User login
Medicaid Insurance Is Associated With Larger Curves in Patients Who Require Scoliosis Surgery
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
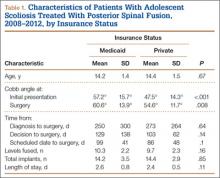
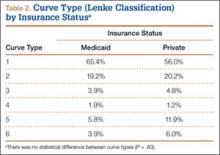
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Open Carpal Tunnel Release With Use of a Nasal Turbinate Speculum
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
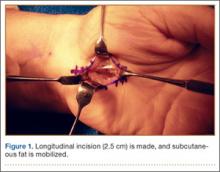
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
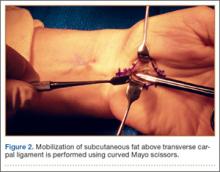
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
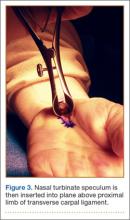
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
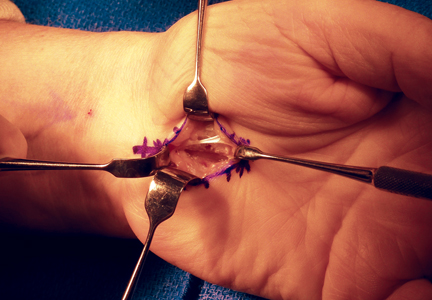
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
TCT: Immobilized leaflets on bioprosthetic aortic valves trigger concern
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM TCT 2015
Key clinical point: CT imaging of recently placed bioprosthetic aortic valves showed several cases of leaflets with reduced motion, suggesting possible clinical consequences.
Major finding: CT imaging showed reduced leaflet motion in 22 of 55 (40%) trial patients and 17 of 132 (13%) registry patients.
Data source: An observational study of CT images collected on 187 patients who had undergone aortic valve replacement from the PORTICO IDE study (55 patients), and the RESOLVE and SAVORY registries (132 total patients).
Disclosures: The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage.
Glutamate-based neuroimaging identifies epileptic foci
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:Glutamate-based neuroimaging could identify epileptic foci in epilepsy patients who are nonlesional on conventional MRI.
Major finding: Glutamate chemical exchange saturation transfer imaging enables lateralization of seizure onset in previously nonlesional patients.
Data source: Imaging study in four individuals with drug-resistant epilepsy.
Disclosures: The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
TCT: FFR-CT improved costs, quality of life in PLATFORM trial
SAN FRANCISCO – Estimating fractional flow reserve with computed tomography appears to reduce resource use and costs when compared with invasive coronary angiography in stable patients with possible symptoms of coronary disease, according to a substudy of the prospective, multicenter PLATFORM trial.
Fractional flow reserve estimated by CT (FFR-CT) was also associated with greater improvement in quality of life measures during the 90-day study period, when compared with usual noninvasive testing, Dr. Mark A. Hlatky of Stanford (Calif.) University reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The PLATFORM trial and substudy data are “game changers,” according to the discussant, Dr. Bernard de Bruyne of Cardiovascular Center Aalst, Belgium, who predicted that if the findings are confirmed in other studies, “this kind of approach will probably largely replace the presently available noninvasive approaches and noninvasive stress testing.”
To assess the effect of using FFR-CT rather than usual care on cost and quality of life, patients with stable symptoms, intermediate probability of CAD (the pretest CAD probability was 49%), and no established CAD diagnosis were enrolled into one of two strata based on whether invasive or noninvasive diagnostic testing was planned. Among 193 patients in the planned invasive testing group who underwent FFR-CT, costs were reduced by 32%, compared with 187 patients in the group who received usual care ($7,343 vs. $10,734). The difference was highly statistically significant.
Among 104 patients in the planned noninvasive testing group who underwent FFR-CT, costs did not differ significantly, compared with 100 in that group who received usual care ($2,679 vs. $2,137), Dr. Hlatky reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These findings persisted after propensity score matching, he noted.
Furthermore, scores on each of three quality of life measures improved in the overall study population, and scores in the noninvasive stratum improved more with FFR-CT than with usual care. For example, Seattle Angina Questionnaire scores were 19.5 vs. 11.4, EuroQOL scores were 0.08 vs. 0.03, and visual analog scale scores were 4.1 vs. 2.3 in the groups, respectively. The improvements in the invasive cohort were similar with FFR-CT and usual care, Dr. Hlatky noted.
The findings, published simultaneously online (J Am Coll Cardiol. 2015. doi:10.1016/j.jacc.2015.09.051), suggest that the combination of anatomic and functional data provided by an FFR-CT–guided testing strategy may lead to more selective use of invasive procedures than relying solely on the anatomic data provided by invasive coronary angiography, Dr. Hlatky reported.
He explained that FFR, which assesses the functional significance of individual coronary lesions, can be estimated noninvasively from standardly acquired CT data based on computational fluid dynamics. FFR-CT was recently approved for clinical use by the Food and Drug Administration and the European Medicines Agency based on its diagnostic accuracy, he said.
The clinical effectiveness of the strategy was demonstrated in the PLATFORM trial which showed a reduction in the rate of invasive angiography without obstructive coronary artery disease from 73% to 12% with the use of FFR-CT. The current findings further demonstrate that the approach improves quality of life outcomes.
Though limited by the use of a consecutive observational design, as opposed to a randomized trial design, the large effect sizes suggest that findings would be similar in a randomized study, Dr. Hlatky said.
“I don’t think this is by chance. The plausibility of it has been explained,” he said, adding that while most people are happy with a normal CT angiography because of the high sensitivity, estimated FFR using the CT technique can be helpful in the setting of uncertainty.
“If you see something and you’re not sure if it’s significant, and if the estimated FFR from this technique is normal, that’s extremely reassuring that it’s just something you’re seeing but it’s not necessarily obstructing flow,” he said.
He added that “this would be best tested by doing a real, true, randomized study,” but said he considers the findings to be “quite interesting and completely in line with the clinical results.”
The technique is “entering progressively into practice in Europe,” said Dr. de Bruyne, who is a PLATFORM coinvestigator. “It is already used in clinical practice. You get the anatomy and physiology at the same time and same place. It is a really important paradigm change,” he said.
Dr. Hlatky and Dr. de Bruyne reported receiving research grants from HeartFlow, which supported the study.
SAN FRANCISCO – Estimating fractional flow reserve with computed tomography appears to reduce resource use and costs when compared with invasive coronary angiography in stable patients with possible symptoms of coronary disease, according to a substudy of the prospective, multicenter PLATFORM trial.
Fractional flow reserve estimated by CT (FFR-CT) was also associated with greater improvement in quality of life measures during the 90-day study period, when compared with usual noninvasive testing, Dr. Mark A. Hlatky of Stanford (Calif.) University reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The PLATFORM trial and substudy data are “game changers,” according to the discussant, Dr. Bernard de Bruyne of Cardiovascular Center Aalst, Belgium, who predicted that if the findings are confirmed in other studies, “this kind of approach will probably largely replace the presently available noninvasive approaches and noninvasive stress testing.”
To assess the effect of using FFR-CT rather than usual care on cost and quality of life, patients with stable symptoms, intermediate probability of CAD (the pretest CAD probability was 49%), and no established CAD diagnosis were enrolled into one of two strata based on whether invasive or noninvasive diagnostic testing was planned. Among 193 patients in the planned invasive testing group who underwent FFR-CT, costs were reduced by 32%, compared with 187 patients in the group who received usual care ($7,343 vs. $10,734). The difference was highly statistically significant.
Among 104 patients in the planned noninvasive testing group who underwent FFR-CT, costs did not differ significantly, compared with 100 in that group who received usual care ($2,679 vs. $2,137), Dr. Hlatky reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These findings persisted after propensity score matching, he noted.
Furthermore, scores on each of three quality of life measures improved in the overall study population, and scores in the noninvasive stratum improved more with FFR-CT than with usual care. For example, Seattle Angina Questionnaire scores were 19.5 vs. 11.4, EuroQOL scores were 0.08 vs. 0.03, and visual analog scale scores were 4.1 vs. 2.3 in the groups, respectively. The improvements in the invasive cohort were similar with FFR-CT and usual care, Dr. Hlatky noted.
The findings, published simultaneously online (J Am Coll Cardiol. 2015. doi:10.1016/j.jacc.2015.09.051), suggest that the combination of anatomic and functional data provided by an FFR-CT–guided testing strategy may lead to more selective use of invasive procedures than relying solely on the anatomic data provided by invasive coronary angiography, Dr. Hlatky reported.
He explained that FFR, which assesses the functional significance of individual coronary lesions, can be estimated noninvasively from standardly acquired CT data based on computational fluid dynamics. FFR-CT was recently approved for clinical use by the Food and Drug Administration and the European Medicines Agency based on its diagnostic accuracy, he said.
The clinical effectiveness of the strategy was demonstrated in the PLATFORM trial which showed a reduction in the rate of invasive angiography without obstructive coronary artery disease from 73% to 12% with the use of FFR-CT. The current findings further demonstrate that the approach improves quality of life outcomes.
Though limited by the use of a consecutive observational design, as opposed to a randomized trial design, the large effect sizes suggest that findings would be similar in a randomized study, Dr. Hlatky said.
“I don’t think this is by chance. The plausibility of it has been explained,” he said, adding that while most people are happy with a normal CT angiography because of the high sensitivity, estimated FFR using the CT technique can be helpful in the setting of uncertainty.
“If you see something and you’re not sure if it’s significant, and if the estimated FFR from this technique is normal, that’s extremely reassuring that it’s just something you’re seeing but it’s not necessarily obstructing flow,” he said.
He added that “this would be best tested by doing a real, true, randomized study,” but said he considers the findings to be “quite interesting and completely in line with the clinical results.”
The technique is “entering progressively into practice in Europe,” said Dr. de Bruyne, who is a PLATFORM coinvestigator. “It is already used in clinical practice. You get the anatomy and physiology at the same time and same place. It is a really important paradigm change,” he said.
Dr. Hlatky and Dr. de Bruyne reported receiving research grants from HeartFlow, which supported the study.
SAN FRANCISCO – Estimating fractional flow reserve with computed tomography appears to reduce resource use and costs when compared with invasive coronary angiography in stable patients with possible symptoms of coronary disease, according to a substudy of the prospective, multicenter PLATFORM trial.
Fractional flow reserve estimated by CT (FFR-CT) was also associated with greater improvement in quality of life measures during the 90-day study period, when compared with usual noninvasive testing, Dr. Mark A. Hlatky of Stanford (Calif.) University reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
The PLATFORM trial and substudy data are “game changers,” according to the discussant, Dr. Bernard de Bruyne of Cardiovascular Center Aalst, Belgium, who predicted that if the findings are confirmed in other studies, “this kind of approach will probably largely replace the presently available noninvasive approaches and noninvasive stress testing.”
To assess the effect of using FFR-CT rather than usual care on cost and quality of life, patients with stable symptoms, intermediate probability of CAD (the pretest CAD probability was 49%), and no established CAD diagnosis were enrolled into one of two strata based on whether invasive or noninvasive diagnostic testing was planned. Among 193 patients in the planned invasive testing group who underwent FFR-CT, costs were reduced by 32%, compared with 187 patients in the group who received usual care ($7,343 vs. $10,734). The difference was highly statistically significant.
Among 104 patients in the planned noninvasive testing group who underwent FFR-CT, costs did not differ significantly, compared with 100 in that group who received usual care ($2,679 vs. $2,137), Dr. Hlatky reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These findings persisted after propensity score matching, he noted.
Furthermore, scores on each of three quality of life measures improved in the overall study population, and scores in the noninvasive stratum improved more with FFR-CT than with usual care. For example, Seattle Angina Questionnaire scores were 19.5 vs. 11.4, EuroQOL scores were 0.08 vs. 0.03, and visual analog scale scores were 4.1 vs. 2.3 in the groups, respectively. The improvements in the invasive cohort were similar with FFR-CT and usual care, Dr. Hlatky noted.
The findings, published simultaneously online (J Am Coll Cardiol. 2015. doi:10.1016/j.jacc.2015.09.051), suggest that the combination of anatomic and functional data provided by an FFR-CT–guided testing strategy may lead to more selective use of invasive procedures than relying solely on the anatomic data provided by invasive coronary angiography, Dr. Hlatky reported.
He explained that FFR, which assesses the functional significance of individual coronary lesions, can be estimated noninvasively from standardly acquired CT data based on computational fluid dynamics. FFR-CT was recently approved for clinical use by the Food and Drug Administration and the European Medicines Agency based on its diagnostic accuracy, he said.
The clinical effectiveness of the strategy was demonstrated in the PLATFORM trial which showed a reduction in the rate of invasive angiography without obstructive coronary artery disease from 73% to 12% with the use of FFR-CT. The current findings further demonstrate that the approach improves quality of life outcomes.
Though limited by the use of a consecutive observational design, as opposed to a randomized trial design, the large effect sizes suggest that findings would be similar in a randomized study, Dr. Hlatky said.
“I don’t think this is by chance. The plausibility of it has been explained,” he said, adding that while most people are happy with a normal CT angiography because of the high sensitivity, estimated FFR using the CT technique can be helpful in the setting of uncertainty.
“If you see something and you’re not sure if it’s significant, and if the estimated FFR from this technique is normal, that’s extremely reassuring that it’s just something you’re seeing but it’s not necessarily obstructing flow,” he said.
He added that “this would be best tested by doing a real, true, randomized study,” but said he considers the findings to be “quite interesting and completely in line with the clinical results.”
The technique is “entering progressively into practice in Europe,” said Dr. de Bruyne, who is a PLATFORM coinvestigator. “It is already used in clinical practice. You get the anatomy and physiology at the same time and same place. It is a really important paradigm change,” he said.
Dr. Hlatky and Dr. de Bruyne reported receiving research grants from HeartFlow, which supported the study.
AT TCT 2015
Key clinical point: Estimating fractional flow reserve with computed tomography appears to reduce resource use and costs when compared with invasive coronary angiography in stable patients with possible symptoms of coronary disease.
Major finding: Costs in patients in the planned invasive testing group who underwent FFR-CT were reduced by 32% compared with those who received usual care ($7,347 vs. $10,734).
Data source: A prospective, multicenter substudy of the PLATFORM trial, involving 584 patients.
Disclosures: Dr. Hlatky reported receiving research grants from HeartFlow, which supported the study.
ESC: What’s the hottest recent advance in cardiology? And the winner is …
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
ESC: Coronary artery calcium score gets an upgrade
LONDON – Coronary artery calcium as assessed by CT scan, widely considered the best marker of cardiovascular risk, just got significantly better.
The standard measure of coronary artery calcium (CAC) has been the Agatson score, which evaluates plaque calcium volume. But new evidence from the large, multicenter, prospective, observational Multi-Ethnic Study of Atherosclerosis (MESA) demonstrates that plaque calcium density is independently and inversely associated with both CHD and stroke risk. In other words, greater calcium density is protective against cardiovascular disease and counteracts the increased risk associated with greater calcium volume, Dr. Michael H. Criqui said at the annual congress of the European Society of Cardiology.
“We no longer believe in the Agatson score. We took a look at it and found out that at any given level of plaque calcium volume, a higher density score is protective. So when we look at our scans now, we no longer use the Agatson. We take the volume, then measure density separately, and we calculate a score that’s based on both,” explained Dr. Criqui, professor and chief of the division of preventive medicine at the University of California, San Diego.
Session moderator Dr. Sidney C. Smith Jr., was favorably impressed by the new analysis.
“Somehow we need to get this information in front of the guideline committees for the ESC, ACC [American College of Cardiology], and AHA [American Heart Association], because this is very interesting,” said Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
The MESA analysis included 3,398 adults followed for an average of 10.3 years. During that time 264 incident CHD events and 126 hard stroke events occurred.
“You find that as the calcium volume gets higher, the CHD risk gets much higher – up to fourfold higher for the fourth quartile. But we all knew that before. The new concept is that as your density score gets higher your risk goes way down. In the fourth quartile of density, you have only half the risk of developing a coronary event at any given calcium volume,” according to Dr. Criqui.
This confirms an earlier preliminary report by Dr. Criqui and coinvestigators based upon 7.6 years of MESA follow-up (JAMA. 2014 Jan 15;311[3]:271-8). The number of cardiovascular events in the update is 47% greater than in the initial report, considerably strengthening the findings.
The predictive power of the combined CAC volume and density score was underscored by the finding that the area under the receiver operating curve for CHD events was 0.674. To put that in perspective, when the investigators applied the ACC/AHA risk calculator tool to the MESA data, the area under the receiver operating characteristic curve was less impressive at 0.654. Combining the risk prediction score and the CAC volume/density score further increases the predictive power, he noted.
Plaque calcium density was similarly predictive in men and women, in younger and older adults, and in all four ethnic groups participating in MESA: Hispanics, African Americans, non-Hispanic whites, and Asians.
For CHD, the hazard ratio was 1.83 for each standard deviation of CAC volume and 0.71 for each standard deviation of CAC density. For stroke, the impacts were slightly less: a hazard ratio of 1.46 for each standard deviation of CAC volume and 0.83 for each standard deviation of density.
Asked if any biomarkers are related to CAC density, Dr. Criqui replied, “Preliminary data show that most of the risk factors we know are bad for us, like diabetes and smoking, are associated with lower CAC density. And the things that are good for us, like exercise and statins, are associated with higher density.”
He and his coworkers are now looking at plaque calcium density versus volume in the abdominal and thoracic aorta to learn if the same relationships seen in the coronary arteries hold true.
MESA is funded by the National Heart, Lung, and Blood Institute. Dr. Criqui reported having no financial conflicts of interest.
LONDON – Coronary artery calcium as assessed by CT scan, widely considered the best marker of cardiovascular risk, just got significantly better.
The standard measure of coronary artery calcium (CAC) has been the Agatson score, which evaluates plaque calcium volume. But new evidence from the large, multicenter, prospective, observational Multi-Ethnic Study of Atherosclerosis (MESA) demonstrates that plaque calcium density is independently and inversely associated with both CHD and stroke risk. In other words, greater calcium density is protective against cardiovascular disease and counteracts the increased risk associated with greater calcium volume, Dr. Michael H. Criqui said at the annual congress of the European Society of Cardiology.
“We no longer believe in the Agatson score. We took a look at it and found out that at any given level of plaque calcium volume, a higher density score is protective. So when we look at our scans now, we no longer use the Agatson. We take the volume, then measure density separately, and we calculate a score that’s based on both,” explained Dr. Criqui, professor and chief of the division of preventive medicine at the University of California, San Diego.
Session moderator Dr. Sidney C. Smith Jr., was favorably impressed by the new analysis.
“Somehow we need to get this information in front of the guideline committees for the ESC, ACC [American College of Cardiology], and AHA [American Heart Association], because this is very interesting,” said Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
The MESA analysis included 3,398 adults followed for an average of 10.3 years. During that time 264 incident CHD events and 126 hard stroke events occurred.
“You find that as the calcium volume gets higher, the CHD risk gets much higher – up to fourfold higher for the fourth quartile. But we all knew that before. The new concept is that as your density score gets higher your risk goes way down. In the fourth quartile of density, you have only half the risk of developing a coronary event at any given calcium volume,” according to Dr. Criqui.
This confirms an earlier preliminary report by Dr. Criqui and coinvestigators based upon 7.6 years of MESA follow-up (JAMA. 2014 Jan 15;311[3]:271-8). The number of cardiovascular events in the update is 47% greater than in the initial report, considerably strengthening the findings.
The predictive power of the combined CAC volume and density score was underscored by the finding that the area under the receiver operating curve for CHD events was 0.674. To put that in perspective, when the investigators applied the ACC/AHA risk calculator tool to the MESA data, the area under the receiver operating characteristic curve was less impressive at 0.654. Combining the risk prediction score and the CAC volume/density score further increases the predictive power, he noted.
Plaque calcium density was similarly predictive in men and women, in younger and older adults, and in all four ethnic groups participating in MESA: Hispanics, African Americans, non-Hispanic whites, and Asians.
For CHD, the hazard ratio was 1.83 for each standard deviation of CAC volume and 0.71 for each standard deviation of CAC density. For stroke, the impacts were slightly less: a hazard ratio of 1.46 for each standard deviation of CAC volume and 0.83 for each standard deviation of density.
Asked if any biomarkers are related to CAC density, Dr. Criqui replied, “Preliminary data show that most of the risk factors we know are bad for us, like diabetes and smoking, are associated with lower CAC density. And the things that are good for us, like exercise and statins, are associated with higher density.”
He and his coworkers are now looking at plaque calcium density versus volume in the abdominal and thoracic aorta to learn if the same relationships seen in the coronary arteries hold true.
MESA is funded by the National Heart, Lung, and Blood Institute. Dr. Criqui reported having no financial conflicts of interest.
LONDON – Coronary artery calcium as assessed by CT scan, widely considered the best marker of cardiovascular risk, just got significantly better.
The standard measure of coronary artery calcium (CAC) has been the Agatson score, which evaluates plaque calcium volume. But new evidence from the large, multicenter, prospective, observational Multi-Ethnic Study of Atherosclerosis (MESA) demonstrates that plaque calcium density is independently and inversely associated with both CHD and stroke risk. In other words, greater calcium density is protective against cardiovascular disease and counteracts the increased risk associated with greater calcium volume, Dr. Michael H. Criqui said at the annual congress of the European Society of Cardiology.
“We no longer believe in the Agatson score. We took a look at it and found out that at any given level of plaque calcium volume, a higher density score is protective. So when we look at our scans now, we no longer use the Agatson. We take the volume, then measure density separately, and we calculate a score that’s based on both,” explained Dr. Criqui, professor and chief of the division of preventive medicine at the University of California, San Diego.
Session moderator Dr. Sidney C. Smith Jr., was favorably impressed by the new analysis.
“Somehow we need to get this information in front of the guideline committees for the ESC, ACC [American College of Cardiology], and AHA [American Heart Association], because this is very interesting,” said Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
The MESA analysis included 3,398 adults followed for an average of 10.3 years. During that time 264 incident CHD events and 126 hard stroke events occurred.
“You find that as the calcium volume gets higher, the CHD risk gets much higher – up to fourfold higher for the fourth quartile. But we all knew that before. The new concept is that as your density score gets higher your risk goes way down. In the fourth quartile of density, you have only half the risk of developing a coronary event at any given calcium volume,” according to Dr. Criqui.
This confirms an earlier preliminary report by Dr. Criqui and coinvestigators based upon 7.6 years of MESA follow-up (JAMA. 2014 Jan 15;311[3]:271-8). The number of cardiovascular events in the update is 47% greater than in the initial report, considerably strengthening the findings.
The predictive power of the combined CAC volume and density score was underscored by the finding that the area under the receiver operating curve for CHD events was 0.674. To put that in perspective, when the investigators applied the ACC/AHA risk calculator tool to the MESA data, the area under the receiver operating characteristic curve was less impressive at 0.654. Combining the risk prediction score and the CAC volume/density score further increases the predictive power, he noted.
Plaque calcium density was similarly predictive in men and women, in younger and older adults, and in all four ethnic groups participating in MESA: Hispanics, African Americans, non-Hispanic whites, and Asians.
For CHD, the hazard ratio was 1.83 for each standard deviation of CAC volume and 0.71 for each standard deviation of CAC density. For stroke, the impacts were slightly less: a hazard ratio of 1.46 for each standard deviation of CAC volume and 0.83 for each standard deviation of density.
Asked if any biomarkers are related to CAC density, Dr. Criqui replied, “Preliminary data show that most of the risk factors we know are bad for us, like diabetes and smoking, are associated with lower CAC density. And the things that are good for us, like exercise and statins, are associated with higher density.”
He and his coworkers are now looking at plaque calcium density versus volume in the abdominal and thoracic aorta to learn if the same relationships seen in the coronary arteries hold true.
MESA is funded by the National Heart, Lung, and Blood Institute. Dr. Criqui reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2015
Key clinical point: Greater coronary artery plaque calcium density protects against cardiovascular events.
Major finding: The risk of a coronary heart disease event decreases by 29% for each standard deviation increase in coronary artery calcium plaque density and rises by 83% with each standard deviation increase in plaque volume.
Data source: This analysis from the multicenter, prospective, observational Multi-Ethnic Study of Atherosclerosis included 3,398 adults followed for an average of 10.3 years.
Disclosures: The MESA study is funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
BCVI: Screen with CT angiography, confirm with DSA
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Use of 64-channel CT angiography screening coupled with digital subtraction angiography for a definitive diagnosis of blunt cerebrovascular injury is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate.
Major finding: None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke.
Data source: A review of 228 patients with possible BCVI.
Disclosures: Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
Case Report: Diagnosis of Small Bowel Obstruction With Bedside Ultrasound
Case
A 64-year-old man presented to the ED seeking assistance in withdrawing from his prescription of oxycodone, which he had been taking to manage chronic pain due to rheumatoid arthritis. He stated that he had discontinued the oxycodone approximately 4 days prior to presentation and over the past 3 days, had been experiencing abdominal pain, nausea, vomiting, diarrhea, and diaphoresis. He noted that his symptoms were identical to those he had during previous unsuccessful attempts to wean himself from the narcotic medication. He denied any fever, dysuria, penile discharge, or any skin changes. Further evaluation revealed a remote history of a cholecystectomy and an appendectomy.
During the initial examination, the patient appeared uncomfortable but in no acute distress. His vital signs were: heart rate, 110 beats/minute; blood pressure, 107/76 mm Hg, respiratory rate, 22 breaths/minute; and temperature, 99˚F. Oxygen saturation was 97% on room air. The abdominal examination revealed moderate diffuse tenderness, mild distension without guarding or rebound, and some well-healed surgical scars.
In light of the abnormal sonographic findings, a computed tomography (CT) scan with intravenous (IV) contrast was performed, which confirmed the diagnosis of a distal small bowel obstruction (SBO). Surgery services were consulted. As the patient’s current symptoms were believed to be the result of an SBO and not from narcotic withdrawal, surgery services instructed nothing by mouth and elected nonsurgical management. They placed a nasogastric tube and administered fluids and analgesics via IV. The patient was discharged 4 days after presentation to the ED, with complete resolution of his symptoms.
Discussion
Annually in the United States, less than 1% of all patients presenting to EDs are subsequently diagnosed with SBO.1 However, this disease comprises 15% of all surgical hospital admissions, costing upward of $1 billion in annual hospital charges.2 Moreover, patients with SBO suffer from a disproportionately high morbidity (eg, bowel strangulation, necrosis) and mortality than the general population,3-5 and delayed diagnosis is associated with a higher risk of bowel resection. One study by Bickell et al6 showed that only 4% of patients appropriately managed less than 24 hours after symptom onset required resection compared with 10% to 14% of patients managed more than 24 hours after symptom onset.
As most patients diagnosed with SBO are first seen in the ED, emergency physicians (EPs) have a distinctive role in lowering the likelihood of a poor outcome by making this diagnosis early.7 Multiple methods of diagnosing SBO are at the disposal of the clinician, including the history and physical examination, abdominal X-ray, ultrasound, CT, and magnetic resonance imaging (MRI).
The history and physical examination can be rapidly performed at the bedside in patients with suspected SBO. The factors typically associated with SBO include constipation, a previous history of abdominal surgeries, abnormal bowel sounds, and abdominal distension.3 However, these findings are not sufficient to accurately and adequately rule in or rule out disease.3,8,9
Diagnostic Imaging
While patient history and physical examination may be helpful in diagnosing SBO, imaging plays a critical role in the definitive diagnosis. The imaging modality that is the de facto gold standard for diagnosis is the CT scan.10 A meta-analysis by Taylor et al,3 which included 64-slice multidetector CT imaging studies (using both oral and IV contrast), demonstrated sensitivities of 93% to 96% and specificities of 93% to 100% in diagnosing SBO.
In patients in whom CT is contraindicated, MRI can be a useful alternative, with studies showing a similar diagnostic accuracy to 64-slice CT.13,14 Both CT and MRI are highly accurate in diagnosing SBO; however, there are disadvantages to their use. Such disadvantages include the inability to perform these studies at bedside; the length of time to perform these studies; the higher cost compared to other modalities; and, in CT, the adverse side effects of radiation and possible contrast reactions.
Bedside Ultrasound
Abdominal X-ray traditionally has been the initial choice in bedside imaging for SBO; however, a recent meta-analysis found this modality to have a summary sensitivity of 75%, specificity of 66%, positive likelihood ratio of 1.6, and negative likelihood ratio of 0.43 in diagnosing SBO.3 Based on these statistics, bedside ultrasound has recently ascended as a viable alternative to abdominal X-ray.
Although there is limited research regarding the accuracy of ultrasound to evaluate SBO, initial study results are encouraging. The previously cited meta-analysis by Taylor et al3 identified six ultrasound studies, two of which were performed in the ED. In one of these two studies, Unlüer et al10 performed a prospective study that enrolled 174 patients in the ED, 90 of whom were subsequently found to have an SBO. In addition, Unlüer et al’s study found that relatively inexperienced emergency medicine (EM) residents were able to use bedside ultrasound in the diagnosis of SBO with a sensitivity of 97.7% and a specificity of 92.7%.
Another ED study by Jang et al15 enrolled 76 patients, 33 of whom were diagnosed with SBO using CT. In this study, the authors found ultrasound to have a 91% sensitivity and 84% specificity for dilated bowel, and a specificity of 98% and sensitivity of 27% for decreased peristalsis.15 Imaging in this study was performed by EM residents, who received only 10 minutes of didactic lecture.
The criteria used in the abovementioned studies varied slightly. The study by Jang et al15 used either fluid-filled dilated bowel >2.5 cm or decreased/absent forward bowel peristalsis, while the study by Unlüer et al10 defined sonographic SBO as two of the three following criteria: greater than 3 dilated loops of either jejunum (>25 mm), or of ileum (>15 mm), increased peristalsis or a collapsed colonic lumen. In cases of higher-grade obstruction, the Tanga sign, fluid seen outside of the dilated loops of bowel, has also been reported (Figure 2).16
Conclusion
There are several distinct advantages to using bedside ultrasound in cases of suspected SBO, including its lack of ionizing radiation, the ability to perform the scan rapidly, and the high accuracy rate in detecting this condition—even in the hands of providers with minimal training. In addition to its cost-effectiveness, ultrasound may be preferred in patients with relative contraindications to CT, such as pregnant patients and patients with contrast allergies. Even in patients in whom there is no contraindication to CT, ultrasound may be used to safely and quickly identify and risk-stratify those who require further imaging versus those who can be safely discharged home—or possibly even finding alternative diagnoses of acute abdominal pain (eg, acute cholecystitis, ureterolithiasis, abdominal aortic aneurysm).
Dr Avila is an attending physician and ultrasound fellow in the department of emergency medicine at the University of Kentucky, Lexington. Dr Smith is the director of emergency ultrasound in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga. Dr Whittle is the director of research in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35 year retrospective. Am J Emerg Med. 201;29(7):711-716.
- Rocha FG, Theman TA, Matros E, Ledbetter SM, Zinner MJ, Ferzoco SJ. Nonoperative management of patients with a diagnosis of high-grade small bowel obstruction by computed tomography. Arch Surg. 2009;144(11):1000-1004.
- Taylor M, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Fevang BT, Fevang J, Stangeland L, Soreide O, Svanes K, Viste A. Complications and death after surgical treatment of small bowel obstruction: a 35-year institutional experience. Ann Surg. 2000;231(4):529-537.
- Cheadle WG, Garr EE, Richardson JD. The importance of early diagnosis of small bowel obstruction. Am Surg. 1988;54(9):565-569.
- Bickell NA, Federman AD, Aufses AH Jr. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203(2):170-176.
- Eskelinen M, Ikonen J, Lipponen P. Contributions of history-taking, physical examination, and computer assistance to diagnosis of acute small-bowel obstruction. Scand J Gastroenterol. 1994;29(8):715-721.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg. 1998;164(10):777-784.
- Unlüer EE, Yavaşi O, Eroğlu O, Yilmaz C, Akarca FK. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Pongpornsup S, Tarachat K, Srisajjakul S. Accuracy of 64-slice multi-detector computed tomography in diagnosis of small bowel obstruction. J Med Assoc Thai. 2009;92(12):1651-1661.
- Shakil O, Zafar SN, Zia-ur-Rehman, Saleem S, Khan R, Pal KM. The role of computed tomography for identifying mechanical bowel obstruction in a Pakistani population. J Pak Med Assoc. 2011;61(9):871-874.
- Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57(8):719-724.
- Regan F, Beall DP, Bohlman ME, Khazan R, Sufi A, Schaefer DC. Fast MR imaging and the detection of small-bowel obstruction. Am J Roentgenol. 1998;170(6):1465-1469.
- Jang TB, Schindler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28(8):676-678.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
Case
A 64-year-old man presented to the ED seeking assistance in withdrawing from his prescription of oxycodone, which he had been taking to manage chronic pain due to rheumatoid arthritis. He stated that he had discontinued the oxycodone approximately 4 days prior to presentation and over the past 3 days, had been experiencing abdominal pain, nausea, vomiting, diarrhea, and diaphoresis. He noted that his symptoms were identical to those he had during previous unsuccessful attempts to wean himself from the narcotic medication. He denied any fever, dysuria, penile discharge, or any skin changes. Further evaluation revealed a remote history of a cholecystectomy and an appendectomy.
During the initial examination, the patient appeared uncomfortable but in no acute distress. His vital signs were: heart rate, 110 beats/minute; blood pressure, 107/76 mm Hg, respiratory rate, 22 breaths/minute; and temperature, 99˚F. Oxygen saturation was 97% on room air. The abdominal examination revealed moderate diffuse tenderness, mild distension without guarding or rebound, and some well-healed surgical scars.
In light of the abnormal sonographic findings, a computed tomography (CT) scan with intravenous (IV) contrast was performed, which confirmed the diagnosis of a distal small bowel obstruction (SBO). Surgery services were consulted. As the patient’s current symptoms were believed to be the result of an SBO and not from narcotic withdrawal, surgery services instructed nothing by mouth and elected nonsurgical management. They placed a nasogastric tube and administered fluids and analgesics via IV. The patient was discharged 4 days after presentation to the ED, with complete resolution of his symptoms.
Discussion
Annually in the United States, less than 1% of all patients presenting to EDs are subsequently diagnosed with SBO.1 However, this disease comprises 15% of all surgical hospital admissions, costing upward of $1 billion in annual hospital charges.2 Moreover, patients with SBO suffer from a disproportionately high morbidity (eg, bowel strangulation, necrosis) and mortality than the general population,3-5 and delayed diagnosis is associated with a higher risk of bowel resection. One study by Bickell et al6 showed that only 4% of patients appropriately managed less than 24 hours after symptom onset required resection compared with 10% to 14% of patients managed more than 24 hours after symptom onset.
As most patients diagnosed with SBO are first seen in the ED, emergency physicians (EPs) have a distinctive role in lowering the likelihood of a poor outcome by making this diagnosis early.7 Multiple methods of diagnosing SBO are at the disposal of the clinician, including the history and physical examination, abdominal X-ray, ultrasound, CT, and magnetic resonance imaging (MRI).
The history and physical examination can be rapidly performed at the bedside in patients with suspected SBO. The factors typically associated with SBO include constipation, a previous history of abdominal surgeries, abnormal bowel sounds, and abdominal distension.3 However, these findings are not sufficient to accurately and adequately rule in or rule out disease.3,8,9
Diagnostic Imaging
While patient history and physical examination may be helpful in diagnosing SBO, imaging plays a critical role in the definitive diagnosis. The imaging modality that is the de facto gold standard for diagnosis is the CT scan.10 A meta-analysis by Taylor et al,3 which included 64-slice multidetector CT imaging studies (using both oral and IV contrast), demonstrated sensitivities of 93% to 96% and specificities of 93% to 100% in diagnosing SBO.
In patients in whom CT is contraindicated, MRI can be a useful alternative, with studies showing a similar diagnostic accuracy to 64-slice CT.13,14 Both CT and MRI are highly accurate in diagnosing SBO; however, there are disadvantages to their use. Such disadvantages include the inability to perform these studies at bedside; the length of time to perform these studies; the higher cost compared to other modalities; and, in CT, the adverse side effects of radiation and possible contrast reactions.
Bedside Ultrasound
Abdominal X-ray traditionally has been the initial choice in bedside imaging for SBO; however, a recent meta-analysis found this modality to have a summary sensitivity of 75%, specificity of 66%, positive likelihood ratio of 1.6, and negative likelihood ratio of 0.43 in diagnosing SBO.3 Based on these statistics, bedside ultrasound has recently ascended as a viable alternative to abdominal X-ray.
Although there is limited research regarding the accuracy of ultrasound to evaluate SBO, initial study results are encouraging. The previously cited meta-analysis by Taylor et al3 identified six ultrasound studies, two of which were performed in the ED. In one of these two studies, Unlüer et al10 performed a prospective study that enrolled 174 patients in the ED, 90 of whom were subsequently found to have an SBO. In addition, Unlüer et al’s study found that relatively inexperienced emergency medicine (EM) residents were able to use bedside ultrasound in the diagnosis of SBO with a sensitivity of 97.7% and a specificity of 92.7%.
Another ED study by Jang et al15 enrolled 76 patients, 33 of whom were diagnosed with SBO using CT. In this study, the authors found ultrasound to have a 91% sensitivity and 84% specificity for dilated bowel, and a specificity of 98% and sensitivity of 27% for decreased peristalsis.15 Imaging in this study was performed by EM residents, who received only 10 minutes of didactic lecture.
The criteria used in the abovementioned studies varied slightly. The study by Jang et al15 used either fluid-filled dilated bowel >2.5 cm or decreased/absent forward bowel peristalsis, while the study by Unlüer et al10 defined sonographic SBO as two of the three following criteria: greater than 3 dilated loops of either jejunum (>25 mm), or of ileum (>15 mm), increased peristalsis or a collapsed colonic lumen. In cases of higher-grade obstruction, the Tanga sign, fluid seen outside of the dilated loops of bowel, has also been reported (Figure 2).16
Conclusion
There are several distinct advantages to using bedside ultrasound in cases of suspected SBO, including its lack of ionizing radiation, the ability to perform the scan rapidly, and the high accuracy rate in detecting this condition—even in the hands of providers with minimal training. In addition to its cost-effectiveness, ultrasound may be preferred in patients with relative contraindications to CT, such as pregnant patients and patients with contrast allergies. Even in patients in whom there is no contraindication to CT, ultrasound may be used to safely and quickly identify and risk-stratify those who require further imaging versus those who can be safely discharged home—or possibly even finding alternative diagnoses of acute abdominal pain (eg, acute cholecystitis, ureterolithiasis, abdominal aortic aneurysm).
Dr Avila is an attending physician and ultrasound fellow in the department of emergency medicine at the University of Kentucky, Lexington. Dr Smith is the director of emergency ultrasound in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga. Dr Whittle is the director of research in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga.
Case
A 64-year-old man presented to the ED seeking assistance in withdrawing from his prescription of oxycodone, which he had been taking to manage chronic pain due to rheumatoid arthritis. He stated that he had discontinued the oxycodone approximately 4 days prior to presentation and over the past 3 days, had been experiencing abdominal pain, nausea, vomiting, diarrhea, and diaphoresis. He noted that his symptoms were identical to those he had during previous unsuccessful attempts to wean himself from the narcotic medication. He denied any fever, dysuria, penile discharge, or any skin changes. Further evaluation revealed a remote history of a cholecystectomy and an appendectomy.
During the initial examination, the patient appeared uncomfortable but in no acute distress. His vital signs were: heart rate, 110 beats/minute; blood pressure, 107/76 mm Hg, respiratory rate, 22 breaths/minute; and temperature, 99˚F. Oxygen saturation was 97% on room air. The abdominal examination revealed moderate diffuse tenderness, mild distension without guarding or rebound, and some well-healed surgical scars.
In light of the abnormal sonographic findings, a computed tomography (CT) scan with intravenous (IV) contrast was performed, which confirmed the diagnosis of a distal small bowel obstruction (SBO). Surgery services were consulted. As the patient’s current symptoms were believed to be the result of an SBO and not from narcotic withdrawal, surgery services instructed nothing by mouth and elected nonsurgical management. They placed a nasogastric tube and administered fluids and analgesics via IV. The patient was discharged 4 days after presentation to the ED, with complete resolution of his symptoms.
Discussion
Annually in the United States, less than 1% of all patients presenting to EDs are subsequently diagnosed with SBO.1 However, this disease comprises 15% of all surgical hospital admissions, costing upward of $1 billion in annual hospital charges.2 Moreover, patients with SBO suffer from a disproportionately high morbidity (eg, bowel strangulation, necrosis) and mortality than the general population,3-5 and delayed diagnosis is associated with a higher risk of bowel resection. One study by Bickell et al6 showed that only 4% of patients appropriately managed less than 24 hours after symptom onset required resection compared with 10% to 14% of patients managed more than 24 hours after symptom onset.
As most patients diagnosed with SBO are first seen in the ED, emergency physicians (EPs) have a distinctive role in lowering the likelihood of a poor outcome by making this diagnosis early.7 Multiple methods of diagnosing SBO are at the disposal of the clinician, including the history and physical examination, abdominal X-ray, ultrasound, CT, and magnetic resonance imaging (MRI).
The history and physical examination can be rapidly performed at the bedside in patients with suspected SBO. The factors typically associated with SBO include constipation, a previous history of abdominal surgeries, abnormal bowel sounds, and abdominal distension.3 However, these findings are not sufficient to accurately and adequately rule in or rule out disease.3,8,9
Diagnostic Imaging
While patient history and physical examination may be helpful in diagnosing SBO, imaging plays a critical role in the definitive diagnosis. The imaging modality that is the de facto gold standard for diagnosis is the CT scan.10 A meta-analysis by Taylor et al,3 which included 64-slice multidetector CT imaging studies (using both oral and IV contrast), demonstrated sensitivities of 93% to 96% and specificities of 93% to 100% in diagnosing SBO.
In patients in whom CT is contraindicated, MRI can be a useful alternative, with studies showing a similar diagnostic accuracy to 64-slice CT.13,14 Both CT and MRI are highly accurate in diagnosing SBO; however, there are disadvantages to their use. Such disadvantages include the inability to perform these studies at bedside; the length of time to perform these studies; the higher cost compared to other modalities; and, in CT, the adverse side effects of radiation and possible contrast reactions.
Bedside Ultrasound
Abdominal X-ray traditionally has been the initial choice in bedside imaging for SBO; however, a recent meta-analysis found this modality to have a summary sensitivity of 75%, specificity of 66%, positive likelihood ratio of 1.6, and negative likelihood ratio of 0.43 in diagnosing SBO.3 Based on these statistics, bedside ultrasound has recently ascended as a viable alternative to abdominal X-ray.
Although there is limited research regarding the accuracy of ultrasound to evaluate SBO, initial study results are encouraging. The previously cited meta-analysis by Taylor et al3 identified six ultrasound studies, two of which were performed in the ED. In one of these two studies, Unlüer et al10 performed a prospective study that enrolled 174 patients in the ED, 90 of whom were subsequently found to have an SBO. In addition, Unlüer et al’s study found that relatively inexperienced emergency medicine (EM) residents were able to use bedside ultrasound in the diagnosis of SBO with a sensitivity of 97.7% and a specificity of 92.7%.
Another ED study by Jang et al15 enrolled 76 patients, 33 of whom were diagnosed with SBO using CT. In this study, the authors found ultrasound to have a 91% sensitivity and 84% specificity for dilated bowel, and a specificity of 98% and sensitivity of 27% for decreased peristalsis.15 Imaging in this study was performed by EM residents, who received only 10 minutes of didactic lecture.
The criteria used in the abovementioned studies varied slightly. The study by Jang et al15 used either fluid-filled dilated bowel >2.5 cm or decreased/absent forward bowel peristalsis, while the study by Unlüer et al10 defined sonographic SBO as two of the three following criteria: greater than 3 dilated loops of either jejunum (>25 mm), or of ileum (>15 mm), increased peristalsis or a collapsed colonic lumen. In cases of higher-grade obstruction, the Tanga sign, fluid seen outside of the dilated loops of bowel, has also been reported (Figure 2).16
Conclusion
There are several distinct advantages to using bedside ultrasound in cases of suspected SBO, including its lack of ionizing radiation, the ability to perform the scan rapidly, and the high accuracy rate in detecting this condition—even in the hands of providers with minimal training. In addition to its cost-effectiveness, ultrasound may be preferred in patients with relative contraindications to CT, such as pregnant patients and patients with contrast allergies. Even in patients in whom there is no contraindication to CT, ultrasound may be used to safely and quickly identify and risk-stratify those who require further imaging versus those who can be safely discharged home—or possibly even finding alternative diagnoses of acute abdominal pain (eg, acute cholecystitis, ureterolithiasis, abdominal aortic aneurysm).
Dr Avila is an attending physician and ultrasound fellow in the department of emergency medicine at the University of Kentucky, Lexington. Dr Smith is the director of emergency ultrasound in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga. Dr Whittle is the director of research in the department of emergency medicine at the University of Tennessee College of Medicine Chattanooga.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35 year retrospective. Am J Emerg Med. 201;29(7):711-716.
- Rocha FG, Theman TA, Matros E, Ledbetter SM, Zinner MJ, Ferzoco SJ. Nonoperative management of patients with a diagnosis of high-grade small bowel obstruction by computed tomography. Arch Surg. 2009;144(11):1000-1004.
- Taylor M, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Fevang BT, Fevang J, Stangeland L, Soreide O, Svanes K, Viste A. Complications and death after surgical treatment of small bowel obstruction: a 35-year institutional experience. Ann Surg. 2000;231(4):529-537.
- Cheadle WG, Garr EE, Richardson JD. The importance of early diagnosis of small bowel obstruction. Am Surg. 1988;54(9):565-569.
- Bickell NA, Federman AD, Aufses AH Jr. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203(2):170-176.
- Eskelinen M, Ikonen J, Lipponen P. Contributions of history-taking, physical examination, and computer assistance to diagnosis of acute small-bowel obstruction. Scand J Gastroenterol. 1994;29(8):715-721.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg. 1998;164(10):777-784.
- Unlüer EE, Yavaşi O, Eroğlu O, Yilmaz C, Akarca FK. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Pongpornsup S, Tarachat K, Srisajjakul S. Accuracy of 64-slice multi-detector computed tomography in diagnosis of small bowel obstruction. J Med Assoc Thai. 2009;92(12):1651-1661.
- Shakil O, Zafar SN, Zia-ur-Rehman, Saleem S, Khan R, Pal KM. The role of computed tomography for identifying mechanical bowel obstruction in a Pakistani population. J Pak Med Assoc. 2011;61(9):871-874.
- Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57(8):719-724.
- Regan F, Beall DP, Bohlman ME, Khazan R, Sufi A, Schaefer DC. Fast MR imaging and the detection of small-bowel obstruction. Am J Roentgenol. 1998;170(6):1465-1469.
- Jang TB, Schindler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28(8):676-678.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35 year retrospective. Am J Emerg Med. 201;29(7):711-716.
- Rocha FG, Theman TA, Matros E, Ledbetter SM, Zinner MJ, Ferzoco SJ. Nonoperative management of patients with a diagnosis of high-grade small bowel obstruction by computed tomography. Arch Surg. 2009;144(11):1000-1004.
- Taylor M, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Fevang BT, Fevang J, Stangeland L, Soreide O, Svanes K, Viste A. Complications and death after surgical treatment of small bowel obstruction: a 35-year institutional experience. Ann Surg. 2000;231(4):529-537.
- Cheadle WG, Garr EE, Richardson JD. The importance of early diagnosis of small bowel obstruction. Am Surg. 1988;54(9):565-569.
- Bickell NA, Federman AD, Aufses AH Jr. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203(2):170-176.
- Eskelinen M, Ikonen J, Lipponen P. Contributions of history-taking, physical examination, and computer assistance to diagnosis of acute small-bowel obstruction. Scand J Gastroenterol. 1994;29(8):715-721.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg. 1998;164(10):777-784.
- Unlüer EE, Yavaşi O, Eroğlu O, Yilmaz C, Akarca FK. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Pongpornsup S, Tarachat K, Srisajjakul S. Accuracy of 64-slice multi-detector computed tomography in diagnosis of small bowel obstruction. J Med Assoc Thai. 2009;92(12):1651-1661.
- Shakil O, Zafar SN, Zia-ur-Rehman, Saleem S, Khan R, Pal KM. The role of computed tomography for identifying mechanical bowel obstruction in a Pakistani population. J Pak Med Assoc. 2011;61(9):871-874.
- Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57(8):719-724.
- Regan F, Beall DP, Bohlman ME, Khazan R, Sufi A, Schaefer DC. Fast MR imaging and the detection of small-bowel obstruction. Am J Roentgenol. 1998;170(6):1465-1469.
- Jang TB, Schindler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28(8):676-678.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
Emergency Ultrasound: Evaluating Right Upper Quadrant Abdominal Pain
When evaluating patients presenting with right upper quadrant abdominal pain, there are several imaging modalities to consider—ultrasound, computed tomography, endoscopic retrograde cholangiopancreatography, and hepatobiliary iminodiacetic acid scan. In addition to the many benefits to choosing bedside ultrasound as an initial modality, it also carries the least amount of imaging-associated risks (eg, lowest amount of radiation, no exposure to ionizing radiation or radionucleotides). Moreover, not only can bedside ultrasound decrease the time to diagnosis, but it also reduces the length of patient stay by an average of 1 hour.1
The patient is typically placed in the recumbent position, but in certain cases, placing the patient in the left lateral decubitus position may improve visualization. The scan should begin by placing the low-frequency probe in a sagittal orientation with the indicator marker pointed toward the head. Starting in the midline subxiphoid position, the clinician should angle the probe underneath the costal margin and then slide it to the patient’s right side. This will typically bring the gallbladder into view. If the gallbladder is not identified in this first pass, a second sweep across the ribs can be performed. If the gallbladder is still not in view, the clinician should then try placing the probe on the patient’s right side near the midaxillary line and fanning though the liver in a coronal plane (Figure 1).
Once the gallbladder is located, it is important to ensure that both the long and short axis planes are completely visualized. This can be accomplished by rotating the probe. There are several techniques to confirm that the structure in view is the gallbladder. One approach is to use the color-flow option, which can help differentiate vascular structures from the gallbladder. The gallbladder, which is connected to the portal vein by the main lobar fissure, will appear as an exclamation point on the ultrasound image (Figure 2).
In ultrasound, pericholecystic fluid will appear around the gallbladder as small black triangular shapes. Gallstones have a hyperechoic anterior rim with posterior dark shadowing. Typically, gallstones are freely mobile unless they are stuck in the neck of the gallbladder, in which case, there is heightened concern for cholecystitis (Figure 2).
Visualization of the common bile duct can be challenging. Fortunately, most cases of acute cholecystitis can be diagnosed even without visualization of the common bile duct. When attempting visualization, however, the common bile duct can be found by tracing the main lobar fissure to the portal vein. The portal triad (commonly referred to as the “Mickey Mouse” sign) comprises the portal vein (“head”), the hepatic artery (left “ear”), and the common bile duct (right “ear”) (Figure 3). Once this sign is identified, the probe should be rotated to bring a long axis view of the portal vein. The common bile duct will run anterior and parallel to the portal vein. The duct should measure less than 4 to 6 mm in diameter; however, in patients older than age 60 years, this range should be adjusted to allow an additional 1 mm per decade).
Summary
Abdominal pain is a common presenting complaint in the ED. Ultrasound is particularly useful in evaluating right upper quadrant pain. This ultrasound is easy to learn and, with practice, it can decrease time to diagnosis and improve the length of a patient’s stay in the ED.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Reference
- Blaivas M, Harwood RA, Lambert MJ. Decreasing length of stay with emergency ultrasound examination of the gallbladder. Acad Emerg Med. 1999;6(10):1020-1023.
Additional examples of ultrasound imaging and videos of the upper right abdominal quadrant may be accessed at https://vimeo.com/136832371
When evaluating patients presenting with right upper quadrant abdominal pain, there are several imaging modalities to consider—ultrasound, computed tomography, endoscopic retrograde cholangiopancreatography, and hepatobiliary iminodiacetic acid scan. In addition to the many benefits to choosing bedside ultrasound as an initial modality, it also carries the least amount of imaging-associated risks (eg, lowest amount of radiation, no exposure to ionizing radiation or radionucleotides). Moreover, not only can bedside ultrasound decrease the time to diagnosis, but it also reduces the length of patient stay by an average of 1 hour.1
The patient is typically placed in the recumbent position, but in certain cases, placing the patient in the left lateral decubitus position may improve visualization. The scan should begin by placing the low-frequency probe in a sagittal orientation with the indicator marker pointed toward the head. Starting in the midline subxiphoid position, the clinician should angle the probe underneath the costal margin and then slide it to the patient’s right side. This will typically bring the gallbladder into view. If the gallbladder is not identified in this first pass, a second sweep across the ribs can be performed. If the gallbladder is still not in view, the clinician should then try placing the probe on the patient’s right side near the midaxillary line and fanning though the liver in a coronal plane (Figure 1).
Once the gallbladder is located, it is important to ensure that both the long and short axis planes are completely visualized. This can be accomplished by rotating the probe. There are several techniques to confirm that the structure in view is the gallbladder. One approach is to use the color-flow option, which can help differentiate vascular structures from the gallbladder. The gallbladder, which is connected to the portal vein by the main lobar fissure, will appear as an exclamation point on the ultrasound image (Figure 2).
In ultrasound, pericholecystic fluid will appear around the gallbladder as small black triangular shapes. Gallstones have a hyperechoic anterior rim with posterior dark shadowing. Typically, gallstones are freely mobile unless they are stuck in the neck of the gallbladder, in which case, there is heightened concern for cholecystitis (Figure 2).
Visualization of the common bile duct can be challenging. Fortunately, most cases of acute cholecystitis can be diagnosed even without visualization of the common bile duct. When attempting visualization, however, the common bile duct can be found by tracing the main lobar fissure to the portal vein. The portal triad (commonly referred to as the “Mickey Mouse” sign) comprises the portal vein (“head”), the hepatic artery (left “ear”), and the common bile duct (right “ear”) (Figure 3). Once this sign is identified, the probe should be rotated to bring a long axis view of the portal vein. The common bile duct will run anterior and parallel to the portal vein. The duct should measure less than 4 to 6 mm in diameter; however, in patients older than age 60 years, this range should be adjusted to allow an additional 1 mm per decade).
Summary
Abdominal pain is a common presenting complaint in the ED. Ultrasound is particularly useful in evaluating right upper quadrant pain. This ultrasound is easy to learn and, with practice, it can decrease time to diagnosis and improve the length of a patient’s stay in the ED.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
When evaluating patients presenting with right upper quadrant abdominal pain, there are several imaging modalities to consider—ultrasound, computed tomography, endoscopic retrograde cholangiopancreatography, and hepatobiliary iminodiacetic acid scan. In addition to the many benefits to choosing bedside ultrasound as an initial modality, it also carries the least amount of imaging-associated risks (eg, lowest amount of radiation, no exposure to ionizing radiation or radionucleotides). Moreover, not only can bedside ultrasound decrease the time to diagnosis, but it also reduces the length of patient stay by an average of 1 hour.1
The patient is typically placed in the recumbent position, but in certain cases, placing the patient in the left lateral decubitus position may improve visualization. The scan should begin by placing the low-frequency probe in a sagittal orientation with the indicator marker pointed toward the head. Starting in the midline subxiphoid position, the clinician should angle the probe underneath the costal margin and then slide it to the patient’s right side. This will typically bring the gallbladder into view. If the gallbladder is not identified in this first pass, a second sweep across the ribs can be performed. If the gallbladder is still not in view, the clinician should then try placing the probe on the patient’s right side near the midaxillary line and fanning though the liver in a coronal plane (Figure 1).
Once the gallbladder is located, it is important to ensure that both the long and short axis planes are completely visualized. This can be accomplished by rotating the probe. There are several techniques to confirm that the structure in view is the gallbladder. One approach is to use the color-flow option, which can help differentiate vascular structures from the gallbladder. The gallbladder, which is connected to the portal vein by the main lobar fissure, will appear as an exclamation point on the ultrasound image (Figure 2).
In ultrasound, pericholecystic fluid will appear around the gallbladder as small black triangular shapes. Gallstones have a hyperechoic anterior rim with posterior dark shadowing. Typically, gallstones are freely mobile unless they are stuck in the neck of the gallbladder, in which case, there is heightened concern for cholecystitis (Figure 2).
Visualization of the common bile duct can be challenging. Fortunately, most cases of acute cholecystitis can be diagnosed even without visualization of the common bile duct. When attempting visualization, however, the common bile duct can be found by tracing the main lobar fissure to the portal vein. The portal triad (commonly referred to as the “Mickey Mouse” sign) comprises the portal vein (“head”), the hepatic artery (left “ear”), and the common bile duct (right “ear”) (Figure 3). Once this sign is identified, the probe should be rotated to bring a long axis view of the portal vein. The common bile duct will run anterior and parallel to the portal vein. The duct should measure less than 4 to 6 mm in diameter; however, in patients older than age 60 years, this range should be adjusted to allow an additional 1 mm per decade).
Summary
Abdominal pain is a common presenting complaint in the ED. Ultrasound is particularly useful in evaluating right upper quadrant pain. This ultrasound is easy to learn and, with practice, it can decrease time to diagnosis and improve the length of a patient’s stay in the ED.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Reference
- Blaivas M, Harwood RA, Lambert MJ. Decreasing length of stay with emergency ultrasound examination of the gallbladder. Acad Emerg Med. 1999;6(10):1020-1023.
Additional examples of ultrasound imaging and videos of the upper right abdominal quadrant may be accessed at https://vimeo.com/136832371
Reference
- Blaivas M, Harwood RA, Lambert MJ. Decreasing length of stay with emergency ultrasound examination of the gallbladder. Acad Emerg Med. 1999;6(10):1020-1023.
Additional examples of ultrasound imaging and videos of the upper right abdominal quadrant may be accessed at https://vimeo.com/136832371