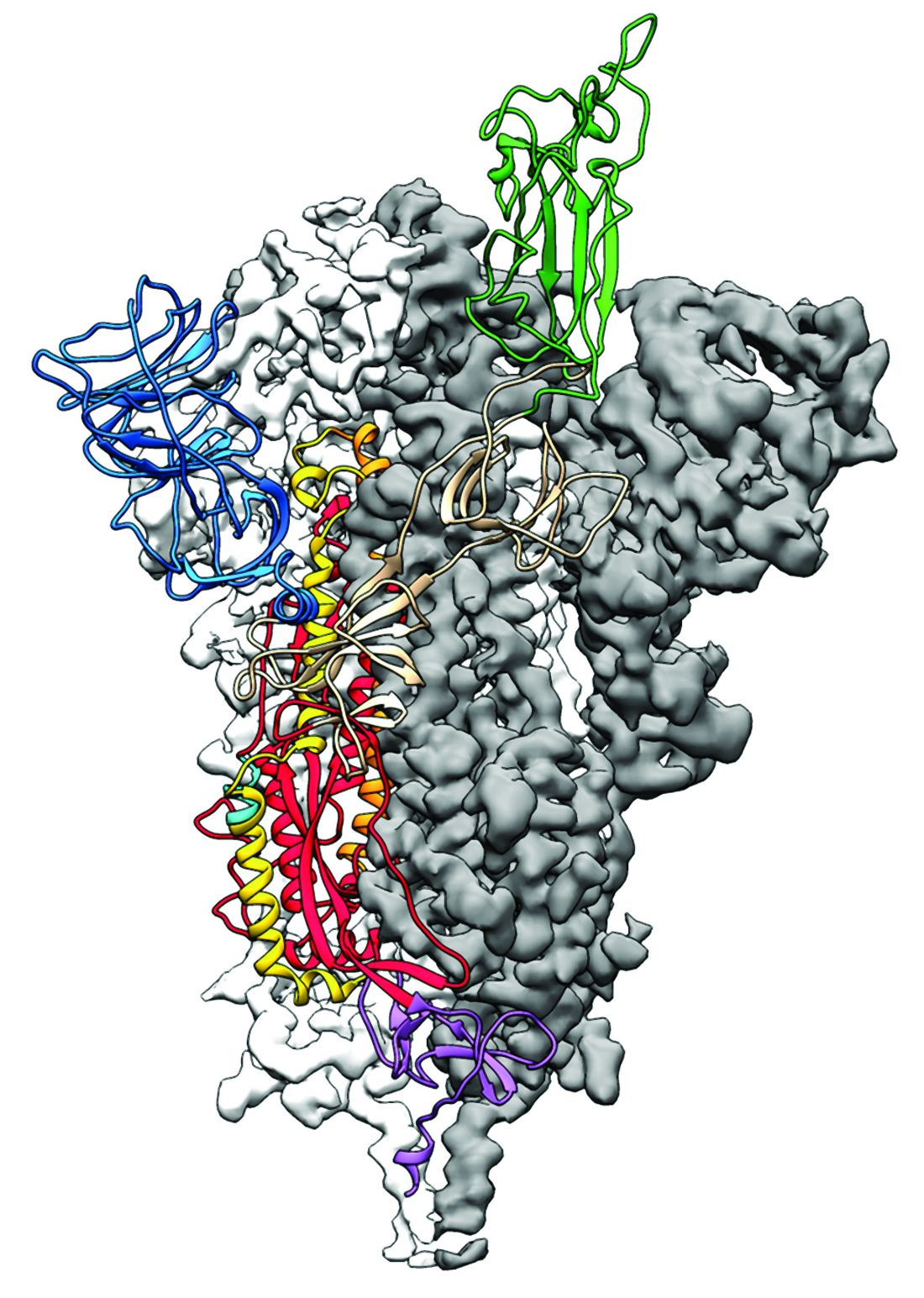
2019-nCoV: Structure, characteristics of key potential therapy target determined
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
Researchers have identified the structure of a protein that could turn out to be a potential vaccine target for the 2019-nCoV.
As is typical of other coronaviruses, 2019-nCoV makes use of a densely glycosylated spike protein to gain entry into host cells. The spike protein is a trimeric class I fusion protein that exists in a metastable prefusion conformation that undergoes a dramatic structural rearrangement to fuse the viral membrane with the host-cell membrane, according to Daniel Wrapp of the University of Texas at Austin and colleagues.
The researchers performed a study to synthesize and determine the 3-D structure of the spike protein because it is a logical target for vaccine development and for the development of targeted therapeutics for COVID-19, the disease caused by the virus.
“As soon as we knew this was a coronavirus, we felt we had to jump at it,” senior author Jason S. McLellan, PhD, associate professor of molecular science, said in a press release from the University, “because we could be one of the first ones to get this structure. We knew exactly what mutations to put into this because we’ve already shown these mutations work for a bunch of other coronaviruses.”
Because recent reports by other researchers demonstrated that 2019-nCoV and SARS-CoV spike proteins share the same functional host-cell receptor–angiotensin-converting enzyme 2 (ACE2), Dr. McLellan and his colleagues examined the relation between the two viruses. They found biophysical and structural evidence that the 2019-nCoV spike protein binds ACE2 with higher affinity than the closely related SARS-CoV spike protein. “The high affinity of 2019-nCoV S for human ACE2 may contribute to the apparent ease with which 2019-nCoV can spread from human-to-human; however, additional studies are needed to investigate this possibility,” the researchers wrote.
Focusing their attention on the receptor-binding domain (RBD) of the 2019-nCoV spike protein, they tested several published SARS-CoV RBD-specific monoclonal antibodies against it and found that these antibodies showed no appreciable binding to 2019-nCoV spike protein, which suggests limited antibody cross-reactivity. For this reason, they suggested that future antibody isolation and therapeutic design efforts will benefit from specifically using 2019-nCoV spike proteins as probes.
“This information will support precision vaccine design and discovery of anti-viral therapeutics, accelerating medical countermeasure development,” they concluded.
The research was supported in part by an National Institutes of Health/National Institute of Allergy and Infectious Diseases grant and by intramural funding from the National Institute of Allergy and Infectious Diseases. Four authors are inventors on US patent application No. 62/412,703 (Prefusion Coronavirus Spike Proteins and Their Use) and all are inventors on US patent application No. 62/972,886 (2019-nCoV Vaccine).
SOURCE: Wrapp D et al. Science. 2020 Feb 19. doi: 10.1126/science.abb2507.
FROM SCIENCE
Doctors look to existing drugs in coronavirus fight
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
COVID-19, the infection caused by the newly identified coronavirus, is a currently a disease with no pharmaceutical weapons against it. There’s no vaccine to prevent it, and no drugs can treat it.
But researchers are racing to change that. A vaccine could be ready to test as soon as April. More than two dozen studies have already been registered on ClinicalTrials.gov, a website that tracks research. These studies aim to test everything from traditional Chinese medicine to vitamin C, stem cells, steroids, and medications that fight other viruses, like the flu and HIV. The hope is that something about how these repurposed remedies work will help patients who are desperately ill with no other prospects.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, says this is all part of the playbook for brand-new diseases. “There’s a lot of empiric guessing,” he says. “They’re going to propose a whole lot of drugs that already exist. They’re going to say, here’s the data that shows it blocks the virus” in a test tube. But test tubes aren’t people, and many drugs that seem to work in a lab won’t end up helping patients.
Coronaviruses are especially hard to stop once they invade the body. Unlike many other kinds of viruses, they have a fail-safe against tampering – a “proofreader” that constantly inspects their code, looking for errors, including the potentially life-saving errors that drugs could introduce.
Dr. Fauci said that researchers will be able to make better guesses about how to help people when they can try drugs in animals. “We don’t have an animal model yet of the new coronavirus. When we do get an animal model, that will be a big boon to drugs because then, you can clearly test them in a physiological way, whether they work,” he says.
Looking to drugs for HIV and flu
One of the drugs already under study is the combination of two HIV medications: lopinavir and ritonavir (Kaletra). Kaletra stops viruses by interfering with the enzymes they need to infect cells, called proteases.
One study being done at the Guangzhou Eighth People’s Hospital in China is testing Kaletra against Arbidol, an antiviral drug approved in China and Russia to treat the flu. Two groups of patients will take the medications along with standard care. A third group in the study will receive only standard care, typically supportive therapy with oxygen and IV fluids that are meant to support the body so the immune system can fight off a virus on its own.
An Ebola drug gets a second look
One repurposed drug generating a lot of buzz is an experimental infusion called remdesivir (Xembify). It was originally tested against the Ebola virus. While it didn’t work for that infection, it has been shown to shut down the new coronavirus, at least in test tubes. It’s been given to a small number of COVID-19 patients already, including one in Washington state.
In order to have better evidence of how well it may work in people, two studies in Beijing are comparing remdesivir to a dummy pill to see if the drug can help patients with both mild and severe symptoms recover from their illnesses. Viruses work by infecting cells, taking over their machinery, and getting them to crank out more copies of the virus, which then goes on to infect more cells. Remdesivir is a mimic that fools a virus into replacing one of its four building blocks with a chemical fake. Once in the virus’s blueprints, the imposter acts like a stop sign that keeps the virus from copying itself.
Other kinds of drugs in the same class – called nucleotide analogs – are used to attack cancer and other infectious viruses like hepatitis.
Last week, Chinese scientists published study showing remdesivir was effective against the new coronavirus, 2019-nCoV. Out of seven drugs tested, only remdesivir and an older drug called chloroquine (Aralen), which is used to treat malaria, worked, at least in test tubes. “It functions like a knife that just cuts off the RNA strand,” says Mark Denison, MD, a pediatric infectious disease specialist at Vanderbilt University in Nashville. “They can’t replicate any more. It stops them from doing that.” Dr. Denison is part of a team of researchers in Tennessee and North Carolina that discovered remdesivir could stop coronaviruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), in test tubes and animals. He has studied coronaviruses in his lab for 30 years. He knew they would pose a threat again. “We’re shocked, but not surprised, that this has happened again,” he says of the China-based outbreak of 2019-nCoV.
After the SARS outbreak, which infected more than 8,000 people in 26 countries during 2002-2003, and MERS, which has infected nearly 2,500 people in 27 countries since 2012, researchers knew they had to start looking for treatments that would work against coronaviruses. Dr. Denison reached out to Gilead Sciences, a company best known for its antiviral medications that treat HIV and hepatitis C, and asked it to send drug candidates for him to test on coronaviruses. “The idea was that we didn’t want a drug that would just work against SARS or MERS,” he says. “We wanted drugs that worked against every coronavirus.”
Many of the agents he tried didn’t work until Dr. Denison and his team knocked out the virus’s pesky proofreader. Remdesivir seems to be able to defeat the proofreader, though Dr. Denison admits that he does not know how the drug gets around a virus’s defenses. He has a grant from the National Institutes of Health to study that. Gilead has been giving remdesivir to “a small number” of coronavirus patients in the United States and Europe on a compassionate basis.
One of those patients was a 35-year-old man in Everett, Wash., who had gotten pneumonia after being infected with the new coronavirus during a trip to see family in Wuhan, China, the epicenter of the outbreak. His doctors started IV remdesivir on the evening of his 7th day in the hospital. On the 8th day, he improved. He was well enough to stop using oxygen. Signs of pneumonia were gone. He got his appetite back. His case was recently published in the New England Journal of Medicine, igniting a firestorm of interest in the therapy.
Unfortunately, though, even Dr. Denison says a single person’s case isn’t enough proof that the medication can treat the new coronavirus. The patient, who has not been identified, was getting expert care. He may have improved on his own, despite getting the drug. He said the challenge in people will be to find out two things: whether the medication can block the spread of virus in the body and whether it can reverse the disease. “You can remove the source of injury, but you still have the injury,” he said. Other important questions include how soon the drug may need to be given after infection for it work and whether it may cause significant side effects.
A promising pill
Another drug, a nucleoside analog, that appears to be able to defeat the coronavirus proofreader, EIDD-2801, was developed by Emory University in Atlanta. It was originally intended to treat the flu but has shown some effectiveness against coronaviruses like SARS and MERS.
The FDA recently reached out to Emory asking if it had any drug candidates that might work against the new coronavirus. “It’s a good shot on goal here,” says George Painter, PhD, CEO of Drug Innovation Ventures at Emory. EIDD-2801 can be taken as a pill, which makes it easier to use outside of a hospital setting.
“The capsules for the trial are being made at the end of this month. So we’re close,” Painter says. “We’re right on the edge.”
While these early tests are just getting started, and it will be months until researchers have results, the World Health Organization has sounded a note of caution.
In new guidelines for the clinical management of COVID-19, the WHO reminded doctors and patients that there’s not enough evidence to recommend any specific treatment for infected patients.
Right now, the guidelines recommend that doctors offer supportive care to help the body fight off an infection on its own.
The organization says unlicensed treatments should be given only in the context of clinical trials that have been ethically reviewed or with strict clinical monitoring in emergencies.
This article first appeared on WebMD.com.
HBV: Surface antigen titer and ALT predict seroconversion
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY
Avoid ‘mutant selection window’ when prescribing antibiotics for acne
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Consider the “mutant selection window” to reduce antibiotic resistance when treating acne, Hilary E. Baldwin, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Dermatologists continue to write a disproportionate number of prescriptions for antibiotics, particularly tetracyclines, noted Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York. In addition to limiting unnecessary use of antimicrobials, strategies for slowing antimicrobial resistance include using anti-inflammatory doses of doxycycline; using more retinoids, isotretinoin, spironolactone, and oral contraceptives; and improving patient compliance with treatment.
Dermatologists can also “pay attention to the bug we are treating and ... make sure the concentration of the drug that we are using is appropriate to the bug we’re trying to kill,” while also targeting resistant organisms. Dr. Baldwin referred to a paper in the infectious disease literature titled: “The mutant selection window and antimicrobial resistance,” which points out that a drug concentration range exists for which mutant strains of bacteria are selected most frequently (J Antimicrob Chemother. 2003 Jul;52[1]:11-7). The dimensions of this range, or “window,” are characteristic of each pathogen-antimicrobial combination. A high enough drug concentration will eliminate both resistant and sensitive strains of the pathogen.
The paper notes that the minimum inhibitory concentration (MIC) is the lowest concentration that will inhibit the visible growth of a microorganism. The mutant prevention concentration (MPC) is the minimum drug concentration needed to prevent the growth of resistant strains, Dr. Baldwin said. The mutant selection window is the concentration range that extends from the MIC up to the MPC, the range “within which resistant mutants are likely to emerge.” If the antimicrobial concentration falls within this window, a mutant strain is likely to develop and “you’re going to add to the problem of antibiotic resistance,” she explained. “So the goal is to treat low or to treat high, but not right in the middle.”
“This is not theoretical,” and has been shown over and over again, with, for example, Streptococcus pneumonia and moxifloxacin, she said (J Antimicrob Chemother. 2003 Oct;52[4]:616-22.).
When the therapeutic window does not extend all the way to the MPC, “toxicity starts to kick in before you can get high enough to kill off the whole group of organisms,” in which case a low-dose strategy would reduce the development of resistant organisms, she noted.
“We’re doing this already,” with topical antifungals, Dr. Baldwin pointed out, asking when the last time anyone heard that a fungus developed resistance to topical antifungal therapy. “Never, because we use our antifungals in such a high dose, that we’re 500 times the MPC.”
Using an anti-inflammatory dose of doxycycline for treating acne or rosacea is a low-dose strategy, and the 40-mg delayed-release dose stays “way below” the antimicrobial threshold, she said, but the 50-mg dose falls “right in the middle of that mutant selection window.”
As more treatments become available, it will be important to determine how to dose topical antibiotics so that they do not fall within the mutant selection window and avoid what happened with clindamycin and erythromycin, “where the topical use of these medications led to the development of resistance such that they no longer work for the treatment” of Cutibacterium acnes.
Dr. Baldwin disclosures included being on the speakers bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Infection with 2019 novel coronavirus extends to infants
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
between Dec. 8, 2019, and Feb. 6, 2020, based on data from the Chinese central government and local health departments.
“As of February 6, 2020, China reported 31,211 confirmed cases of COVID-19 and 637 fatalities,” wrote Min Wei, MD, of Wuhan University, China, and colleagues. However, “few infections in children have been reported.”
In a research letter published in JAMA, the investigators reviewed data from nine infants aged 28 days to 1 year who were hospitalized with a diagnosis of COVID-19 between Dec. 8, 2019, and Feb. 6, 2020. The ages of the infants ranged from 1 month to 11 months, and seven were female. The patients included two children from Beijing, two from Hainan, and one each from the areas of Guangdong, Anhui, Shanghai, Zhejiang, and Guizhou.
All infected infants had at least one infected family member, and the infants’ infections occurred after the family members’ infections; seven infants lived in Wuhan or had family members who had visited Wuhan.
One of the infants had no symptoms but tested positive for the 2019 novel coronavirus, and two others had a diagnosis but missing information on any symptoms. Fever occurred in four patients, and mild upper respiratory tract symptoms occurred in two patients.
None of the infants died, and none reported severe complications or the need for intensive care or mechanical ventilation, the investigators said. The fact that most of the infants were female might suggest that they are more susceptible to the virus than males, although overall COVID-19 viral infections have been more common in adult men, especially those with chronic comorbidities, Dr. Wei and associates noted.
The study findings were limited by the small sample size and lack of symptom data for some patients, the researchers said. However, the results confirm that the COVID-19 virus is transmissible to infants younger than 1 year, and adult caregivers should exercise protective measures including wearing masks, washing hands before contact with infants, and routinely sterilizing toys and tableware, they emphasized.
The study was supported by the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities. The researchers had no financial conflicts to disclose.
SOURCE: Wei M et al. JAMA. 2020 Feb 14. doi:10.1001/jama.2020.2131.
FROM JAMA
As novel coronavirus outbreak evolves, critical care providers need to be prepared
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
EXPERT ANALYSIS FROM CCC49
Flu increases activity but not its severity

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.
ACC issues guidance on cardiac implications of coronavirus
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
An epidemic of fear and misinformation
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.