User login
Shoulder Pain After a Cycling Accident
Traumatic Posterior Hip Instability and Femoroacetabular Impingement in Athletes
Cardiac CT Trims Triage Time for ACS, but Not Costs
CHICAGO – Triaging chest-pain patients with cardiac CT angiography increased direct emergency room discharges and shortened hospital stays, but did not reduce costs, compared with a standard emergency department evaluation, in the prospective, randomized ROMICAT-II trial.
There were no missed cases of acute coronary syndrome with either strategy in ROMICAT II, the Rule Out Myocardial Infarction using Computer Assisted Computed Tomography II trial.
Incorporating cardiac computed tomography angiography (CCTA) early into an emergency department (ED) evaluation strategy improves clinical decision making for ED triage compared to current practice and is safe, principal investigator Dr. Udo Hoffmann said at the annual meeting of the American College of Cardiology.
ROMICAT II evenly randomized 1,000 patients, aged 40-74 years, with suspected acute coronary syndrome to the CCTA-based strategy or standard ED evaluation. Their mean age was 54 years and half had 2-3 major cardiovascular risk factors.
The average time to diagnosis was shortened by 8 hours with CCTA from 18.7 hours with a standard evaluation to 10.4 hours. A diagnosis could not be made in 4% of CT scans.
The average length of stay, the study’s primary end point, was significantly shorter at 23 hours vs. 31 hours with a standard evaluation. This was driven largely by patients without ACS as a final diagnosis, who averaged 17 hours in the hospital with CCTA vs. 27 hours with a standard evaluation, said Dr. Hoffmann, a cardiac radiologist at Massachusetts General Hospital in Boston.
Patients with an ACS diagnosis had similar lengths of stay, averaging 86.3 hours vs. 84 hours, respectively. ROMICAT I reported an 8% prevalence of ACS in acute chest-pain patients at low to intermediate risk of ACS.
"An interesting data point here is that it took about 8 hours to discharge 50% of patients in the CT arm, while it took about 18 hours longer to discharge 50% of patients in the standard-of-care arm," he said.
The number CCTA patients discharged directly from the emergency department was triple that of the standard-evaluation group (46.7% vs. 12.4%), with no missed ACS. At 28 days, major adverse cardiovascular events were similar, reported in two CCTA and five standard-care patients. In all, 13 CCTA patients and 19 standard-care patients returned to the ED, with seven repeat hospitalizations in both groups.
Invited discussant Dr. Matthew Budoff, director of the University of California, Los Angeles BioMed CT Reading Center, observed that similar trends were just reported at the meeting from the American College of Radiology Imaging Network PA 4005 trial and were reported last year from the CT-STAT (Computed Tomography for Systematic Triage of Acute Chest Pain Patients to Treatment trial. (J. Am. Coll. Cardiol. 2011;58:1414-22).
"We’re now garnering significant clinical evidence with three randomized trials that are all very consistent," he said.
Financial Costs
While cost analyses are still awaited from American College of Radiology Imaging Network PA 4005, hospital billing data from a subset of patients in ROMICAT II showed that ED costs with the CCTA strategy were significantly lower, at 19% less per patient at a mean of $2,053 vs. $2,532 with a standard evaluation, Dr. Hoffmann reported. Hospital costs, however, were 50% higher with the CCTA strategy ($1,950 vs. $1,297), driven largely by significantly more diagnostic testing during the index stay and more invasive coronary angiography (12% vs. 8%).
Dr. Hoffmann said Medicare data have shown a doubling in procedures and costs after CCTA. He described the absence of an increase in costs as "a major step forward," but also acknowledged "there’s always room for improvement."
He went on to say that CCTA may become more cost effective as technology evolves and the effectiveness of CT increases and as emergency room experience with CCTA improves. ROMICAT II was conducted at centers without ED CCTA experience, and required training by Dr. Hoffmann and a coauthor was not included in the cost analysis, he acknowledged.
Dr. Michael Crawford, professor of medicine and chief of clinical cardiology at the University of California, San Francisco Medical Center, pointed out that CCTA use in ROMICAT II was very contained since enrollment was limited to patients coming to the emergency room on weekday business hours only.
"So if you expand this to 24/7 coverage, the cost has to go up," he told reporters. "The question of what you save downstream by avoiding further tests remains to be shown in the future."
Radiation Costs
Invited discussant Dr. Elliott Antman expressed concerns about the cost of radiation exposure, noting that there was roughly a threefold increase in the cumulative radiation dose with the CCTA strategy (14.3 5.3 mSv vs. 5.3 mSv), and that chest-pain patients often present to different emergency departments.
"If in fact one adopts a strategy using cardiac CT, there is the potential for patients to have repeated exposures," said Dr. Antman, professor of medicine at Harvard University and senior faculty member in the cardiovascular division at Brigham and Women’s Hospital in Boston.
Dr. Hoffmann said there is a cost to performing a CT and to being able to discharge patients early, but also noted the collateral effect early discharge of chest-pain patients can have on getting other patients seen in the emergency room.
Dr. Budoff commented that there have been less repeat visits with CCTA in the three trials to date, and thus if one looked at cumulative radiation exposure over the subsequent year, "it might actually start to balance a little better."
This study was sponsored by Massachusetts General Hospital. Dr. Hoffmann reported research grants from the National Institutes of Health and Siemens Medical Systems. Five of his coauthors reported consulting and research funding from a variety of sources.
CHICAGO – Triaging chest-pain patients with cardiac CT angiography increased direct emergency room discharges and shortened hospital stays, but did not reduce costs, compared with a standard emergency department evaluation, in the prospective, randomized ROMICAT-II trial.
There were no missed cases of acute coronary syndrome with either strategy in ROMICAT II, the Rule Out Myocardial Infarction using Computer Assisted Computed Tomography II trial.
Incorporating cardiac computed tomography angiography (CCTA) early into an emergency department (ED) evaluation strategy improves clinical decision making for ED triage compared to current practice and is safe, principal investigator Dr. Udo Hoffmann said at the annual meeting of the American College of Cardiology.
ROMICAT II evenly randomized 1,000 patients, aged 40-74 years, with suspected acute coronary syndrome to the CCTA-based strategy or standard ED evaluation. Their mean age was 54 years and half had 2-3 major cardiovascular risk factors.
The average time to diagnosis was shortened by 8 hours with CCTA from 18.7 hours with a standard evaluation to 10.4 hours. A diagnosis could not be made in 4% of CT scans.
The average length of stay, the study’s primary end point, was significantly shorter at 23 hours vs. 31 hours with a standard evaluation. This was driven largely by patients without ACS as a final diagnosis, who averaged 17 hours in the hospital with CCTA vs. 27 hours with a standard evaluation, said Dr. Hoffmann, a cardiac radiologist at Massachusetts General Hospital in Boston.
Patients with an ACS diagnosis had similar lengths of stay, averaging 86.3 hours vs. 84 hours, respectively. ROMICAT I reported an 8% prevalence of ACS in acute chest-pain patients at low to intermediate risk of ACS.
"An interesting data point here is that it took about 8 hours to discharge 50% of patients in the CT arm, while it took about 18 hours longer to discharge 50% of patients in the standard-of-care arm," he said.
The number CCTA patients discharged directly from the emergency department was triple that of the standard-evaluation group (46.7% vs. 12.4%), with no missed ACS. At 28 days, major adverse cardiovascular events were similar, reported in two CCTA and five standard-care patients. In all, 13 CCTA patients and 19 standard-care patients returned to the ED, with seven repeat hospitalizations in both groups.
Invited discussant Dr. Matthew Budoff, director of the University of California, Los Angeles BioMed CT Reading Center, observed that similar trends were just reported at the meeting from the American College of Radiology Imaging Network PA 4005 trial and were reported last year from the CT-STAT (Computed Tomography for Systematic Triage of Acute Chest Pain Patients to Treatment trial. (J. Am. Coll. Cardiol. 2011;58:1414-22).
"We’re now garnering significant clinical evidence with three randomized trials that are all very consistent," he said.
Financial Costs
While cost analyses are still awaited from American College of Radiology Imaging Network PA 4005, hospital billing data from a subset of patients in ROMICAT II showed that ED costs with the CCTA strategy were significantly lower, at 19% less per patient at a mean of $2,053 vs. $2,532 with a standard evaluation, Dr. Hoffmann reported. Hospital costs, however, were 50% higher with the CCTA strategy ($1,950 vs. $1,297), driven largely by significantly more diagnostic testing during the index stay and more invasive coronary angiography (12% vs. 8%).
Dr. Hoffmann said Medicare data have shown a doubling in procedures and costs after CCTA. He described the absence of an increase in costs as "a major step forward," but also acknowledged "there’s always room for improvement."
He went on to say that CCTA may become more cost effective as technology evolves and the effectiveness of CT increases and as emergency room experience with CCTA improves. ROMICAT II was conducted at centers without ED CCTA experience, and required training by Dr. Hoffmann and a coauthor was not included in the cost analysis, he acknowledged.
Dr. Michael Crawford, professor of medicine and chief of clinical cardiology at the University of California, San Francisco Medical Center, pointed out that CCTA use in ROMICAT II was very contained since enrollment was limited to patients coming to the emergency room on weekday business hours only.
"So if you expand this to 24/7 coverage, the cost has to go up," he told reporters. "The question of what you save downstream by avoiding further tests remains to be shown in the future."
Radiation Costs
Invited discussant Dr. Elliott Antman expressed concerns about the cost of radiation exposure, noting that there was roughly a threefold increase in the cumulative radiation dose with the CCTA strategy (14.3 5.3 mSv vs. 5.3 mSv), and that chest-pain patients often present to different emergency departments.
"If in fact one adopts a strategy using cardiac CT, there is the potential for patients to have repeated exposures," said Dr. Antman, professor of medicine at Harvard University and senior faculty member in the cardiovascular division at Brigham and Women’s Hospital in Boston.
Dr. Hoffmann said there is a cost to performing a CT and to being able to discharge patients early, but also noted the collateral effect early discharge of chest-pain patients can have on getting other patients seen in the emergency room.
Dr. Budoff commented that there have been less repeat visits with CCTA in the three trials to date, and thus if one looked at cumulative radiation exposure over the subsequent year, "it might actually start to balance a little better."
This study was sponsored by Massachusetts General Hospital. Dr. Hoffmann reported research grants from the National Institutes of Health and Siemens Medical Systems. Five of his coauthors reported consulting and research funding from a variety of sources.
CHICAGO – Triaging chest-pain patients with cardiac CT angiography increased direct emergency room discharges and shortened hospital stays, but did not reduce costs, compared with a standard emergency department evaluation, in the prospective, randomized ROMICAT-II trial.
There were no missed cases of acute coronary syndrome with either strategy in ROMICAT II, the Rule Out Myocardial Infarction using Computer Assisted Computed Tomography II trial.
Incorporating cardiac computed tomography angiography (CCTA) early into an emergency department (ED) evaluation strategy improves clinical decision making for ED triage compared to current practice and is safe, principal investigator Dr. Udo Hoffmann said at the annual meeting of the American College of Cardiology.
ROMICAT II evenly randomized 1,000 patients, aged 40-74 years, with suspected acute coronary syndrome to the CCTA-based strategy or standard ED evaluation. Their mean age was 54 years and half had 2-3 major cardiovascular risk factors.
The average time to diagnosis was shortened by 8 hours with CCTA from 18.7 hours with a standard evaluation to 10.4 hours. A diagnosis could not be made in 4% of CT scans.
The average length of stay, the study’s primary end point, was significantly shorter at 23 hours vs. 31 hours with a standard evaluation. This was driven largely by patients without ACS as a final diagnosis, who averaged 17 hours in the hospital with CCTA vs. 27 hours with a standard evaluation, said Dr. Hoffmann, a cardiac radiologist at Massachusetts General Hospital in Boston.
Patients with an ACS diagnosis had similar lengths of stay, averaging 86.3 hours vs. 84 hours, respectively. ROMICAT I reported an 8% prevalence of ACS in acute chest-pain patients at low to intermediate risk of ACS.
"An interesting data point here is that it took about 8 hours to discharge 50% of patients in the CT arm, while it took about 18 hours longer to discharge 50% of patients in the standard-of-care arm," he said.
The number CCTA patients discharged directly from the emergency department was triple that of the standard-evaluation group (46.7% vs. 12.4%), with no missed ACS. At 28 days, major adverse cardiovascular events were similar, reported in two CCTA and five standard-care patients. In all, 13 CCTA patients and 19 standard-care patients returned to the ED, with seven repeat hospitalizations in both groups.
Invited discussant Dr. Matthew Budoff, director of the University of California, Los Angeles BioMed CT Reading Center, observed that similar trends were just reported at the meeting from the American College of Radiology Imaging Network PA 4005 trial and were reported last year from the CT-STAT (Computed Tomography for Systematic Triage of Acute Chest Pain Patients to Treatment trial. (J. Am. Coll. Cardiol. 2011;58:1414-22).
"We’re now garnering significant clinical evidence with three randomized trials that are all very consistent," he said.
Financial Costs
While cost analyses are still awaited from American College of Radiology Imaging Network PA 4005, hospital billing data from a subset of patients in ROMICAT II showed that ED costs with the CCTA strategy were significantly lower, at 19% less per patient at a mean of $2,053 vs. $2,532 with a standard evaluation, Dr. Hoffmann reported. Hospital costs, however, were 50% higher with the CCTA strategy ($1,950 vs. $1,297), driven largely by significantly more diagnostic testing during the index stay and more invasive coronary angiography (12% vs. 8%).
Dr. Hoffmann said Medicare data have shown a doubling in procedures and costs after CCTA. He described the absence of an increase in costs as "a major step forward," but also acknowledged "there’s always room for improvement."
He went on to say that CCTA may become more cost effective as technology evolves and the effectiveness of CT increases and as emergency room experience with CCTA improves. ROMICAT II was conducted at centers without ED CCTA experience, and required training by Dr. Hoffmann and a coauthor was not included in the cost analysis, he acknowledged.
Dr. Michael Crawford, professor of medicine and chief of clinical cardiology at the University of California, San Francisco Medical Center, pointed out that CCTA use in ROMICAT II was very contained since enrollment was limited to patients coming to the emergency room on weekday business hours only.
"So if you expand this to 24/7 coverage, the cost has to go up," he told reporters. "The question of what you save downstream by avoiding further tests remains to be shown in the future."
Radiation Costs
Invited discussant Dr. Elliott Antman expressed concerns about the cost of radiation exposure, noting that there was roughly a threefold increase in the cumulative radiation dose with the CCTA strategy (14.3 5.3 mSv vs. 5.3 mSv), and that chest-pain patients often present to different emergency departments.
"If in fact one adopts a strategy using cardiac CT, there is the potential for patients to have repeated exposures," said Dr. Antman, professor of medicine at Harvard University and senior faculty member in the cardiovascular division at Brigham and Women’s Hospital in Boston.
Dr. Hoffmann said there is a cost to performing a CT and to being able to discharge patients early, but also noted the collateral effect early discharge of chest-pain patients can have on getting other patients seen in the emergency room.
Dr. Budoff commented that there have been less repeat visits with CCTA in the three trials to date, and thus if one looked at cumulative radiation exposure over the subsequent year, "it might actually start to balance a little better."
This study was sponsored by Massachusetts General Hospital. Dr. Hoffmann reported research grants from the National Institutes of Health and Siemens Medical Systems. Five of his coauthors reported consulting and research funding from a variety of sources.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: The average length of stay was 23.2 hours with cardiac CT angiography screening and 30.8 hours with a standard evaluation.
Data Source: ROMICAT II, a multicenter, randomized comparative-effectiveness trial, included 1,000 chest-pain patients presenting to the ED with suspected acute coronary syndrome.
Disclosures: This study was sponsored by Massachusetts General Hospital. Dr. Hoffmann reported research grants from the National Institutes of Health and Siemens Medical Systems. Five of his coauthors reported consulting and research funding from a variety of sources.
Trial Supports Cardiac CT for Acute Chest Pain
CHICAGO – Cardiac CT angiography in the emergency department safely redirects to home the many patients who would otherwise be admitted for acute chest pain, according to results of the prospective, randomized ACRIN PA 4005 trial.
Low- to intermediate-risk patients who receive cardiac computed tomographic angiography (CCTA) were more likely to be discharged directly from the emergency department (ED), to have shorter hospital stays, and to have more than double the coronary artery disease (CAD) diagnosed than were those receiving a traditional evaluation.
Moreover, none of the 640 patients who had a negative CCTA died or had a myocardial infarction within 30 days after presentation (95% confidence interval, 0-0.57), Dr. Harold Litt, principal investigator of ACRIN (American College of Radiology Imaging Network) PA 4005, said at the annual meeting of the American College of Cardiology.
The upper limit of the confidence interval met the study’s prespecified safety threshold of less than 1%, and may be robust enough to help sway ED physicians who have been unwilling to adopt a CT-based strategy because similar findings from other randomized trials were not sufficiently powered.
"This is a large public health problem," Dr. Litt said, noting that roughly 2%-3% of patients are discharged from the ED with an unrecognized MI.
Conversely, more than 6 million Americans visit the ED for chest pain each year. Only 10%-15% are ultimately diagnosed with acute coronary syndrome (ACS), with most admitted to hospitals at a staggering cost of more than $3 billion annually.
The ACRIN PA 4005 trial randomized 1,370 patients with symptoms consistent with possible ACS from five clinical sites to undergo at least 64-slice CCTA or traditional evaluation, comprising mostly – but not limited to – exercise treadmill test, stress test with imaging, and stress echocardiography. They had an average TIMI (Thrombolysis in Myocardial Infarction) risk score of 0-2 and an electrocardiogram without acute ischemia. Their average age was about 50 years, and 60% were black.
Half of the 908 CCTA patients were discharged directly from the ED, compared with 23% of the 642 traditional-care patients (95% CI, 21.4-32.2).
The overall length of stay was 18 hours and 25 hours, respectively, but decreased even further to 12 hours for the 602 CCTA patients who had a negative scan, said Dr. Litt, chief of cardiovascular imaging at the University of Pennsylvania Health System in Philadelphia.
The CCTA group was also less likely than was the traditional-care group to have negative findings on invasive angiography (29% vs. 53%; 95% CI, –48.8-3.3).
The finding of more incidental CAD diagnoses in the CCTA arm vs. the traditional-care arm (9% vs. 3.5%; 95% CI, 0-11.2) is more problematic to interpret.
"Will this result in better prevention for them as they go on?" he asked. "Will they be encouraged to have lifestyle modifications and be put on statins, etc., resulting in lower future event rates and not showing up in the emergency room? Or will it just result in more testing that won’t be a benefit to them? We don’t know the answer to that."
No significant differences were observed in a 30-day resource utilization that included catheterization, revascularization, repeat ED visit, rehospitalization, and cardiologist visit. A 1-year follow-up is being obtained, and cost modeling will be conducted, he said. The possibility for substantial health care savings exists, however, as low- to intermediate-risk patients account for 50%-70% of cases presenting with possible ACS.
Overall, MI was reported within 30 days after presentation in 10 CCTA patientsand 5 traditional-care patients (1% vs. 11%; CI, –5.6-5.7). One serious adverse event (bradyarrhythmia) occurred in each group. There were no cardiac deaths in the traditional-care group.
Dr. Litt acknowledged that CCTA does increase radiation exposure, but said that radiation dosage is very technology dependent and that current technology has reduced the average radiation dose to less than that from nuclear myocardial perfusion studies. He also cautioned that the ACRIN PA 4005 results should not be extrapolated to groups with a higher risk of clinically significant coronary disease.
Invited discussant Dr. Thomas Gerber, a professor of medicine and radiology at Mayo Clinic in Jacksonville, Fla., asked why the investigators chose to focus on coronary CT angiography instead of the "triple rule-out" CT angiography strategy to evaluate the coronary arteries, pulmonary arteries, and thoracic aorta, and whether there were any patients who had pulmonary embolism or aortic dissection on subsequent evaluation.
Dr. Litt said they did track PE and acute aortic syndromes, and will report these findings in the future. The investigators used CCTA because they wanted to focus on patients in whom exclusion of ACS was the primary diagnostic question. He acknowledged that not all dissections are visible on CT, but added that "we are getting to the point where the radiology dose from a triple rule-out isn’t all that much higher than from a coronary CT. So in light of that new technology, that question may need to be reevaluated."
In a separate interview, Dr. James G. Adams, professor and chair of emergency medicine at Northwestern University in Chicago, said that no test is perfect, that all the evidence shows that coronary CT is at least as good as other testing strategies used in the initial evaluation of patients with acute chest pain, and that there are fewer hospital admissions.
"This [study] will certainly be used to promote coronary CT for patients at low to moderate risk of coronary disease," he said. "I believe that emergency physicians will increase their use."
Adoption of the CCTA approach depends on much more than whether emergency physicians find the results convincing, emergency physician Dr. Robert Solomon of Allegheny General Hospital in Pittsburgh said in an interview.
"Cardiology and radiology must also buy into it, and the resources necessary to enable clinicians to use this approach must be made available," he said. "This includes cardiologists, radiologists, and trained technologists. The necessary resources will not be available 24-7, even at tertiary care centers, so timing will always be an issue."
This study was sponsored by the Commonwealth of Pennsylvania Department of Health and the American Radiology Imaging Network Foundation. Dr. Litt reported grant funding and travel reimbursement from Siemens Medical Solutions and consulting fees from Medrad-Bayer. The study was simultaneously published online in the New England Journal of Medicine (2012 March 26 [doi:10.1056/NEJMoa1201163]).
CHICAGO – Cardiac CT angiography in the emergency department safely redirects to home the many patients who would otherwise be admitted for acute chest pain, according to results of the prospective, randomized ACRIN PA 4005 trial.
Low- to intermediate-risk patients who receive cardiac computed tomographic angiography (CCTA) were more likely to be discharged directly from the emergency department (ED), to have shorter hospital stays, and to have more than double the coronary artery disease (CAD) diagnosed than were those receiving a traditional evaluation.
Moreover, none of the 640 patients who had a negative CCTA died or had a myocardial infarction within 30 days after presentation (95% confidence interval, 0-0.57), Dr. Harold Litt, principal investigator of ACRIN (American College of Radiology Imaging Network) PA 4005, said at the annual meeting of the American College of Cardiology.
The upper limit of the confidence interval met the study’s prespecified safety threshold of less than 1%, and may be robust enough to help sway ED physicians who have been unwilling to adopt a CT-based strategy because similar findings from other randomized trials were not sufficiently powered.
"This is a large public health problem," Dr. Litt said, noting that roughly 2%-3% of patients are discharged from the ED with an unrecognized MI.
Conversely, more than 6 million Americans visit the ED for chest pain each year. Only 10%-15% are ultimately diagnosed with acute coronary syndrome (ACS), with most admitted to hospitals at a staggering cost of more than $3 billion annually.
The ACRIN PA 4005 trial randomized 1,370 patients with symptoms consistent with possible ACS from five clinical sites to undergo at least 64-slice CCTA or traditional evaluation, comprising mostly – but not limited to – exercise treadmill test, stress test with imaging, and stress echocardiography. They had an average TIMI (Thrombolysis in Myocardial Infarction) risk score of 0-2 and an electrocardiogram without acute ischemia. Their average age was about 50 years, and 60% were black.
Half of the 908 CCTA patients were discharged directly from the ED, compared with 23% of the 642 traditional-care patients (95% CI, 21.4-32.2).
The overall length of stay was 18 hours and 25 hours, respectively, but decreased even further to 12 hours for the 602 CCTA patients who had a negative scan, said Dr. Litt, chief of cardiovascular imaging at the University of Pennsylvania Health System in Philadelphia.
The CCTA group was also less likely than was the traditional-care group to have negative findings on invasive angiography (29% vs. 53%; 95% CI, –48.8-3.3).
The finding of more incidental CAD diagnoses in the CCTA arm vs. the traditional-care arm (9% vs. 3.5%; 95% CI, 0-11.2) is more problematic to interpret.
"Will this result in better prevention for them as they go on?" he asked. "Will they be encouraged to have lifestyle modifications and be put on statins, etc., resulting in lower future event rates and not showing up in the emergency room? Or will it just result in more testing that won’t be a benefit to them? We don’t know the answer to that."
No significant differences were observed in a 30-day resource utilization that included catheterization, revascularization, repeat ED visit, rehospitalization, and cardiologist visit. A 1-year follow-up is being obtained, and cost modeling will be conducted, he said. The possibility for substantial health care savings exists, however, as low- to intermediate-risk patients account for 50%-70% of cases presenting with possible ACS.
Overall, MI was reported within 30 days after presentation in 10 CCTA patientsand 5 traditional-care patients (1% vs. 11%; CI, –5.6-5.7). One serious adverse event (bradyarrhythmia) occurred in each group. There were no cardiac deaths in the traditional-care group.
Dr. Litt acknowledged that CCTA does increase radiation exposure, but said that radiation dosage is very technology dependent and that current technology has reduced the average radiation dose to less than that from nuclear myocardial perfusion studies. He also cautioned that the ACRIN PA 4005 results should not be extrapolated to groups with a higher risk of clinically significant coronary disease.
Invited discussant Dr. Thomas Gerber, a professor of medicine and radiology at Mayo Clinic in Jacksonville, Fla., asked why the investigators chose to focus on coronary CT angiography instead of the "triple rule-out" CT angiography strategy to evaluate the coronary arteries, pulmonary arteries, and thoracic aorta, and whether there were any patients who had pulmonary embolism or aortic dissection on subsequent evaluation.
Dr. Litt said they did track PE and acute aortic syndromes, and will report these findings in the future. The investigators used CCTA because they wanted to focus on patients in whom exclusion of ACS was the primary diagnostic question. He acknowledged that not all dissections are visible on CT, but added that "we are getting to the point where the radiology dose from a triple rule-out isn’t all that much higher than from a coronary CT. So in light of that new technology, that question may need to be reevaluated."
In a separate interview, Dr. James G. Adams, professor and chair of emergency medicine at Northwestern University in Chicago, said that no test is perfect, that all the evidence shows that coronary CT is at least as good as other testing strategies used in the initial evaluation of patients with acute chest pain, and that there are fewer hospital admissions.
"This [study] will certainly be used to promote coronary CT for patients at low to moderate risk of coronary disease," he said. "I believe that emergency physicians will increase their use."
Adoption of the CCTA approach depends on much more than whether emergency physicians find the results convincing, emergency physician Dr. Robert Solomon of Allegheny General Hospital in Pittsburgh said in an interview.
"Cardiology and radiology must also buy into it, and the resources necessary to enable clinicians to use this approach must be made available," he said. "This includes cardiologists, radiologists, and trained technologists. The necessary resources will not be available 24-7, even at tertiary care centers, so timing will always be an issue."
This study was sponsored by the Commonwealth of Pennsylvania Department of Health and the American Radiology Imaging Network Foundation. Dr. Litt reported grant funding and travel reimbursement from Siemens Medical Solutions and consulting fees from Medrad-Bayer. The study was simultaneously published online in the New England Journal of Medicine (2012 March 26 [doi:10.1056/NEJMoa1201163]).
CHICAGO – Cardiac CT angiography in the emergency department safely redirects to home the many patients who would otherwise be admitted for acute chest pain, according to results of the prospective, randomized ACRIN PA 4005 trial.
Low- to intermediate-risk patients who receive cardiac computed tomographic angiography (CCTA) were more likely to be discharged directly from the emergency department (ED), to have shorter hospital stays, and to have more than double the coronary artery disease (CAD) diagnosed than were those receiving a traditional evaluation.
Moreover, none of the 640 patients who had a negative CCTA died or had a myocardial infarction within 30 days after presentation (95% confidence interval, 0-0.57), Dr. Harold Litt, principal investigator of ACRIN (American College of Radiology Imaging Network) PA 4005, said at the annual meeting of the American College of Cardiology.
The upper limit of the confidence interval met the study’s prespecified safety threshold of less than 1%, and may be robust enough to help sway ED physicians who have been unwilling to adopt a CT-based strategy because similar findings from other randomized trials were not sufficiently powered.
"This is a large public health problem," Dr. Litt said, noting that roughly 2%-3% of patients are discharged from the ED with an unrecognized MI.
Conversely, more than 6 million Americans visit the ED for chest pain each year. Only 10%-15% are ultimately diagnosed with acute coronary syndrome (ACS), with most admitted to hospitals at a staggering cost of more than $3 billion annually.
The ACRIN PA 4005 trial randomized 1,370 patients with symptoms consistent with possible ACS from five clinical sites to undergo at least 64-slice CCTA or traditional evaluation, comprising mostly – but not limited to – exercise treadmill test, stress test with imaging, and stress echocardiography. They had an average TIMI (Thrombolysis in Myocardial Infarction) risk score of 0-2 and an electrocardiogram without acute ischemia. Their average age was about 50 years, and 60% were black.
Half of the 908 CCTA patients were discharged directly from the ED, compared with 23% of the 642 traditional-care patients (95% CI, 21.4-32.2).
The overall length of stay was 18 hours and 25 hours, respectively, but decreased even further to 12 hours for the 602 CCTA patients who had a negative scan, said Dr. Litt, chief of cardiovascular imaging at the University of Pennsylvania Health System in Philadelphia.
The CCTA group was also less likely than was the traditional-care group to have negative findings on invasive angiography (29% vs. 53%; 95% CI, –48.8-3.3).
The finding of more incidental CAD diagnoses in the CCTA arm vs. the traditional-care arm (9% vs. 3.5%; 95% CI, 0-11.2) is more problematic to interpret.
"Will this result in better prevention for them as they go on?" he asked. "Will they be encouraged to have lifestyle modifications and be put on statins, etc., resulting in lower future event rates and not showing up in the emergency room? Or will it just result in more testing that won’t be a benefit to them? We don’t know the answer to that."
No significant differences were observed in a 30-day resource utilization that included catheterization, revascularization, repeat ED visit, rehospitalization, and cardiologist visit. A 1-year follow-up is being obtained, and cost modeling will be conducted, he said. The possibility for substantial health care savings exists, however, as low- to intermediate-risk patients account for 50%-70% of cases presenting with possible ACS.
Overall, MI was reported within 30 days after presentation in 10 CCTA patientsand 5 traditional-care patients (1% vs. 11%; CI, –5.6-5.7). One serious adverse event (bradyarrhythmia) occurred in each group. There were no cardiac deaths in the traditional-care group.
Dr. Litt acknowledged that CCTA does increase radiation exposure, but said that radiation dosage is very technology dependent and that current technology has reduced the average radiation dose to less than that from nuclear myocardial perfusion studies. He also cautioned that the ACRIN PA 4005 results should not be extrapolated to groups with a higher risk of clinically significant coronary disease.
Invited discussant Dr. Thomas Gerber, a professor of medicine and radiology at Mayo Clinic in Jacksonville, Fla., asked why the investigators chose to focus on coronary CT angiography instead of the "triple rule-out" CT angiography strategy to evaluate the coronary arteries, pulmonary arteries, and thoracic aorta, and whether there were any patients who had pulmonary embolism or aortic dissection on subsequent evaluation.
Dr. Litt said they did track PE and acute aortic syndromes, and will report these findings in the future. The investigators used CCTA because they wanted to focus on patients in whom exclusion of ACS was the primary diagnostic question. He acknowledged that not all dissections are visible on CT, but added that "we are getting to the point where the radiology dose from a triple rule-out isn’t all that much higher than from a coronary CT. So in light of that new technology, that question may need to be reevaluated."
In a separate interview, Dr. James G. Adams, professor and chair of emergency medicine at Northwestern University in Chicago, said that no test is perfect, that all the evidence shows that coronary CT is at least as good as other testing strategies used in the initial evaluation of patients with acute chest pain, and that there are fewer hospital admissions.
"This [study] will certainly be used to promote coronary CT for patients at low to moderate risk of coronary disease," he said. "I believe that emergency physicians will increase their use."
Adoption of the CCTA approach depends on much more than whether emergency physicians find the results convincing, emergency physician Dr. Robert Solomon of Allegheny General Hospital in Pittsburgh said in an interview.
"Cardiology and radiology must also buy into it, and the resources necessary to enable clinicians to use this approach must be made available," he said. "This includes cardiologists, radiologists, and trained technologists. The necessary resources will not be available 24-7, even at tertiary care centers, so timing will always be an issue."
This study was sponsored by the Commonwealth of Pennsylvania Department of Health and the American Radiology Imaging Network Foundation. Dr. Litt reported grant funding and travel reimbursement from Siemens Medical Solutions and consulting fees from Medrad-Bayer. The study was simultaneously published online in the New England Journal of Medicine (2012 March 26 [doi:10.1056/NEJMoa1201163]).
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: None of 640 patients who had been cleared with a negative cardiac CT angiogram died or had an MI within 30 days after ED presentation.
Data Source: Data are from a prospective, randomized trial of 1,370 low- to intermediate-risk patients presenting to the ED with potential acute coronary syndromes.
Disclosures: This study was sponsored by the Commonwealth of Pennsylvania Department of Health and the American Radiology Imaging Network Foundation. Dr. Litt reported consulting fees from Medrad-Bayer and grant funding and travel reimbursement from Siemens Medical Solutions.
Primary Hyperparathyroidism May Be Misdiagnosed
MADISON, WIS. – Nearly one-fifth of all patients referred for parathyroidectomy were misdiagnosed with primary hyperparathyroidism in a retrospective study of 324 consecutive patients.
Notably, many of these patients had undergone comprehensive work-ups prior to surgical referral that clearly diagnosed secondary hyperparathyroidism.
"This demonstrates that there’s a prevalent misunderstanding of parathyroid pathophysiology," Dr. James Iannuzzi said at the annual meeting of the Central Surgical Association. "In particular, vitamin D deficiency accounted for the majority of misdiagnosis."
Using ICD-9 codes for hyperparathyroidism, benign, or malignant parathyroid tumors or hypercalcemia, the authors identified 381 patients who were referred to the endocrine surgery division at the University of Rochester (N.Y.) Medical Center between 2008 and 2011 for parathyroidectomy. Primary hyperparathyroidism (HPT) was defined by a calcium level of at least 10 mg/dL plus a parathyroid hormone (PTH) level of more than 50 pg/mL. Thirty patients were excluded for renal failure and 27, for coding errors.
Among the remaining 324 patients, 264 were correctly diagnosed and 60 (18.5%) were misdiagnosed with primary HPT, said Dr. Iannuzzi, a general surgery resident at the university. Fifty-four (90%) of the misdiagnosed patients had secondary HPT at the time of the referral. For 43 patients, this was clear at the time of referral, and 11 had secondary HPT as their sole diagnosis after follow-up. Six patients had hypercalcemia but not HPT. Half of the hypercalcemic patients were referred after a single spuriously elevated calcium level, only to have their calcium drop to normal levels after follow-up, he said.
Most of the 54 patients with secondary HPT had vitamin D deficiency (37 patients). Other causes were gastric bypass (4 patients), celiac disease (2 patients), and unclear etiology (11 patients). Mean calcium and PTH levels among patients with secondary HPT were 9.3 mg/dL and 92 pg/mL, respectively.
In all, 42 (70%) of the 60 misdiagnosed patients underwent inappropriate localized imaging, of which 57% were falsely positive.
"These studies are not indicated; likely to be falsely positive, as we show; and cause patients and referring physicians to more aggressively push for unindicated and potentially harmful surgery because of a finding on a scan," Dr. Jacob Moalem said in an interview. Dr. Moalem, an endocrine surgeon at the University of Rochester, was the senior author of the paper.
Part of the problem is that ultrasound is highly user dependent, and sonographers can be easily misled by exophytic nodules, hypoechoic structures that were subcentimeters, or lymph nodes. Sestamibi scans in this clinical setting also are likely to be positive because of the activated mitochondria. Thus, it is very important that the pretest diagnosis be correct, Dr. Moalem and Dr. Iannuzzi stressed.
Many patients were found to have an elevated PTH during evaluation for symptoms that could have been consistent with primary HPT. Such symptoms were present in 46 of the 60 misdiagnosed patients, the majority of which were from nephrolithiasis (22 patients), he said. Also relevant were bone loss in 15 and vague symptoms such as fatigue or depression in 11.
Although surgery may seem like a simple solution for patients who have an elevated PTH level, symptoms, and positive imaging findings, the operation can be harmful for those with secondary HPT because they’re dependent on increased PTH levels to maintain calcium homeostasis, Dr. Iannuzzi said. Parathyroidectomy is associated with 95%-98% cure rates for primary HPT, but patients with secondary HPT are unlikely to have an intraoperative drop in PTH levels. As a result, they often undergo a bilateral neck exploration that predisposes them to nerve injury. More importantly, if the parathyroid tissue they’re dependent on is removed, it may create irreversible hypoparathyroidism.
"Vigilance is absolutely necessary to avoid unindicated and potentially harmful surgery," Dr. Iannuzzi cautioned.
Invited discussant Dr. Herbert Chen, chair of general surgery and leader of the endocrine oncology group at the University of Wisconsin in Madison, said that the results underscore the role of surgeons as diagnosticians.
"This finding really emphasizes that as surgeons we are not merely technicians operating at the whim of our referring physicians, but have a responsibility to lead in the diagnostic phase of patient care," he said.
Those thoughts were echoed by fellow discussant Dr. Allan Siperstein, chair of endocrine surgery at the Cleveland Clinic, who also asked what lab panels are used to assess patients with mild hyperparathyroidism. Dr. Iannuzzi said that they use an extensive panel including ionized calcium, magnesium, phosphorus, vitamin D, and urinary calcium. Dr. Moalem emphasized that the diagnosis of primary HPT must be made based on simultaneously high or high-normal calcium and PTH measurements. Imaging studies have no role in making or refuting the diagnosis and should be used to guide the operative approach only when the diagnosis of primary HPT has been definitively established.
Dr. Iannuzzi and his coauthors reported no relevant financial disclosures.
MADISON, WIS. – Nearly one-fifth of all patients referred for parathyroidectomy were misdiagnosed with primary hyperparathyroidism in a retrospective study of 324 consecutive patients.
Notably, many of these patients had undergone comprehensive work-ups prior to surgical referral that clearly diagnosed secondary hyperparathyroidism.
"This demonstrates that there’s a prevalent misunderstanding of parathyroid pathophysiology," Dr. James Iannuzzi said at the annual meeting of the Central Surgical Association. "In particular, vitamin D deficiency accounted for the majority of misdiagnosis."
Using ICD-9 codes for hyperparathyroidism, benign, or malignant parathyroid tumors or hypercalcemia, the authors identified 381 patients who were referred to the endocrine surgery division at the University of Rochester (N.Y.) Medical Center between 2008 and 2011 for parathyroidectomy. Primary hyperparathyroidism (HPT) was defined by a calcium level of at least 10 mg/dL plus a parathyroid hormone (PTH) level of more than 50 pg/mL. Thirty patients were excluded for renal failure and 27, for coding errors.
Among the remaining 324 patients, 264 were correctly diagnosed and 60 (18.5%) were misdiagnosed with primary HPT, said Dr. Iannuzzi, a general surgery resident at the university. Fifty-four (90%) of the misdiagnosed patients had secondary HPT at the time of the referral. For 43 patients, this was clear at the time of referral, and 11 had secondary HPT as their sole diagnosis after follow-up. Six patients had hypercalcemia but not HPT. Half of the hypercalcemic patients were referred after a single spuriously elevated calcium level, only to have their calcium drop to normal levels after follow-up, he said.
Most of the 54 patients with secondary HPT had vitamin D deficiency (37 patients). Other causes were gastric bypass (4 patients), celiac disease (2 patients), and unclear etiology (11 patients). Mean calcium and PTH levels among patients with secondary HPT were 9.3 mg/dL and 92 pg/mL, respectively.
In all, 42 (70%) of the 60 misdiagnosed patients underwent inappropriate localized imaging, of which 57% were falsely positive.
"These studies are not indicated; likely to be falsely positive, as we show; and cause patients and referring physicians to more aggressively push for unindicated and potentially harmful surgery because of a finding on a scan," Dr. Jacob Moalem said in an interview. Dr. Moalem, an endocrine surgeon at the University of Rochester, was the senior author of the paper.
Part of the problem is that ultrasound is highly user dependent, and sonographers can be easily misled by exophytic nodules, hypoechoic structures that were subcentimeters, or lymph nodes. Sestamibi scans in this clinical setting also are likely to be positive because of the activated mitochondria. Thus, it is very important that the pretest diagnosis be correct, Dr. Moalem and Dr. Iannuzzi stressed.
Many patients were found to have an elevated PTH during evaluation for symptoms that could have been consistent with primary HPT. Such symptoms were present in 46 of the 60 misdiagnosed patients, the majority of which were from nephrolithiasis (22 patients), he said. Also relevant were bone loss in 15 and vague symptoms such as fatigue or depression in 11.
Although surgery may seem like a simple solution for patients who have an elevated PTH level, symptoms, and positive imaging findings, the operation can be harmful for those with secondary HPT because they’re dependent on increased PTH levels to maintain calcium homeostasis, Dr. Iannuzzi said. Parathyroidectomy is associated with 95%-98% cure rates for primary HPT, but patients with secondary HPT are unlikely to have an intraoperative drop in PTH levels. As a result, they often undergo a bilateral neck exploration that predisposes them to nerve injury. More importantly, if the parathyroid tissue they’re dependent on is removed, it may create irreversible hypoparathyroidism.
"Vigilance is absolutely necessary to avoid unindicated and potentially harmful surgery," Dr. Iannuzzi cautioned.
Invited discussant Dr. Herbert Chen, chair of general surgery and leader of the endocrine oncology group at the University of Wisconsin in Madison, said that the results underscore the role of surgeons as diagnosticians.
"This finding really emphasizes that as surgeons we are not merely technicians operating at the whim of our referring physicians, but have a responsibility to lead in the diagnostic phase of patient care," he said.
Those thoughts were echoed by fellow discussant Dr. Allan Siperstein, chair of endocrine surgery at the Cleveland Clinic, who also asked what lab panels are used to assess patients with mild hyperparathyroidism. Dr. Iannuzzi said that they use an extensive panel including ionized calcium, magnesium, phosphorus, vitamin D, and urinary calcium. Dr. Moalem emphasized that the diagnosis of primary HPT must be made based on simultaneously high or high-normal calcium and PTH measurements. Imaging studies have no role in making or refuting the diagnosis and should be used to guide the operative approach only when the diagnosis of primary HPT has been definitively established.
Dr. Iannuzzi and his coauthors reported no relevant financial disclosures.
MADISON, WIS. – Nearly one-fifth of all patients referred for parathyroidectomy were misdiagnosed with primary hyperparathyroidism in a retrospective study of 324 consecutive patients.
Notably, many of these patients had undergone comprehensive work-ups prior to surgical referral that clearly diagnosed secondary hyperparathyroidism.
"This demonstrates that there’s a prevalent misunderstanding of parathyroid pathophysiology," Dr. James Iannuzzi said at the annual meeting of the Central Surgical Association. "In particular, vitamin D deficiency accounted for the majority of misdiagnosis."
Using ICD-9 codes for hyperparathyroidism, benign, or malignant parathyroid tumors or hypercalcemia, the authors identified 381 patients who were referred to the endocrine surgery division at the University of Rochester (N.Y.) Medical Center between 2008 and 2011 for parathyroidectomy. Primary hyperparathyroidism (HPT) was defined by a calcium level of at least 10 mg/dL plus a parathyroid hormone (PTH) level of more than 50 pg/mL. Thirty patients were excluded for renal failure and 27, for coding errors.
Among the remaining 324 patients, 264 were correctly diagnosed and 60 (18.5%) were misdiagnosed with primary HPT, said Dr. Iannuzzi, a general surgery resident at the university. Fifty-four (90%) of the misdiagnosed patients had secondary HPT at the time of the referral. For 43 patients, this was clear at the time of referral, and 11 had secondary HPT as their sole diagnosis after follow-up. Six patients had hypercalcemia but not HPT. Half of the hypercalcemic patients were referred after a single spuriously elevated calcium level, only to have their calcium drop to normal levels after follow-up, he said.
Most of the 54 patients with secondary HPT had vitamin D deficiency (37 patients). Other causes were gastric bypass (4 patients), celiac disease (2 patients), and unclear etiology (11 patients). Mean calcium and PTH levels among patients with secondary HPT were 9.3 mg/dL and 92 pg/mL, respectively.
In all, 42 (70%) of the 60 misdiagnosed patients underwent inappropriate localized imaging, of which 57% were falsely positive.
"These studies are not indicated; likely to be falsely positive, as we show; and cause patients and referring physicians to more aggressively push for unindicated and potentially harmful surgery because of a finding on a scan," Dr. Jacob Moalem said in an interview. Dr. Moalem, an endocrine surgeon at the University of Rochester, was the senior author of the paper.
Part of the problem is that ultrasound is highly user dependent, and sonographers can be easily misled by exophytic nodules, hypoechoic structures that were subcentimeters, or lymph nodes. Sestamibi scans in this clinical setting also are likely to be positive because of the activated mitochondria. Thus, it is very important that the pretest diagnosis be correct, Dr. Moalem and Dr. Iannuzzi stressed.
Many patients were found to have an elevated PTH during evaluation for symptoms that could have been consistent with primary HPT. Such symptoms were present in 46 of the 60 misdiagnosed patients, the majority of which were from nephrolithiasis (22 patients), he said. Also relevant were bone loss in 15 and vague symptoms such as fatigue or depression in 11.
Although surgery may seem like a simple solution for patients who have an elevated PTH level, symptoms, and positive imaging findings, the operation can be harmful for those with secondary HPT because they’re dependent on increased PTH levels to maintain calcium homeostasis, Dr. Iannuzzi said. Parathyroidectomy is associated with 95%-98% cure rates for primary HPT, but patients with secondary HPT are unlikely to have an intraoperative drop in PTH levels. As a result, they often undergo a bilateral neck exploration that predisposes them to nerve injury. More importantly, if the parathyroid tissue they’re dependent on is removed, it may create irreversible hypoparathyroidism.
"Vigilance is absolutely necessary to avoid unindicated and potentially harmful surgery," Dr. Iannuzzi cautioned.
Invited discussant Dr. Herbert Chen, chair of general surgery and leader of the endocrine oncology group at the University of Wisconsin in Madison, said that the results underscore the role of surgeons as diagnosticians.
"This finding really emphasizes that as surgeons we are not merely technicians operating at the whim of our referring physicians, but have a responsibility to lead in the diagnostic phase of patient care," he said.
Those thoughts were echoed by fellow discussant Dr. Allan Siperstein, chair of endocrine surgery at the Cleveland Clinic, who also asked what lab panels are used to assess patients with mild hyperparathyroidism. Dr. Iannuzzi said that they use an extensive panel including ionized calcium, magnesium, phosphorus, vitamin D, and urinary calcium. Dr. Moalem emphasized that the diagnosis of primary HPT must be made based on simultaneously high or high-normal calcium and PTH measurements. Imaging studies have no role in making or refuting the diagnosis and should be used to guide the operative approach only when the diagnosis of primary HPT has been definitively established.
Dr. Iannuzzi and his coauthors reported no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Major Finding: Sixty patients (18.5%) were misdiagnosed with primary hyperparathyroidism and 264 were correctly diagnosed.
Data Source: This retrospective analysis involved 324 patients diagnosed with primary hyperparathyroidism who were referred for surgery.
Disclosures: Dr. Iannuzzi and his coauthors reported no relevant financial disclosures.
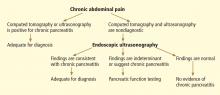
Endoscopic ultrasonography to evaluate pancreatitis
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
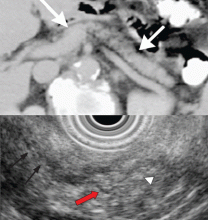
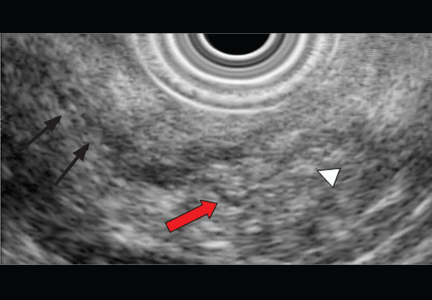
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
KEY POINTS
- EUS can identify the cause of acute pancreatitis when other imaging tests (computed tomography, transabdominal ultrasonography) are unrevealing.
- EUS can safely and accurately detect bile duct stones and other causes of recurrent acute pancreatitis. It can also detect mild and severe structural features of chronic pancreatitis.
- An endoscopic pancreatic function test may be a useful adjunct to EUS to detect mild exocrine insufficiency in early chronic pancreatitis.
Nausea and Epigastric Pain in a Renal Failure Patient
An Unusual Presentation of Subacute Osteomyelitis: A Talus Brodie Abscess With Tendon Involvement
Her Chief Complaint Is ... And by the Way She’s Also Pregnant
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
MRI Inflammation 'Marginally' Predicts AS Bone Growth
Evidence of inflammation on spinal MRIs was "marginally predictive" of new syndesmophyte formation 2 years later at the vertebral sites where inflammation was observed, in a study of over 100 patients with ankylosing spondylitis.
However, growth of syndesmophytes that were present at vertebral sites at baseline was not associated with evidence of inflammation on MRIs, reported Dr. Désirée van der Heijde, of Leiden University Medical Center, Leiden, the Netherlands, and her associates, in the March issue of the Annals of the Rheumatic Diseases (Ann. Rheum. Dis. 2012;71:369-73).
"MRI inflammation in a vertebral unit slightly increases the likelihood of finding a new syndesmophyte in the same vertebral unit 2 years later, but does not predict the growth of already existing syndesmophytes," they wrote. The finding that most of the syndesmophytes grew in the vertebral units with no MRI evidence of inflammation suggests that "the relationship between MRI inflammation and syndesmophyte formation is not straightforward," they added.
The goal of the study was to address the association between inflammation of a vertebral unit (defined as "the region between two virtual lines through the middle of each vertebra") on MRI and the subsequent formation and growth of syndesmophytes, the excessive bone formation that represents the structural damage seen in ankylosing spondylitis (AS). The processes behind the formation of syndesmophytes in AS are not well understood and are not inhibited by treatment with tumor necrosis factor (TNF) blockers, despite the dramatic effects these agents have on inflammation, they noted.
They used data that included spinal MRI activity scores, from a random sample of people enrolled in ASSERT (AS Study for the Evaluation of Recombinant Infliximab Therapy), a 24-week study published in 2005 that compared infliximab to placebo in patients with active AS and followed patients in a open extension period up to 102 weeks, during which time everyone was treated with infliximab (Arthritis Rheum. 2005;52:582-91).
They analyzed information from 1,827 to 2,070 vertebral units from 177 to 183 patients, (the numbers varied, depending on the case definition and the reader of the MRI). MRI images of all 23 vertebral units of the spine – C2 to S1 – were scored on a scale of 0 to 3 (the presence of inflammation in a vertebral unit was defined as a score greater than 0), and a score from 4 to 6 if erosions were also evident.
After adjusting for possible confounders, the risk of developing a syndesmophyte in a vertebral unit with MRI evidence of inflammation was increased 1.5- to 2-fold, a statistically significant increase, they said.
Depending on the syndesmophyte definition used, the reader of the MRI, and the MRI case definition, 6%-32% of new syndesmophytes developed at the vertebral sites with evidence of active inflammation and 68%-94% of new syndesmophytes developed at the vertebral sites that had no evidence of active inflammation. Similar findings were observed for the syndesmophytes that were present at baseline: 0%-30% of the syndesmophytes that were present at baseline and grew were at sites of active inflammation, and 70%-100% of the syndesmophytes that grew "did so in vertebral units without inflammation," they reported.
"The subtle association between MRI activity and new syndesmophytes is in conflict with the absence of an effect of TNF blockers on structural damage," the authors wrote. One explanation they proposed was that the formation of syndesmophytes is "a post-inflammatory repair reaction that may only be inhibited if a TNF blocker is started early before inflammation gives way to repair."
Based on the finding that evidence of inflammation at the vertebral unit "only marginally predicts" the formation of new syndesmophytes at that site, "if inflammation is indeed the principle trigger of repair responses, a strong case can be made for early and aggressive anti-inflammatory treatment," they speculated. However, they added, "if inflammation and repair are independent pathways triggered by common factors, new therapies targeting the pathologically enhanced repair responses need to be developed."
One of the eight authors was supported by a grant from the Fundação para a Ciência e a Tecnologia (FCT); otherwise, the authors had no disclosures to report.
In an accompanying editorial titled, "Inflammation and Ankylosis: still an enigmatic relationship in spondyloarthritis," Dr. Rik Lories and Dr. Maxime Dougados referred to TNF-blocker therapy’s apparent lack of an effect on the structural progression of disease in patients with spondyloarthritis as a "clinical conundrum," noting that anti-TNF therapy slows progression in rheumatoid arthritis.
One hypothesis that has been proposed to address this issue is that inflammation, including TNF upregulation, triggers local damage and repair, which results in the formation of new bone and ankylosis, "establishing a causal coupling between inflammation" and the ankylosing process. Another hypothesis is that inflammation and new bone formation are caused by a "common trigger," but "further develop in a largely molecular independent way."
"Taken together, recent MRI studies on the structural progression of disease in spondyloarthritis and its relation to inflammation are another important step forward in our understanding of a disease that was neglected by most of the rheumatology research community for too long," they wrote, adding: "The complex nature of the disease and the many factors that have recently been implicated in playing a role in its onset and progression indicate that further research is needed" (Ann. Rheum. Dis. 2012;71:317-8).
Dr. Lories is K. U. Leuven is research professor at Universiteit Leuven (Belgium) and Dr. Dougados is professor of rheumatology at René Descartes University in Paris. They said they had no disclosures.
In an accompanying editorial titled, "Inflammation and Ankylosis: still an enigmatic relationship in spondyloarthritis," Dr. Rik Lories and Dr. Maxime Dougados referred to TNF-blocker therapy’s apparent lack of an effect on the structural progression of disease in patients with spondyloarthritis as a "clinical conundrum," noting that anti-TNF therapy slows progression in rheumatoid arthritis.
One hypothesis that has been proposed to address this issue is that inflammation, including TNF upregulation, triggers local damage and repair, which results in the formation of new bone and ankylosis, "establishing a causal coupling between inflammation" and the ankylosing process. Another hypothesis is that inflammation and new bone formation are caused by a "common trigger," but "further develop in a largely molecular independent way."
"Taken together, recent MRI studies on the structural progression of disease in spondyloarthritis and its relation to inflammation are another important step forward in our understanding of a disease that was neglected by most of the rheumatology research community for too long," they wrote, adding: "The complex nature of the disease and the many factors that have recently been implicated in playing a role in its onset and progression indicate that further research is needed" (Ann. Rheum. Dis. 2012;71:317-8).
Dr. Lories is K. U. Leuven is research professor at Universiteit Leuven (Belgium) and Dr. Dougados is professor of rheumatology at René Descartes University in Paris. They said they had no disclosures.
In an accompanying editorial titled, "Inflammation and Ankylosis: still an enigmatic relationship in spondyloarthritis," Dr. Rik Lories and Dr. Maxime Dougados referred to TNF-blocker therapy’s apparent lack of an effect on the structural progression of disease in patients with spondyloarthritis as a "clinical conundrum," noting that anti-TNF therapy slows progression in rheumatoid arthritis.
One hypothesis that has been proposed to address this issue is that inflammation, including TNF upregulation, triggers local damage and repair, which results in the formation of new bone and ankylosis, "establishing a causal coupling between inflammation" and the ankylosing process. Another hypothesis is that inflammation and new bone formation are caused by a "common trigger," but "further develop in a largely molecular independent way."
"Taken together, recent MRI studies on the structural progression of disease in spondyloarthritis and its relation to inflammation are another important step forward in our understanding of a disease that was neglected by most of the rheumatology research community for too long," they wrote, adding: "The complex nature of the disease and the many factors that have recently been implicated in playing a role in its onset and progression indicate that further research is needed" (Ann. Rheum. Dis. 2012;71:317-8).
Dr. Lories is K. U. Leuven is research professor at Universiteit Leuven (Belgium) and Dr. Dougados is professor of rheumatology at René Descartes University in Paris. They said they had no disclosures.
Evidence of inflammation on spinal MRIs was "marginally predictive" of new syndesmophyte formation 2 years later at the vertebral sites where inflammation was observed, in a study of over 100 patients with ankylosing spondylitis.
However, growth of syndesmophytes that were present at vertebral sites at baseline was not associated with evidence of inflammation on MRIs, reported Dr. Désirée van der Heijde, of Leiden University Medical Center, Leiden, the Netherlands, and her associates, in the March issue of the Annals of the Rheumatic Diseases (Ann. Rheum. Dis. 2012;71:369-73).
"MRI inflammation in a vertebral unit slightly increases the likelihood of finding a new syndesmophyte in the same vertebral unit 2 years later, but does not predict the growth of already existing syndesmophytes," they wrote. The finding that most of the syndesmophytes grew in the vertebral units with no MRI evidence of inflammation suggests that "the relationship between MRI inflammation and syndesmophyte formation is not straightforward," they added.
The goal of the study was to address the association between inflammation of a vertebral unit (defined as "the region between two virtual lines through the middle of each vertebra") on MRI and the subsequent formation and growth of syndesmophytes, the excessive bone formation that represents the structural damage seen in ankylosing spondylitis (AS). The processes behind the formation of syndesmophytes in AS are not well understood and are not inhibited by treatment with tumor necrosis factor (TNF) blockers, despite the dramatic effects these agents have on inflammation, they noted.
They used data that included spinal MRI activity scores, from a random sample of people enrolled in ASSERT (AS Study for the Evaluation of Recombinant Infliximab Therapy), a 24-week study published in 2005 that compared infliximab to placebo in patients with active AS and followed patients in a open extension period up to 102 weeks, during which time everyone was treated with infliximab (Arthritis Rheum. 2005;52:582-91).
They analyzed information from 1,827 to 2,070 vertebral units from 177 to 183 patients, (the numbers varied, depending on the case definition and the reader of the MRI). MRI images of all 23 vertebral units of the spine – C2 to S1 – were scored on a scale of 0 to 3 (the presence of inflammation in a vertebral unit was defined as a score greater than 0), and a score from 4 to 6 if erosions were also evident.
After adjusting for possible confounders, the risk of developing a syndesmophyte in a vertebral unit with MRI evidence of inflammation was increased 1.5- to 2-fold, a statistically significant increase, they said.
Depending on the syndesmophyte definition used, the reader of the MRI, and the MRI case definition, 6%-32% of new syndesmophytes developed at the vertebral sites with evidence of active inflammation and 68%-94% of new syndesmophytes developed at the vertebral sites that had no evidence of active inflammation. Similar findings were observed for the syndesmophytes that were present at baseline: 0%-30% of the syndesmophytes that were present at baseline and grew were at sites of active inflammation, and 70%-100% of the syndesmophytes that grew "did so in vertebral units without inflammation," they reported.
"The subtle association between MRI activity and new syndesmophytes is in conflict with the absence of an effect of TNF blockers on structural damage," the authors wrote. One explanation they proposed was that the formation of syndesmophytes is "a post-inflammatory repair reaction that may only be inhibited if a TNF blocker is started early before inflammation gives way to repair."
Based on the finding that evidence of inflammation at the vertebral unit "only marginally predicts" the formation of new syndesmophytes at that site, "if inflammation is indeed the principle trigger of repair responses, a strong case can be made for early and aggressive anti-inflammatory treatment," they speculated. However, they added, "if inflammation and repair are independent pathways triggered by common factors, new therapies targeting the pathologically enhanced repair responses need to be developed."
One of the eight authors was supported by a grant from the Fundação para a Ciência e a Tecnologia (FCT); otherwise, the authors had no disclosures to report.
Evidence of inflammation on spinal MRIs was "marginally predictive" of new syndesmophyte formation 2 years later at the vertebral sites where inflammation was observed, in a study of over 100 patients with ankylosing spondylitis.
However, growth of syndesmophytes that were present at vertebral sites at baseline was not associated with evidence of inflammation on MRIs, reported Dr. Désirée van der Heijde, of Leiden University Medical Center, Leiden, the Netherlands, and her associates, in the March issue of the Annals of the Rheumatic Diseases (Ann. Rheum. Dis. 2012;71:369-73).
"MRI inflammation in a vertebral unit slightly increases the likelihood of finding a new syndesmophyte in the same vertebral unit 2 years later, but does not predict the growth of already existing syndesmophytes," they wrote. The finding that most of the syndesmophytes grew in the vertebral units with no MRI evidence of inflammation suggests that "the relationship between MRI inflammation and syndesmophyte formation is not straightforward," they added.
The goal of the study was to address the association between inflammation of a vertebral unit (defined as "the region between two virtual lines through the middle of each vertebra") on MRI and the subsequent formation and growth of syndesmophytes, the excessive bone formation that represents the structural damage seen in ankylosing spondylitis (AS). The processes behind the formation of syndesmophytes in AS are not well understood and are not inhibited by treatment with tumor necrosis factor (TNF) blockers, despite the dramatic effects these agents have on inflammation, they noted.
They used data that included spinal MRI activity scores, from a random sample of people enrolled in ASSERT (AS Study for the Evaluation of Recombinant Infliximab Therapy), a 24-week study published in 2005 that compared infliximab to placebo in patients with active AS and followed patients in a open extension period up to 102 weeks, during which time everyone was treated with infliximab (Arthritis Rheum. 2005;52:582-91).
They analyzed information from 1,827 to 2,070 vertebral units from 177 to 183 patients, (the numbers varied, depending on the case definition and the reader of the MRI). MRI images of all 23 vertebral units of the spine – C2 to S1 – were scored on a scale of 0 to 3 (the presence of inflammation in a vertebral unit was defined as a score greater than 0), and a score from 4 to 6 if erosions were also evident.
After adjusting for possible confounders, the risk of developing a syndesmophyte in a vertebral unit with MRI evidence of inflammation was increased 1.5- to 2-fold, a statistically significant increase, they said.
Depending on the syndesmophyte definition used, the reader of the MRI, and the MRI case definition, 6%-32% of new syndesmophytes developed at the vertebral sites with evidence of active inflammation and 68%-94% of new syndesmophytes developed at the vertebral sites that had no evidence of active inflammation. Similar findings were observed for the syndesmophytes that were present at baseline: 0%-30% of the syndesmophytes that were present at baseline and grew were at sites of active inflammation, and 70%-100% of the syndesmophytes that grew "did so in vertebral units without inflammation," they reported.
"The subtle association between MRI activity and new syndesmophytes is in conflict with the absence of an effect of TNF blockers on structural damage," the authors wrote. One explanation they proposed was that the formation of syndesmophytes is "a post-inflammatory repair reaction that may only be inhibited if a TNF blocker is started early before inflammation gives way to repair."
Based on the finding that evidence of inflammation at the vertebral unit "only marginally predicts" the formation of new syndesmophytes at that site, "if inflammation is indeed the principle trigger of repair responses, a strong case can be made for early and aggressive anti-inflammatory treatment," they speculated. However, they added, "if inflammation and repair are independent pathways triggered by common factors, new therapies targeting the pathologically enhanced repair responses need to be developed."
One of the eight authors was supported by a grant from the Fundação para a Ciência e a Tecnologia (FCT); otherwise, the authors had no disclosures to report.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major Finding: Evidence of inflammation on spinal MRIs of patients with ankylosing spondylitis (AS) was associated with a 1.5- to 2-fold increased risk of new syndesmophyte formation at the vertebral sites of inflammation 2 years later, which was statistically significant. But the growth of syndesmophytes that were present at vertebral sites at baseline was not associated with evidence of inflammation on MRIs.
Data Source: A study using a random sample of patients enrolled in ASSERT (AS Study for the Evaluation of Recombinant Infliximab Therapy), a randomized controlled study published in 2005 that compared infliximab to placebo in patients with active AS for 24 weeks, then followed patients until 102 weeks, during which time everyone was treated with infliximab.
Disclosures: One of the eight authors was supported by a grant from the Fundação para a Ciência e a Tecnologia (FCT); otherwise, the authors had no disclosures.