User login
Coronavirus stays in aerosols for hours, on surfaces for days
according to a new study.
The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
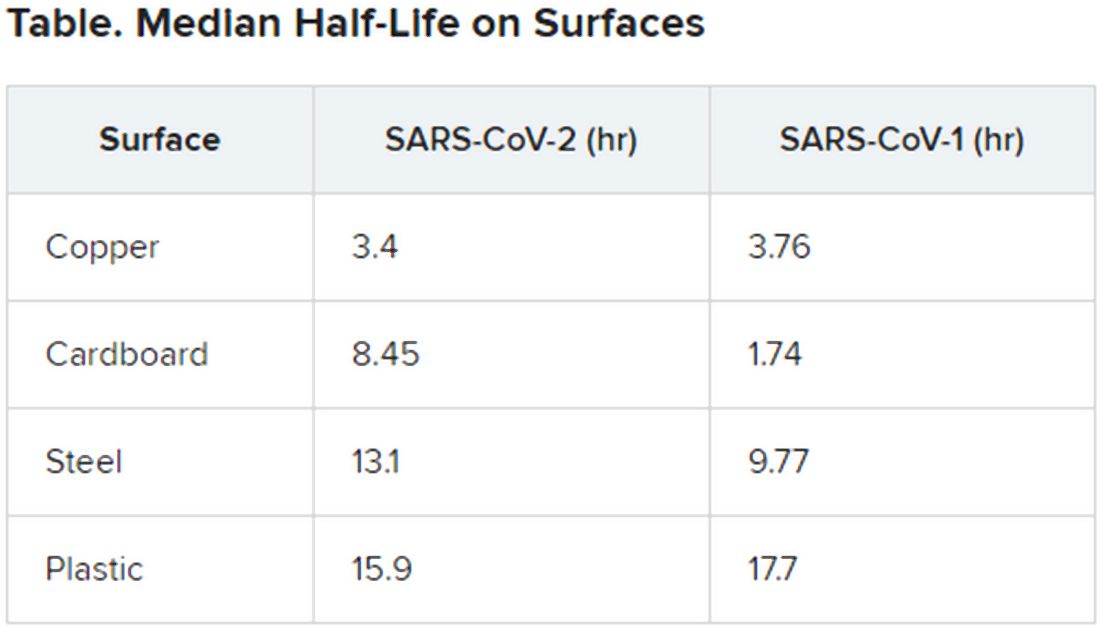
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
according to a new study.

The data indicate that the stability of the new virus is similar to that of SARS-CoV-1, which caused the SARS epidemic, researchers report in an article published on the medRxivpreprint server. (The posted article has been submitted for journal publication but has not been peer reviewed.)
Transmission of SARS-CoV-2, which causes COVID-19, has quickly outstripped the pace of the 2003 SARS epidemic. “Superspread” of the earlier disease arose from infection during medical procedures, in which a single infected individual seeded many secondary cases. In contrast, the novel coronavirus appears to be spread more through human-to-human transmission in a variety of settings.
However, it’s not yet known the extent to which asymptomatic or presymptomatic individuals spread the new virus through daily routine.
To investigate how long SARS-CoV-2 remains infective in the environment, Neeltje van Doremalen, PhD, of the Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, in Hamilton, Montana, and colleagues conducted simulation experiments in which they compared the viability of SARS-CoV-2 with that of SARS-CoV-1 in aerosols and on surfaces.
Among patients infected with SARS-CoV-2, viral loads in the upper respiratory tract are high; as a consequence, respiratory secretion in the form of aerosols (<5 μm) or droplets (>5 mcm) is likely, the authors note.
van Doremalen and colleagues used nebulizers to generate aerosols. Samples of SARS-CoV-1 and SARS-CoV-2 were collecting at 0, 30, 60, 120, and 180 minutes on a gelatin filter. The researchers then tested the infectivity of the viruses on Vero cells grown in culture.
They found that SARS-CoV-2 was largely stable through the full 180-minute test, with only a slight decline at 3 hours. This time course is similar to that of SARS-CoV-1; both viruses have a median half-life in aerosols of 2.7 hours (range, 1.65 hr for SARS-CoV-1, vs 7.24 hr for SARS-CoV-2).
The researchers then tested the viruses on a variety of surfaces for up to 7 days, using humidity values and temperatures designed to mimic “a variety of household and hospital situations.” The volumes of viral exposures that the team used were consistent with amounts found in the human upper and lower respiratory tracts.
For example, they applied 50 mcL of virus-containing solution to a piece of cardboard and then swabbed the surface, at different times, with an additional 1 mcL of medium. Each surface assay was replicated three times.
The novel coronavirus was most stable on plastic and stainless steel, with some virus remaining viable up to 72 hours. However, by that time the viral load had fallen by about three orders of magnitude, indicating exponential decay. This profile was remarkably similar to that of SARS-CoV-1, according to the authors.
However, the two viruses differed in staying power on copper and cardboard. No viable SARS-CoV-2 was detectable on copper after 4 hours or on cardboard after 24 hours. In contrast, SARS-CoV-1 was not viable beyond 8 hours for either copper or cardboard.
“Taken together, our results indicate that aerosol and fomite transmission of HCoV-19 [SARS-CoV-2] are plausible, as the virus can remain viable in aerosols for multiple hours and on surfaces up to days,” the authors conclude.
Andrew Pekosz, PhD, codirector of the Center of Excellence in Influenza Research and Surveillance and director of the Center for Emerging Viruses and Infectious Diseases at the Johns Hopkins Center for Global Health, Baltimore, Maryland, applauds the real-world value of the experiments.
“The PCR [polymerase chain reaction] test used [in other studies] to detect SARS-CoV-2 just detects the virus genome. It doesn’t tell you if the virus was still infectious, or ‘viable.’ That’s why this study is interesting,” Pekosz said. “It focuses on infectious virus, which is the virus that has the potential to transmit and infect another person. What we don’t know yet is how much infectious (viable) virus is needed to initiate infection in another person.”
He suggests that further investigations evaluate other types of environmental surfaces, including lacquered wood that is made into desks and ceramic tiles found in bathrooms and kitchens.
One limitation of the study is that the data for experiments on cardboard were more variable than the data for other surfaces tested.
The investigators and Pekosz have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Potential GI manifestation, transmission of coronavirus
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
The novel coronavirus (2019-nCoV) shows evidence of causing gastrointestinal symptoms and has the potential to be transmitted by the fecal-oral route, according to a new report from physicians at Shanghai Jiao Tong University, published online (Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054).
The virus’s respiratory symptoms are well documented and suggest primary transmission by droplet or contact, while other symptoms such as diarrhea, nausea, vomiting, and abdominal discomfort are less common and appear to vary between populations. The SARS coronavirus showed up in stool, even sometimes in patients discharged from the hospital. In a study of hospitalized patients in Wuhan, China, 10.1% of coronavirus patients had diarrhea and nausea in the 1-2 days before onset of fever and dyspnea. The first U.S. patient to be diagnosed had a 2-day history of nausea and vomiting, and had a loose bowel movement on the second day in the hospital. Clinicians later confirmed the presence of viral RNA in both the patient’s stool and airway.
The authors say that researchers in China have isolated viral RNA from the stool of two patients (unpublished), and it has been found in saliva, suggesting the possibility of the salivary gland as an infection or transmission route.
The authors maintain that previous studies likely overlooked or neglected patients who had mild intestinal symptoms. “Many efforts should be made to be alert on the initial digestive symptoms of COVID-19 for early detection, early diagnosis, early isolation and early intervention,” the authors wrote.
Like other coronaviruses, it appears that 2019-nCoV infects cells through an interaction between viral transmembrane spike glycoprotein (S-protein) receptor-binding domain, and the cell receptors angiotensin-converting enzyme 2 (ACE-2) and host cellular transmembrane serine protease (TMPRSS). Transcriptome analysis has shown that human lung AT2 cells express ACE-2 and TMPRSS, but esophagus upper and stratified epithelial cells also express both factors, as do stratified epithelial cells and absorptive enterocytes in the ileum and colon.
The researchers call for investigation into ACE-2 fusion proteins and TMPRSS inhibitors for diagnosis, prophylaxis, or treatment of COVID-19.
The authors also noted that COVID-19 has been linked to mild to moderate liver injury as revealed by elevated aminotransferases, hypoproteinemia and prothrombin time prolongation. This also has precedent in that the SARS coronavirus can infect the liver, and biopsies revealed mitoses and apoptosis, along with other abnormalities. SARS-associated hepatitis may be the result of viral hepatitis, immune overreaction, or a secondary effect of antiviral medications or other drugs. Little is known to date about the ability of 2019-nCoV to infect the liver, but single-cell RNA sequencing data from two distinct cohorts showed more ACE-2 expression in cholangiocytes (59.7%) than hepatocytes (2.6%), which indicates that the virus might directly affect intrahepatic bile ducts.
The authors had no sources of funding or financial conflicts.
SOURCE: GU J et al. Gastroenterology. 2020 March 3. doi: 10.1053/j.gastro.2020.02.054.
*This story was updated on 4/10.2020.
FROM GASTROENTEROLOGY
Treating COVID-19 in patients with diabetes
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
Patients with diabetes may be at extra risk for coronavirus disease (COVID-19) mortality, and doctors treating them need to keep up with the latest guidelines and expert advice.
Most health advisories about COVID-19 mention diabetes as one of the high-risk categories for the disease, likely because early data coming out of China, where the disease was first reported, indicated an elevated case-fatality rate for COVID-19 patients who also had diabetes.
In an article published in JAMA, Zunyou Wu, MD, and Jennifer M. McGoogan, PhD, summarized the findings from a February report on 44,672 confirmed cases of the disease from the Chinese Center for Disease Control and Prevention. The overall case-fatality rate (CFR) at that stage was 2.3% (1,023 deaths of the 44,672 confirmed cases). The data indicated that the CFR was elevated among COVID-19 patients with preexisting comorbid conditions, specifically, cardiovascular disease (CFR, 10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6%), and cancer (5.6%).
The data also showed an aged-related trend in the CFR, with patients aged 80 years or older having a CFR of 14.8% and those aged 70-79 years, a rate of 8.0%, while there were no fatal cases reported in patients aged 9 years or younger (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
Those findings have been echoed by the U.S. Centers of Disease Control and Prevention. The American Diabetes Association and the American Association of Clinical Endocrinologists have in turn referenced the CDC in their COVID-19 guidance recommendations for patients with diabetes.
Guidelines were already in place for treatment of infections in patients with diabetes, and
In general, patients with diabetes – especially those whose disease is not controlled, or not well controlled – can be more susceptible to more common infections, such as influenza and pneumonia, possibly because hyperglycemia can subdue immunity by disrupting function of the white blood cells.
Glucose control is key
An important factor in any form of infection control in patients with diabetes seems to be whether or not a patient’s glucose levels are well controlled, according to comments from members of the editorial advisory board for Clinical Endocrinology News. Good glucose control, therefore, could be instrumental in reducing both the risk for and severity of infection.
Paul Jellinger, MD, of the Center for Diabetes & Endocrine Care, Hollywood, Fla., said that, over the years, he had not observed higher infection rates in general in patients with hemoglobin A1c levels below 7, or even higher. However, “a bigger question for me, given the broad category of ‘diabetes’ listed as a risk for serious coronavirus complications by the CDC, has been: Just which individuals with diabetes are really at risk? Are patients with well-controlled diabetes at increased risk as much as those with significant hyperglycemia and uncontrolled diabetes? In my view, not likely.”
Alan Jay Cohen, MD, agreed with Dr. Jellinger. “Many patients have called the office in the last 10 days to ask if there are special precautions they should take because they are reading that they are in the high-risk group because they have diabetes. Many of them are in superb, or at least pretty good, control. I have not seen where they have had a higher incidence of infection than the general population, and I have not seen data with COVID-19 that specifically demonstrates that a person with diabetes in good control has an increased risk,” he said.
“My recommendations to these patients have been the same as those given to the general population,” added Dr. Cohen, medical director at Baptist Medical Group: The Endocrine Clinic, Memphis.
Herbert I. Rettinger, MD, also conceded that poorly controlled blood sugars and confounding illnesses, such as renal and cardiac conditions, are common in patients with long-standing diabetes, but “there is a huge population of patients with type 1 diabetes, and very few seem to be more susceptible to infection. Perhaps I am missing those with poor diet and glucose control.”
Philip Levy, MD, picked up on that latter point, emphasizing that “endocrinologists take care of fewer patients with diabetes than do primary care physicians. Most patients with type 2 diabetes are not seen by us unless the PCP has problems [treating them],” so it could be that PCPs may see a higher number of patients who are at a greater risk for infections.
Ultimately, “good glucose control is very helpful in avoiding infections,” said Dr. Levy, of the Banner University Medical Group Endocrinology & Diabetes, Phoenix.
For sick patients
Guidelines for patients at the Joslin Diabetes Center in Boston advise patients who are feeling sick to continue taking their diabetes medications, unless instructed otherwise by their providers, and to monitor their glucose more frequently because it can spike suddenly.
Patients with type 1 diabetes should check for ketones if their glucose passes 250 mg/dL, according to the guidelines, and patients should remain hydrated at all times and get plenty of rest.
“Sick-day guidelines definitely apply, but patients should be advised to get tested if they have any symptoms they are concerned about,” said Dr. Rettinger, of the Endocrinology Medical Group of Orange County, Orange, Calif.
If patients with diabetes develop COVID-19, then home management may still be possible, according to Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues (Diabetes Metab Syndr. 2020 Mar 10;14[3]:211-2. doi: 10.1016/j.dsx.2020.03.002).
Dr. Rettinger agreed, noting that home management would be feasible as long as “everything is going well, that is, the patient is not experiencing respiratory problems or difficulties in controlling glucose levels. Consider patients with type 1 diabetes who have COVID-19 as you would a nursing home patient – ever vigilant.”
Dr. Gupta and coauthors also recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve. However, the ADA warns in its guidelines that patients should “be aware that some constant glucose monitoring sensors (Dexcom G5, Medtronic Enlite, and Guardian) are impacted by acetaminophen (Tylenol), and that patients should check with finger sticks to ensure accuracy [if they are taking acetaminophen].”
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often, the authors wrote, cautioning that “frequent changes in dosage and correctional bolus may be required to maintain normoglycemia.” Dr Rettinger emphasized that “hyperglycemia, as always, is best treated with fluids and insulin and frequent checks of sugars to be sure the treatment regimen is successful.”
In regard to diabetic drug regimens, patients with type 1 or 2 disease should continue on their current medications, advised Yehuda Handelsman, MD. “Some, especially those on insulin, may require more of it. And the patient should increase fluid intake to prevent fluid depletion. We do not reduce antihyperglycemic medication to preserve fluids.
“As for hypoglycemia, we always aim for less to no hypoglycemia,” he continued. “Monitoring glucose and appropriate dosage is the way to go. In other words, do not reduce medications in sick patients who typically need more medication.”
Dr. Handelsman, medical director and principal investigator at Metabolic Institute of America, Tarzana, Calif., added that very sick patients who are hospitalized should be managed with insulin and that oral agents – particularly metformin and sodium-glucose transporter 2 inhibitors – should be stopped.
“Once the patient has recovered and stabilized, you can return to the prior regimen, and, even if the patient is still in hospital, noninsulin therapy can be reintroduced,” he said.
“This is standard procedure in very sick patients, especially those in critical care. Metformin may raise lactic acid levels, and the SGLT2 inhibitors cause volume contraction, fat metabolism, and acidosis,” he explained. “We also stop the glucagon-like peptide receptor–1 analogues, which can cause nausea and vomiting, and pioglitazone because it causes fluid overload.
“Only insulin can be used for acutely sick patients – those with sepsis, for example. The same would apply if they have severe breathing disorders, and definitely, if they are on a ventilator. This is also the time we stop aromatase inhibitor orals and we use insulin.”
Preventive measures
In the interest of maintaining good glucose control, patients also should monitor their glucose levels more frequently so that fluctuations can be detected early and quickly addressed with the appropriate medication adjustments, according to guidelines from the ADA and AACE. They should continue to follow a healthy diet that includes adequate protein and they should exercise regularly.
Patients should ensure that they have enough medication and testing supplies – for at least 14 days, and longer, if costs permit – in case they have to go into quarantine.
General preventive measures, such as frequent hand washing with soap and water, practicing good respiratory hygiene by sneezing or coughing into a facial tissue or bent elbow, also apply for reducing the risk of infection. Touching of the face should be avoided, as should nonessential travel and contact with infected individuals.
Patients with diabetes should always be current with their influenza and pneumonia shots.
Dr. Rettinger said that he always recommends the following preventative measures to his patients and he is using the current health crisis to reinforce them:
- Eat lots of multicolored fruits and vegetables.
- Eat yogurt and take probiotics to keep the intestinal biome strong and functional.
- Be extra vigilant regarding sugars and sugar control to avoid peaks and valleys wherever possible.
- Keep the immune system strong with at least 7-8 hours sleep and reduce stress levels whenever possible.
- Avoid crowds and handshaking.
- Wash hands regularly.
Possible therapies
There are currently no drugs that have been approved specifically for the treatment of COVID-19, although a vaccine against the disease is currently under development.
Dr. Gupta and his colleagues noted in their article that there have been reports of the anecdotal use of antiviral drugs such as lopinavir, ritonavir, interferon-beta, the RNA polymerase inhibitor remdesivir, and chloroquine.
However, Dr. Handelsman said that, as far as he knows, none of these drugs has been shown to be beneficial for COVID-19. “Some [providers] have tried Tamiflu, but with no clear outcomes, and for severely sick patients, they tried medications for anti-HIV, hepatitis C, and malaria, but so far, there has been no breakthrough.”
Dr. Cohen, Dr. Handelsman, Dr. Jellinger, Dr. Levy, and Dr. Rettinger are members of the editorial advisory board of Clinical Endocrinology News. Dr. Gupta and Dr. Wu, and their colleagues, reported no conflicts of interest.
COVID-19: Extra caution needed for patients with diabetes
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
Patients with diabetes may have an increased risk of developing coronavirus infection (COVID-19), along with increased risks of morbidity and mortality, according to researchers writing in Diabetes & Metabolic Syndrome.
Although relevant clinical data remain scarce, patients with diabetes should take extra precautions to avoid infection and, if infected, may require special care, reported Ritesh Gupta, MD, of Fortis C-DOC Hospital, New Delhi, and colleagues.
“The disease severity [with COVID-19] has varied from mild, self-limiting, flu-like illness to fulminant pneumonia, respiratory failure, and death,” the authors wrote.
As of March 16, 2020, the World Health Organization reported 167,515 confirmed cases of COVID-19 and 6,606 deaths from around the world, with a mortality rate of 3.9%. But the actual mortality rate may be lower, the authors suggested, because a study involving more than 1,000 confirmed cases reported a mortality rate of 1.4%.
“Considering that the number of unreported and unconfirmed cases is likely to be much higher than the reported cases, the actual mortality may be less than 1%, which is similar to that of severe seasonal influenza,” the authors said, in reference to an editorial by Anthony S. Fauci, MD, and colleagues in the New England Journal of Medicine. In addition, they noted, mortality rates may vary by region.
The largest study relevant to patients with diabetes, which involved 72,314 cases of COVID-19, showed that patients with diabetes had a threefold higher mortality rate than did those without diabetes (7.3% vs. 2.3%, respectively). These figures were reported by the Chinese Centre for Disease Control and Prevention.
However, data from smaller cohorts with diabetes and COVID-19 have yielded mixed results. For instance, one study, involving 140 patients from Wuhan, suggested that diabetes was not a risk factor for severe disease, and in an analysis of 11 studies reporting on laboratory abnormalities in patients with a diagnosis of COVID-19, raised blood sugar levels or diabetes were not mentioned among the predictors of severe disease.
“Our knowledge about the prevalence of COVID-19 and disease course in people with diabetes will evolve as more detailed analyses are carried out,” the authors wrote. “For now, it is reasonable to assume that people with diabetes are at increased risk of developing infection. Coexisting heart disease, kidney disease, advanced age, and frailty are likely to further increase the severity of disease.”
Prevention first
“It is important that people with diabetes maintain good glycemic control, because it might help in reducing the risk of infection and the severity,” the authors wrote.
In addition to more frequent monitoring of blood glucose levels, they recommended other preventive measures, such as getting adequate nutrition, exercising, and being current with vaccinations for influenza and pneumonia. The latter, they said, may also reduce the risk of secondary bacterial pneumonia after a respiratory viral infection.
In regard to nutrition, adequate protein intake is important and “any deficiencies of minerals and vitamins need to be taken care of,” they advised. Likewise, exercise is known to improve immunity and should continue, but they suggest avoiding gyms and swimming pools.
For patients with coexisting heart and/or kidney disease, they also recommended efforts to stabilize cardiac/renal status.
In addition, the general preventive measures, such as regular and thorough hand washing with soap and water, practicing good respiratory hygiene by sneezing and coughing into a bent elbow or a facial tissue, and avoiding contact with anyone who is infected, should be observed.
As with other patients with chronic diseases that are managed long-term medications, patients with diabetes should always ensure that they have a sufficient supply of their medications and refills, if possible.
After a diagnosis
If patients with diabetes develop COVID-19, then home management may still be possible, wrote the authors, who recommended basic treatment measures such as maintaining hydration and managing symptoms with acetaminophen and steam inhalation, and home isolation for 14 days or until the symptoms resolve.
In the event of hyperglycemia with fever in patients with type 1 diabetes, blood glucose and urinary ketones should be monitored often. “Frequent changes in dosage and correctional bolus may be required to maintain normoglycemia,” they cautioned.
Concerning diabetic drug regimens, they suggest patients avoid antihyperglycemic agents that can cause volume depletion or hypoglycemia and, if necessary, that they reduce oral antidiabetic drugs and follow sick-day guidelines.
For hospitalized patients, the investigators strengthened that statement, advising that oral agents need to be stopped, particularly sodium-glucose cotransporter 2 inhibitors and metformin. “Insulin is the preferred agent for control of hyperglycemia in hospitalized sick patients,” they wrote.
Untested therapies
The authors also discussed a range of untested therapies that may help fight COVID-19, such as antiviral drugs (such as lopinavir and ritonavir), zinc nanoparticles, and vitamin C. Supplementing those recommendations, Dr. Gupta and colleagues provided a concise review of COVID-19 epidemiology and extant data relevant to patients with diabetes.
The investigators reported no conflicts of interest.
SOURCE: Gupta et al. Diabetes Metab Syndr. 2020;14(3):211-12.
FROM DIABETES & METABOLIC SYNDROME
FDA provides flexibility to improve COVID-19 test availability
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
CDC expert answers top COVID-19 questions
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
Trump to governors: Don’t wait for feds on medical supplies
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
Nearly half of STI events go without HIV testing
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
FROM PEDIATRICS
Stick with the full 12-week DAA course for acute HCV
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
FROM CROI 2020
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
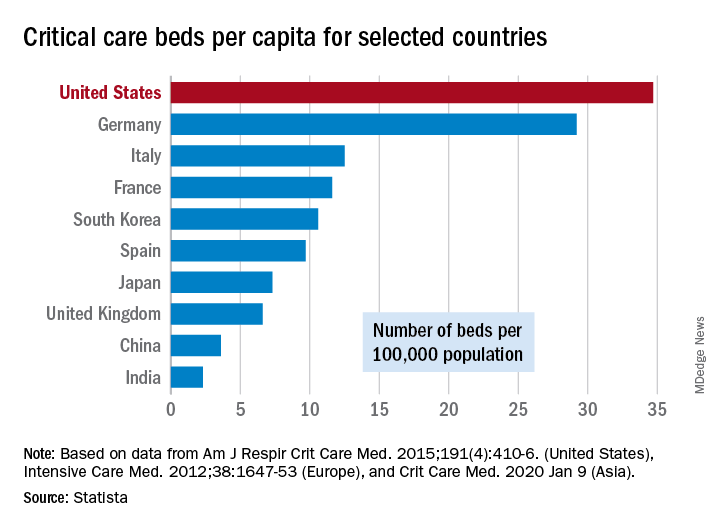
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”