User login
Antibody testing suggests COVID-19 cases are being missed
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.
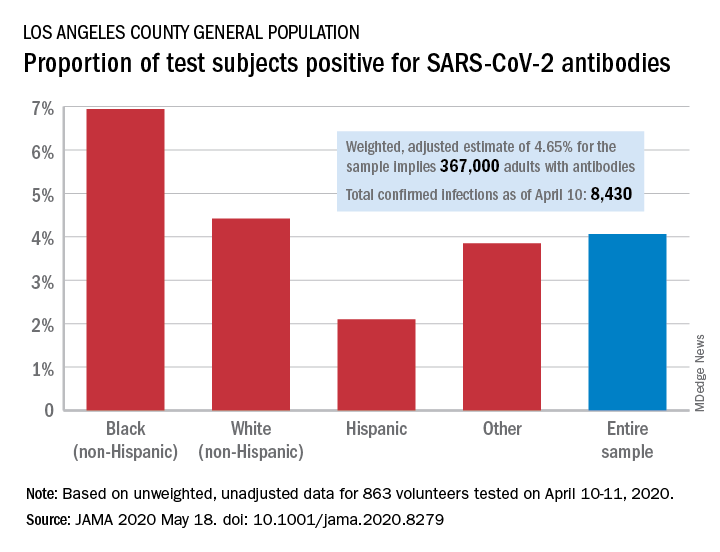
Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
FROM JAMA
COVID-19 in kids: Severe illness most common in infants, teens
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
FROM THE JOURNAL OF PEDIATRICS
Today's top news highlights
Dermatologic changes with COVID-19: What we do and don’t know
From qurantine toes to patients with chilblains, the skin manifestaions of COVID-19 are being seen and documented. "It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?" READ MORE
Glucose control linked to COVID-19 outcomes in largest study yet
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes, according to findings published in Cell Metabolism. “We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li said in a statement. READ MORE
FDA approves pomalidomide for Kaposi sarcoma
The FDA has granted accelerated approval to pomalidomide for the treatment of AIDS-related Kaposi sarcoma that is resistant to HAART or that occurs in HIV-negative patients. Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company. READ MORE
ER docs ask, 'Where are our patients?'
Across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more, noted Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh. He is concerned for a coming “tsunami of untreated illness," adding that "the safest place in the world to be right now is the ED." READ MORE
Obesity can shift severe COVID-19 to younger groups
The younger an ICU patient with severe COVID-19 is, the more obese that patient tends to be, according to a new analysis published in The Lancet. "If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer than your mother or grandparents or others in their 60s or 70s,” noted David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore. READ MORE
Many hydroxychloroquine prophylaxis trials lack ECG screening
As of April 30, 155 randomized, control trials listed on clinicaltrials.gov had designs that intended to randomize a total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis. All three agents potentially produce lengthening of the corrected QT interval (QTc), Michael H. Gollob, MD, said in an article posted by JAAC. If this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob. “It is ... inexcusable that clinical investigators would dare to include healthy individuals ... without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Dermatologic changes with COVID-19: What we do and don’t know
From qurantine toes to patients with chilblains, the skin manifestaions of COVID-19 are being seen and documented. "It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?" READ MORE
Glucose control linked to COVID-19 outcomes in largest study yet
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes, according to findings published in Cell Metabolism. “We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li said in a statement. READ MORE
FDA approves pomalidomide for Kaposi sarcoma
The FDA has granted accelerated approval to pomalidomide for the treatment of AIDS-related Kaposi sarcoma that is resistant to HAART or that occurs in HIV-negative patients. Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company. READ MORE
ER docs ask, 'Where are our patients?'
Across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more, noted Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh. He is concerned for a coming “tsunami of untreated illness," adding that "the safest place in the world to be right now is the ED." READ MORE
Obesity can shift severe COVID-19 to younger groups
The younger an ICU patient with severe COVID-19 is, the more obese that patient tends to be, according to a new analysis published in The Lancet. "If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer than your mother or grandparents or others in their 60s or 70s,” noted David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore. READ MORE
Many hydroxychloroquine prophylaxis trials lack ECG screening
As of April 30, 155 randomized, control trials listed on clinicaltrials.gov had designs that intended to randomize a total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis. All three agents potentially produce lengthening of the corrected QT interval (QTc), Michael H. Gollob, MD, said in an article posted by JAAC. If this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob. “It is ... inexcusable that clinical investigators would dare to include healthy individuals ... without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Dermatologic changes with COVID-19: What we do and don’t know
From qurantine toes to patients with chilblains, the skin manifestaions of COVID-19 are being seen and documented. "It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?" READ MORE
Glucose control linked to COVID-19 outcomes in largest study yet
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes, according to findings published in Cell Metabolism. “We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li said in a statement. READ MORE
FDA approves pomalidomide for Kaposi sarcoma
The FDA has granted accelerated approval to pomalidomide for the treatment of AIDS-related Kaposi sarcoma that is resistant to HAART or that occurs in HIV-negative patients. Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company. READ MORE
ER docs ask, 'Where are our patients?'
Across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more, noted Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh. He is concerned for a coming “tsunami of untreated illness," adding that "the safest place in the world to be right now is the ED." READ MORE
Obesity can shift severe COVID-19 to younger groups
The younger an ICU patient with severe COVID-19 is, the more obese that patient tends to be, according to a new analysis published in The Lancet. "If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer than your mother or grandparents or others in their 60s or 70s,” noted David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore. READ MORE
Many hydroxychloroquine prophylaxis trials lack ECG screening
As of April 30, 155 randomized, control trials listed on clinicaltrials.gov had designs that intended to randomize a total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis. All three agents potentially produce lengthening of the corrected QT interval (QTc), Michael H. Gollob, MD, said in an article posted by JAAC. If this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob. “It is ... inexcusable that clinical investigators would dare to include healthy individuals ... without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
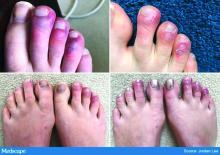
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
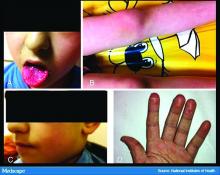
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
Hazard pay included in new COVID-19 relief bill
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Summary of the IDSA guidelines on the diagnosis of COVID-19
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
Even with mild COVID-19, athletes need cardiac testing before returning to play
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
FROM JAMA CARDIOLOGY
Comparing COVID-19, flu death tolls ‘extremely dangerous’
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
Masks, fear, and loss of connection in the era of COVID-19
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Doctors advise asthmatics to continue therapy during pandemic
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.