User login
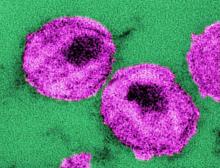
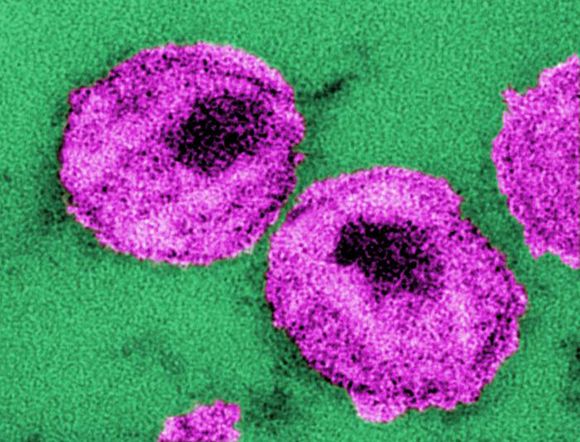
Infection control protects hospital staff from COVID-19, study shows
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hospital-related infections have been widely reported during the ongoing coronavirus outbreak, with healthcare professionals bearing a disproportionate risk. However, a proactive response in Hong Kong’s public hospital system appears to have bucked this trend and successfully protected both patients and staff from SARS-CoV-2, according to a study published online today in Infection Control & Hospital Epidemiology.
During the first 42 days of the outbreak, the 43 hospitals in the network tested 1275 suspected cases and treated 42 patients with confirmed COVID-19, the disease caused by SARS-CoV-2 infection. Yet, there were no nosocomial infections or infections among healthcare personnel, report Vincent C.C. Cheng, MD, FRCPath, the hospital’s infection control officer, and colleagues.
Cheng and colleagues note that 11 out of 413 healthcare workers who treat patients with confirmed infections had unprotected exposure and were in quarantine for 14 days, but none became ill.
In comparison, they note, the 2003 SARS outbreak saw almost 60% of nosocomial cases occurring in healthcare workers.
Proactive bundle
The Hong Kong success story may be due to a stepped-up proactive bundle of measures that included enhanced laboratory surveillance, early airborne infection isolation, and rapid-turnaround molecular diagnostics. Other strategies included staff forums and one-on-one discussions about infection control, employee training in protective equipment use, hand-hygiene compliance enforcement, and contact tracing for workers with unprotected exposure.
In addition, surgical masks were provided for all healthcare workers, patients, and visitors to clinical areas, a practice previously associated with reduced in-hospital transmission during influenza outbreaks, the authors note.
Hospitals also mandated use of personal protective equipment (PPE) for aerosol-generating procedures (AGPs), such as endotracheal intubation, open suctioning, and high-flow oxygen use, as AGPs had been linked to nosocomial transmission to healthcare workers during the 2003 SARS outbreak.
The infection control measures, which were part of a preparedness plan developed after the SARS outbreak, were initiated on December 31, when the first reports of a cluster of infections came from Wuhan, China.
As the outbreak evolved, the Hong Kong hospitals quickly widened the epidemiologic criteria for screening, from initially including only those who had been to a wet market in Wuhan within 14 days of symptom onset, to eventually including anyone who had been to Hubei province, been in a medical facility in mainland China, or in contact with a known case.
All suspected cases were sent to an airborne-infection isolation room (AIIR) or a ward with at least a meter of space between patients.
“Appropriate hospital infection control measures could prevent nosocomial transmission of SARS-CoV-2,” the authors write. “Vigilance in hand hygiene practice, wearing of surgical mask in the hospital, and appropriate use of PPE in patient care, especially [when] performing AGPs, are the key infection control measures to prevent nosocomial transmission of SARS-CoV-2 even before the availability of effective antiviral agents and vaccine.”
Asked for his perspective on the report, Aaron E. Glatt, MD, chairman of the department of medicine and chief of infectious diseases at Mount Sinai South Nassau in Oceanside, New York, said that apart from the widespread issuing of surgical masks to workers, patients, and visitors, the measures taken in Hong Kong are not different from standard infection-control practices in American hospitals. Glatt, who is also a hospital epidemiologist, said it was unclear how much impact the masks would have.
“Although the infection control was impressive, I don’t see any evidence of a difference in care,” he told Medscape Medical News.
Could zero infection transmission be achieved in the more far-flung and variable settings of hospitals across the United States? “The ability to get zero transmission is only possible if people adhere to the strictest infection-control guidelines,” Glatt said. “That is clearly the goal, and it will take time to see if our existing strict guidelines are sufficient to maintain zero or close to zero contamination and transmission rates in our hospitals.”
Rather than looking to change US practices, he stressed adherence to widely established tenets of care. “It’s critically important to keep paying close attention to the basics, to the simple blocking and tackling, and to identify which patients are at risk, and therefore, when workers need protective equipment,” he said.
“Follow the recommended standards,” continued Glatt, who is also a spokesperson for the Infectious Diseases Society of America and did not participate in this study.
In a finding from an ancillary pilot experiment, the Hong Kong researchers found exhaled air from a patient with a moderate coronavirus load showed no evidence of the virus, whether the patient was breathing normally or heavily, speaking, or coughing. And spot tests around the room detected the virus in just one location.
“We may not be able to make a definite conclusion based on the analysis of a single patient,” the authors write. “However, it may help to reassure our staff that the exhaled air may be rapidly diluted inside the AIIR with 12 air changes per hour, or probably the SARS-CoV-2 may not be predominantly transmitted by [the] airborne route.”
However, a recent Singapore study showed widespread environmental contamination by SARS-CoV-2 through respiratory droplets and fecal shedding, underlining the need for strict adherence to environmental and hand hygiene. Post-cleaning samples tested negative, suggesting that standard decontamination practices are effective.
This work was partly supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases of the Department of Health, Hong Kong Special Administrative Region; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Ministry of Education of China. The authors and Glatt have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Monthly injection therapy for HIV found noninferior to daily oral dosing
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
Two international phase 3 randomized trials of according to reports published in the New England Journal of Medicine.
The Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection (FLAIR) study and the Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression (ATLAS) study looked at a separate facet of the use of a monthly therapeutic injection as a replacement for daily oral HIV therapy.
The FLAIR trial (ClinicalTrials.gov number, NCT02938520) was a phase 3, randomized, open-label study in which adults with HIV-1 infection who had not previously received antiretroviral therapy were given 20 weeks of daily oral induction therapy with dolutegravir–abacavir–lamivudine. Those patients with an HIV-1 RNA level of less than 50 copies per milliliter after 16 weeks were then randomly assigned (1:1) to continue their current oral therapy or switch to oral cabotegravir plus rilpivirine for 1 month followed by monthly intramuscular injections of long-acting cabotegravir, an HIV-1 integrase strand-transfer inhibitor, and rilpivirine, a nonnucleoside reverse-transcriptase inhibitor.
At week 48, an HIV-1 RNA level of 50 copies per milliliter or higher was found in 6 of 283 patients (2.1%) who received the long-acting therapy and in 7 of 283 (2.5%) who received oral therapy, which met the criterion for noninferiority for the primary endpoint. An HIV-1 RNA level of less than 50 copies per milliliter at week 48 was found in 93.6% of patients who received long-acting therapy and in 93.3% who received oral therapy, which also met the criterion for noninferiority, according to the study published in the New England Journal of Medicine.
Injection site reactions were reported in 86% of the long-acting therapy patients, 4 of whom withdrew from the trial for injection-related reasons. Grade 3 or higher adverse events and events that met liver-related stopping criteria occurred in 11% and 2%, respectively, of those who received long-acting therapy and in 4% and 1% of those who received oral therapy.
An assessment of treatment satisfaction at 48 weeks showed that 91% of the patients who switched to long-acting therapy preferred it to their daily oral therapy.
The ATLAS trial (ClinicalTrials.gov number, NCT02951052) was a phase 3, open-label, multicenter, noninferiority trial involving patients who had plasma HIV-1 RNA levels of less than 50 copies per milliliter for at least 6 months while taking standard oral antiretroviral therapy. These patients were randomized (308 in each group) to the long-acting cabotegravir plus rilpivirine injection therapy or daily oral therapy.
At 48 weeks, HIV-1 RNA levels of 50 copies per milliliter or higher were found in five participants (1.6%) receiving long-acting therapy and in three (1.0%) receiving oral therapy, which met the criterion for noninferiority for the primary endpoint, according to a study reported in the New England Journal of Medicine.
HIV-1 RNA levels of less than 50 copies per milliliter at week 48 occurred in 92.5% of patients on long-acting therapy and in 95.5% of those receiving oral therapy, which also met the criterion for noninferiority for this endpoint. Three patients in the long-acting therapy group had virologic failure, compared with four participants who received oral therapy.
Adverse events were more common in the long-acting–therapy group and included injection-site pain, which occurred in 231 recipients (75%) of long-acting therapy. This was mild or moderate in most cases, according to the authors. However, 1% of the participants in this group withdrew because of it. Overall, serious adverse events were reported in no more than 5% of participants in each group.
Together, the ATLAS and the FLAIR trials show that long-acting intramuscular injection therapy is noninferior to oral therapy as both an early regimen for HIV treatment, as well as for later, maintenance dosing. The use of long-acting therapy may improve patient adherence to treatment, according to both sets of study authors.
The ATLAS and FLAIR trials were funded by ViiV Healthcare and Janssen. The authors of both studies reported ties to pharmaceutical associations, and some authors are employees of the two funding sources.
SOURCE: Orkin C et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1909512 and Swindells S et al. N Engl J Med. 2020 Mar 4. doi: 10.1056/NEJMoa1904398.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
What’s Eating You? Human Body Lice (Pediculus humanus corporis)
Epidemiology and Transmission
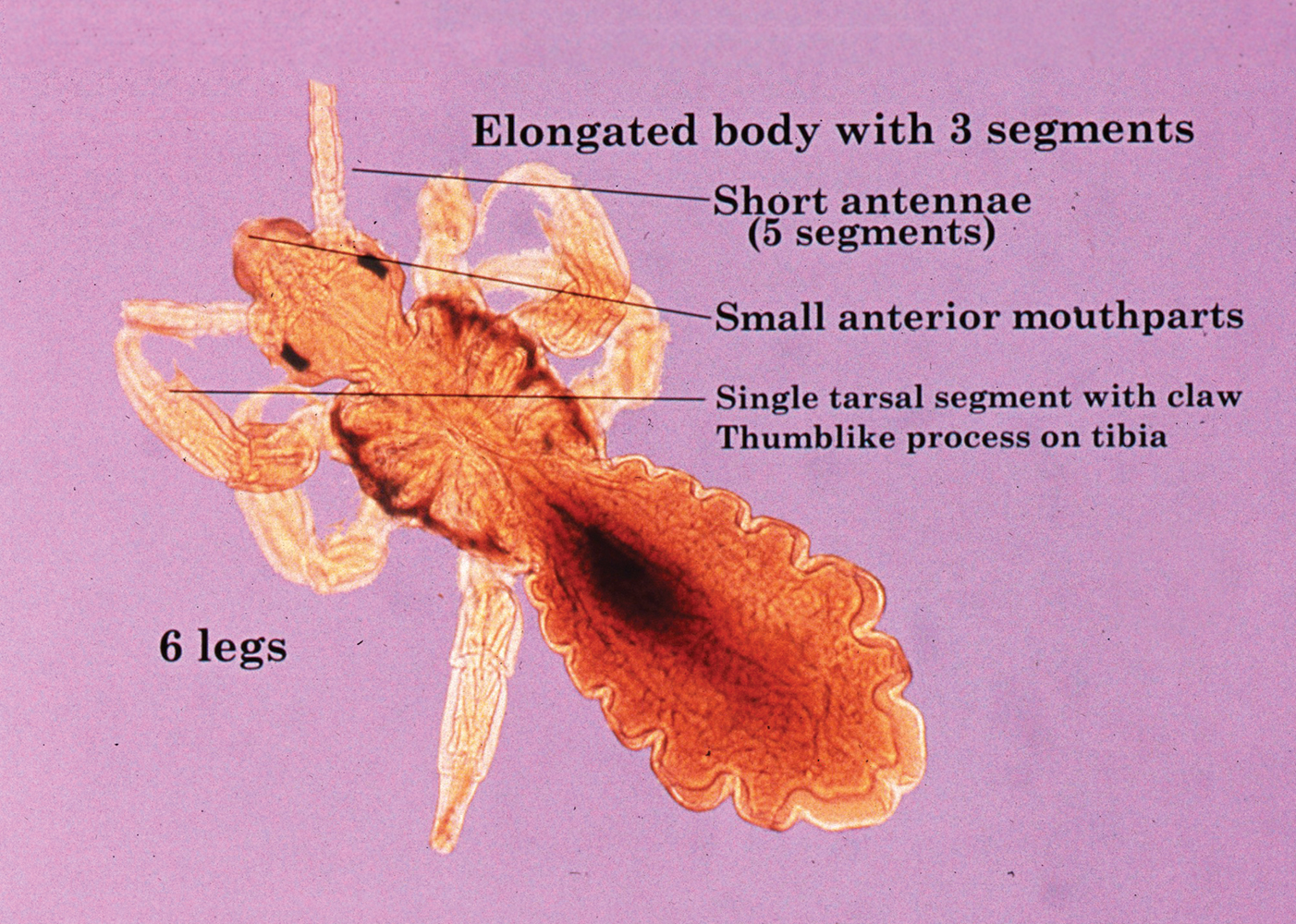
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3

Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
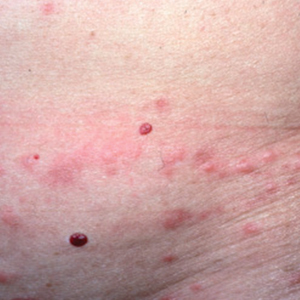
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3


Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3


Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15

Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Practice Points
- Body lice reside in clothing, particularly folds and seams, and migrate to the host for blood meals. To evaluate for infestation, the clinician should not only look at the skin but also closely examine the patient’s clothing. Clothes also are a target for treatment via washing in hot water.
- Due to observed and theoretical adverse effects of other chemical treatments, benzyl alcohol is the authors’ choice for treatment of head lice.
- Oral ivermectin is a promising future treatment for body lice.
In a public health crisis, obstetric collaboration is mission-critical
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at [email protected].
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at [email protected].
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at [email protected].
Recurrent Vesicles on the Palm
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
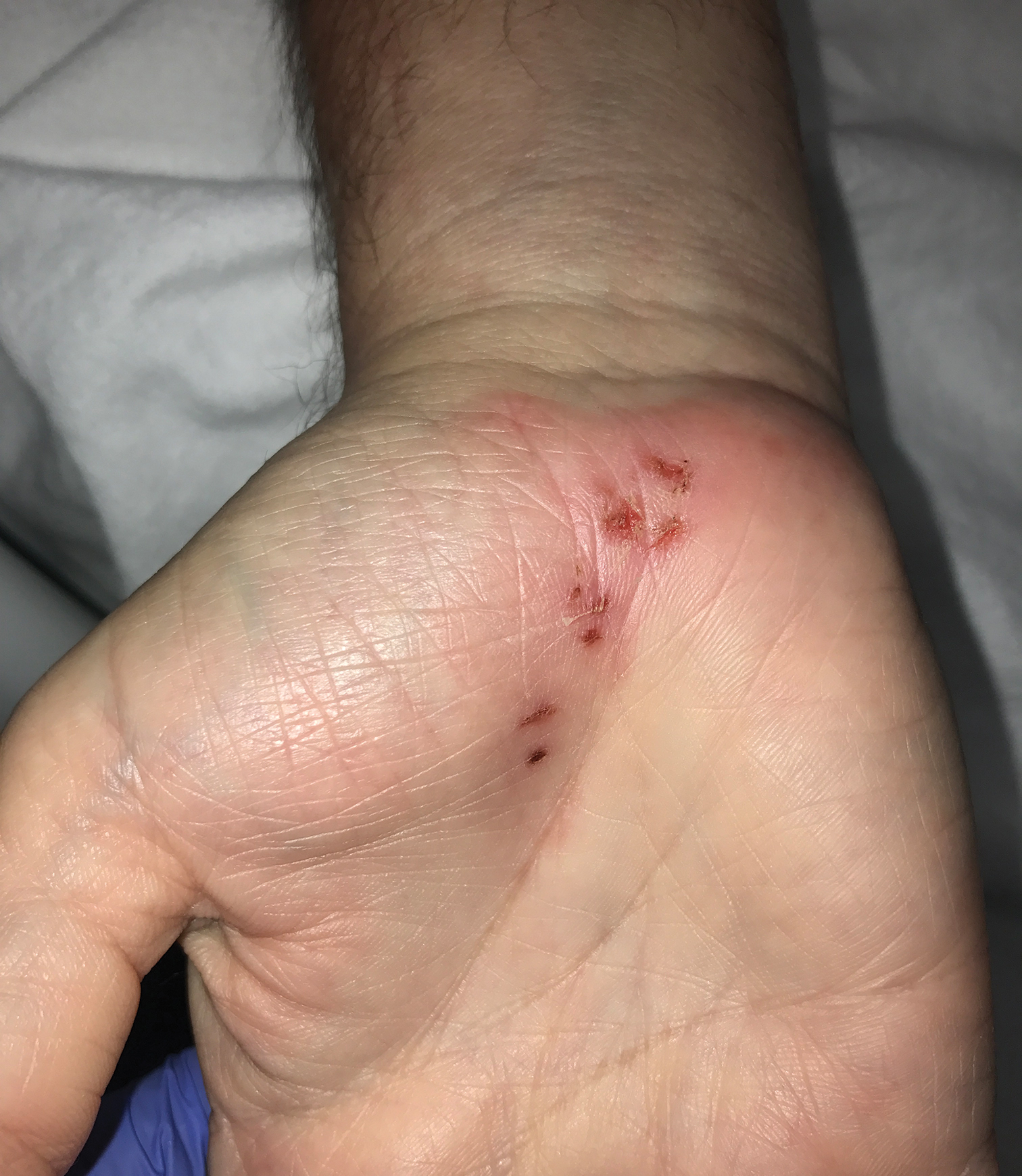
A 54-year-old man presented to the emergency department with painful lesions at the base of the right palm that had progressed to include areas of erythema and warmth migrating proximally along the right forearm and distal right arm. He stated that similar lesions had occurred episodically in the same location approximately 100 times over the last 20 years. Each time, the lesions began as painful vesicles that he subsequently popped with a sewing needle. He denied any history of orolabial or genital herpes simplex virus infection. Physical examination revealed erythematous scattered papules with dry hemorrhagic crust over the base of the right palm with expressible serous fluid upon forceful pressure. Swelling, erythema, and warmth of the distal right forearm also were observed.
FDA moves to expand coronavirus testing capacity; CDC clarifies testing criteria
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
FROM A PRESS BRIEFING BY THE WHITE HOUSE CORONAVIRUS TASK FORCE
What medical conferences are being canceled by coronavirus?
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
Upcoming vaccine may offset surge in polio subtypes
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP vaccination update
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
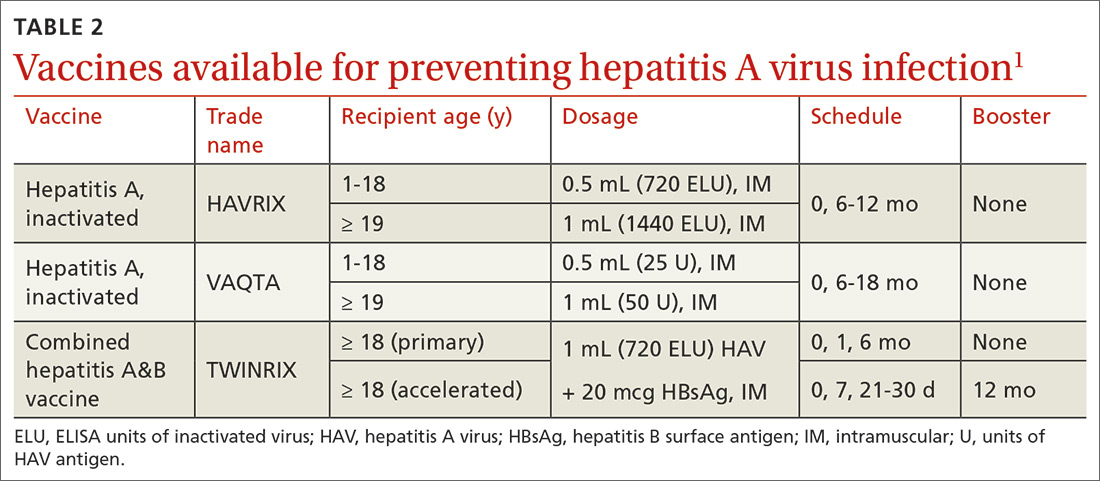
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12
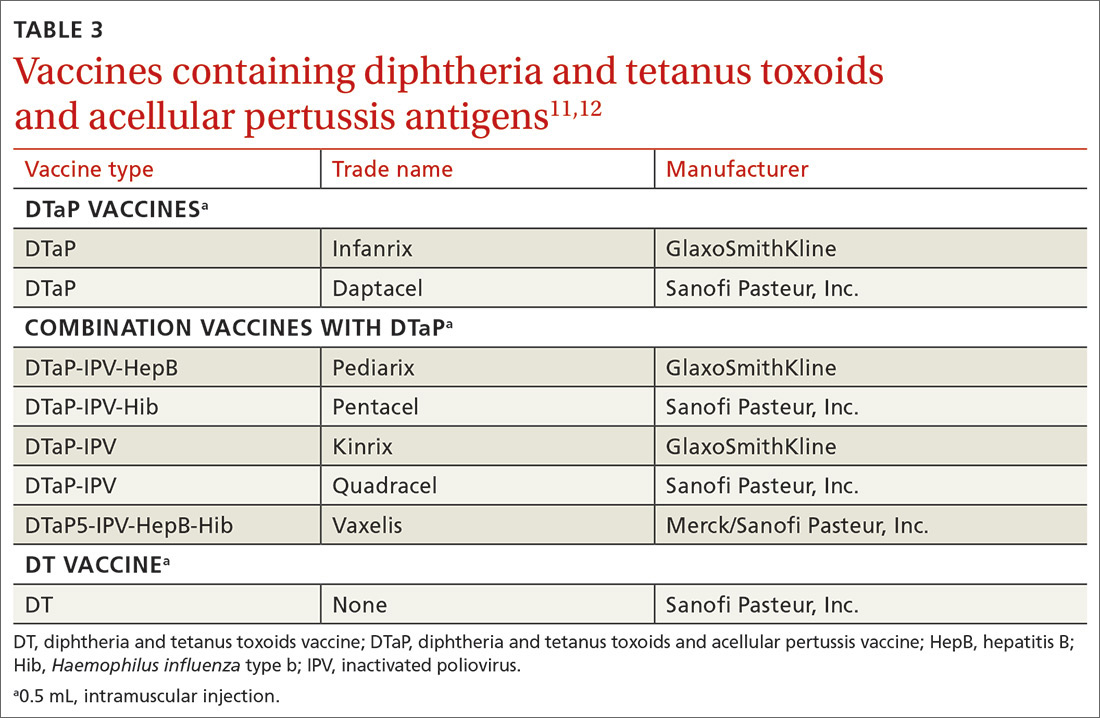
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1

ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2

At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12

Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1

ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2

At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12

Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Dengue vaccine deemed acceptable by most doctors, fewer parents
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
FROM AN ACIP MEETING