User login
AHA: Coronary calcium personalizes ACC/AHA risk calculator
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
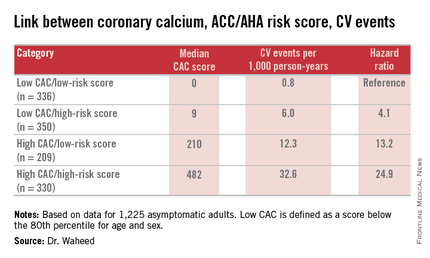
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.
“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.

“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
ORLANDO – Combining the coronary artery calcium score with the ACC/AHA cardiovascular risk calculator tool enables physicians to refine their decision making about who to recommend for statin therapy, Dr. Salman Waheed reported at the American Heart Association scientific sessions.
He presented an analysis of 1,225 asymptomatic adults followed for a median of 3.9 years in the observational arm of the St. Francis Health Study, an early landmark prospective study of the relationship between electron beam CT coronary artery calcium (CAC) score and cardiovascular event risk.
The 217 subjects who today would not be recommended for statin therapy on the basis of a 10-year atherosclerotic cardiovascular disease risk of 5%-7.4% as determined by the risk calculator included in the 2013 ACC/AHA cholesterol management guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45) would be reclassified as warranting statin therapy if they had a CAC greater than 0, as was the case for 169 of the 217 (78%). Indeed, the presence of a CAC of 1 or more boosted their estimated 10-year risk to 10.8%.
On the other hand, there were 510 patients who would be classified as high risk by the ACC/AHA clinical risk calculator, with a 10-year risk of 7.5%-20%. Taking their CAC score into account would result in 73 being reclassified as low risk and becoming no longer statin candidates because their CAC of 0 was associated with a 10-year event risk of less than 1%. In contrast, for the 447 remaining subjects with a CAC greater than 0, the 10-year risk climbed to 21.9%, according to Dr. Waheed of the University of Kansas, Kansas City.
The composite outcome utilized in this analysis from the St. Francis Heart Study was comprised of nonfatal MI, coronary death, stroke, peripheral arterial revascularization, or coronary revascularization. Of note, heart failure wasn’t included.
Among the 545 subjects deemed at low cardiovascular risk because they weren’t eligible for statin therapy according to the 2013 ACC/AHA guidelines, 209 would be recategorized as high risk on the basis of a CAC score at or above the 80th percentile adjusted for age and gender. The adjusted risk of a cardiovascular event in the high CAC/low clinical risk group was 24.9-fold greater than in the low CAC/low clinical risk group.

“Among those eligible for statin therapy based upon current guidelines, high CAC portends a sixfold higher outcome risk than low CAC,” Dr. Waheed added.
The magnitude of CAC progression over the course of 4 years was similar across the baseline risk categories; however, the absolute CAC progression was greater among those with a high CAC at baseline.
Audience member Dr. Daniel S. Berman observed that the results of Dr. Waheed’s study are highly concordant with an earlier report from the Multi-Ethnic Study of Atherosclerosis in which a CAC of 0 was quite common in patients for whom statins would be recommended under the current ACC/AHA guidelines.
“Your data support the idea that stratification based upon CAC could more personalize the statin recommendations,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles.
Another audience member, Dr. Donald M. Lloyd-Jones, one of the architects of the current guidelines, commented that the analyses from the St. Francis Heart Study and the Multi-Ethnic Study of Atherosclerosis suggest CAC screening coupled with the ACC/AHA risk score provides added value in a select group of patients.
“Your analysis continues to reinforce the point that we probably shouldn’t be doing universal CAC screening because in the people with a 10-year risk of less than 5% the yield is low and the CAC didn’t change anything, while in the people with a 10-year risk of 20% or higher the yield is incredibly high and the CAC didn’t change anything,” observed Dr. Lloyd-Jones, professor and chair of the department of preventive medicine at Northwestern University, Chicago.
“So CAC really is for those intermediate-risk folks where we’re on the bubble, where we might consider withholding therapy. And I’d love to see a trial to show that’s safe, by the way, but maybe that day will come. But we certainly would be comfortable up-classifying somebody if more CAC is present than there should be,” he added.
Dr. Waheed reported having no financial conflicts regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Measuring coronary artery calcium provides added value in refining the 10-year atherosclerotic cardiovascular disease risk, especially in patients with an intermediate-risk score on the ACC/AHA risk calculator.
Major finding: Seventy-eight percent of a group of patients for whom statin therapy wouldn’t be recommended under the current ACC/AHA guidelines because their estimated 10-year event risk was 5%-7.5% would be reclassified as warranting statin therapy because their coronary artery calcium score was greater than 0, pushing their estimated risk to 10.8%.
Data source: A retrospective analysis of data on 1,225 asymptomatic adults followed prospectively with coronary artery calcium measurements in the St. Francis Health Study.
Disclosures: The study presenter reported having no financial conflicts of interest.
AHA: PCI renal complications keep climbing
ORLANDO – Cases of contrast-induced nephropathy increased dramatically among Medicare patients undergoing percutaneous coronary intervention (PCI) during a recent 5-year period, despite the increased attention that has been drawn to the problem.
“These findings suggest that despite a longstanding focus on preventing CIN [contrast-induced nephropathy], the complication is increasing steadily and new efforts to reduce PCI-related CIN are warranted,” Dr. Phillip P. Brown said at the American Heart Association scientific sessions.
A fresh approach is a priority for Medicare, in part because new-onset renal failure requiring hemodialysis as a result of CIN increases health care costs substantially for the remainder of the patient’s life, noted Dr. Brown of Cardiac Data Solutions in Atlanta.
He presented a retrospective analysis of Medicare data files for 2009-2013. Among 1,552,960 Medicare beneficiaries who underwent PCI without valve surgery or coronary artery bypass graft surgery, 275,471 were admitted for nonelective PCI.
The rate of new hemodialysis as a complication of nonelective PCI increased by 24.8% annually during the study period, climbing to an incidence of 1.15% in 2013. Among patients admitted for elective PCI, the rate of new-onset renal failure requiring hemodialysis essentially doubled from 1% to 2% during the 5-year period.
The rate of new-onset acute renal failure as a complication of nonelective PCI increased by an average of 6.9% annually, reaching 7.67% in 2013. The increase in acute renal failure as a complication of elective PCI was even steeper: an average of 10.6% per year.
In addition to the rising rates of acute renal failure and need for dialysis as a complication of PCI, the proportion of patients who presented with prior dialysis or acute renal failure at admission for the procedure also rose year by year. In 2013, acute renal failure was present at admission in 6.12% of patients undergoing elective and 7.02% having nonelective PCI. Prior dialysis at admission was present in 2.61% and 0.94%, respectively.
Dr. Brown reported having no financial conflicts regarding this descriptive study.
ORLANDO – Cases of contrast-induced nephropathy increased dramatically among Medicare patients undergoing percutaneous coronary intervention (PCI) during a recent 5-year period, despite the increased attention that has been drawn to the problem.
“These findings suggest that despite a longstanding focus on preventing CIN [contrast-induced nephropathy], the complication is increasing steadily and new efforts to reduce PCI-related CIN are warranted,” Dr. Phillip P. Brown said at the American Heart Association scientific sessions.
A fresh approach is a priority for Medicare, in part because new-onset renal failure requiring hemodialysis as a result of CIN increases health care costs substantially for the remainder of the patient’s life, noted Dr. Brown of Cardiac Data Solutions in Atlanta.
He presented a retrospective analysis of Medicare data files for 2009-2013. Among 1,552,960 Medicare beneficiaries who underwent PCI without valve surgery or coronary artery bypass graft surgery, 275,471 were admitted for nonelective PCI.
The rate of new hemodialysis as a complication of nonelective PCI increased by 24.8% annually during the study period, climbing to an incidence of 1.15% in 2013. Among patients admitted for elective PCI, the rate of new-onset renal failure requiring hemodialysis essentially doubled from 1% to 2% during the 5-year period.
The rate of new-onset acute renal failure as a complication of nonelective PCI increased by an average of 6.9% annually, reaching 7.67% in 2013. The increase in acute renal failure as a complication of elective PCI was even steeper: an average of 10.6% per year.
In addition to the rising rates of acute renal failure and need for dialysis as a complication of PCI, the proportion of patients who presented with prior dialysis or acute renal failure at admission for the procedure also rose year by year. In 2013, acute renal failure was present at admission in 6.12% of patients undergoing elective and 7.02% having nonelective PCI. Prior dialysis at admission was present in 2.61% and 0.94%, respectively.
Dr. Brown reported having no financial conflicts regarding this descriptive study.
ORLANDO – Cases of contrast-induced nephropathy increased dramatically among Medicare patients undergoing percutaneous coronary intervention (PCI) during a recent 5-year period, despite the increased attention that has been drawn to the problem.
“These findings suggest that despite a longstanding focus on preventing CIN [contrast-induced nephropathy], the complication is increasing steadily and new efforts to reduce PCI-related CIN are warranted,” Dr. Phillip P. Brown said at the American Heart Association scientific sessions.
A fresh approach is a priority for Medicare, in part because new-onset renal failure requiring hemodialysis as a result of CIN increases health care costs substantially for the remainder of the patient’s life, noted Dr. Brown of Cardiac Data Solutions in Atlanta.
He presented a retrospective analysis of Medicare data files for 2009-2013. Among 1,552,960 Medicare beneficiaries who underwent PCI without valve surgery or coronary artery bypass graft surgery, 275,471 were admitted for nonelective PCI.
The rate of new hemodialysis as a complication of nonelective PCI increased by 24.8% annually during the study period, climbing to an incidence of 1.15% in 2013. Among patients admitted for elective PCI, the rate of new-onset renal failure requiring hemodialysis essentially doubled from 1% to 2% during the 5-year period.
The rate of new-onset acute renal failure as a complication of nonelective PCI increased by an average of 6.9% annually, reaching 7.67% in 2013. The increase in acute renal failure as a complication of elective PCI was even steeper: an average of 10.6% per year.
In addition to the rising rates of acute renal failure and need for dialysis as a complication of PCI, the proportion of patients who presented with prior dialysis or acute renal failure at admission for the procedure also rose year by year. In 2013, acute renal failure was present at admission in 6.12% of patients undergoing elective and 7.02% having nonelective PCI. Prior dialysis at admission was present in 2.61% and 0.94%, respectively.
Dr. Brown reported having no financial conflicts regarding this descriptive study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Renal complication rates in Medicare patients undergoing PCI continue to rise dramatically.
Major finding: The combined rate of acute renal failure and need for hemodialysis as a complication of elective PCI in Medicare patients climbed by 18% per year during a recent 5-year period and by nearly 32% annually in those undergoing nonelective PCI.
Data source: A retrospective study of 1.5 million Medicare beneficiaries who underwent elective or nonelective PCI during 2009-2013.
Disclosures: The presenter of this study reported having no financial conflicts.
Glial fibrillary acidic protein may help identify youth with TBI
Glial fibrillary acidic protein appears to be a candidate biomarker for detecting traumatic intracranial lesions on head CT after mild to moderate head trauma in youth, a study showed.
Previous studies have found that head CT scans in children less than 5 years old may contribute to the risk for brain cancer and leukemia because children are more sensitive to ionizing radiation. Ninety-nine different pediatric biomarkers have been researched for traumatic brain injury (TBI); some studies have indicated glial fibrillary acidic protein (GFAP) may be a promising biomarker for mild to moderate TBI in adults.
Dr. Linda Papa of the department of emergency medicine at Orlando Regional Medical Center and colleagues compared the GFAP level in the serum of children and youth evaluated for mild to moderate TBI with pediatric trauma patients without brain injury to see how these levels were related to evidence of traumatic lesions on head CT. Their results were published in Academic Emergency Medicine (2015 Nov;22[11]:1274-82. doi: 10.1111/acem.12795).
They conducted a prospective cohort study of 197 children and youth who presented with a Glasgow Coma Scale (GCS) score of 9-15 after blunt head trauma. The 60 control patients included those without head trauma and a GSC score of 15. A head CT scan was obtained in 152 patients, with 11% demonstrating traumatic intracranial lesions. Serum samples were drawn within 6 hours of injury, at a mean 3.3 hours in those with head injury and 4.1 hours in those without head injury.
Children with traumatic intracranial lesions on CT scan had higher median GFAP levels (1.01, interquartile range = 0.59-1.48), compared with those without lesions on CT (0.18, IQR = 0.06-0.47).
When GFAP was used to detect traumatic lesions on head CT, the area under the receiver operating characteristic curve (AUC) was 0.82 (95% confidence interval, 0.71-0.93); it was 0.80 (95% CI, 0.68-0.92) for those with a GCS of 15, and 0.83 (95% CI, 0.56-1.00) in those younger than 5 years old.
Using a cutoff level of 0.15 ng/mL for GFAP, Dr. Papa and colleagues noted a negative predictive value of 98%, a specificity of 47%, and a sensitivity of 94% for detecting intracranial lesions.
Several limitations to the study included not having research assistants available to enroll participants 24/7, a lack of long-term outcome data, and a small cohort without any participants requiring neurosurgical intervention.
The next steps would involve clinical validation with a large, multicenter study.
This study was supported by an award from the National Institute of Neurological Disorders and Stroke. Dr. Papa reported consulting for Banyan Biomarkers.
Out of the 99 markers studied previously, glial fibrillary acidic protein appears to be the most promising in adults. The authors wanted to examine its usefulness in children.

|
Dr. Gregory L. Landry |
This serum marker may be helpful to clinicians in deciding who needs a CT scan and who does not, but a larger study is still needed.
Serum markers seem to be most useful in patients with moderate to severe head trauma. The typical sports concussion (mild traumatic brain injury) does not cause enough damage to raise serum markers, and so far studies of markers in that group have shown that they are not helpful in determining severity of the injury.
Dr. Gregory L. Landry is affiliated with the University of Wisconsin–Madison, specializing in pediatric and adolescent primary care sports medicine. These comments were taken from an interview with Dr. Landry, who said he had no relevant financial disclosures.
Out of the 99 markers studied previously, glial fibrillary acidic protein appears to be the most promising in adults. The authors wanted to examine its usefulness in children.

|
Dr. Gregory L. Landry |
This serum marker may be helpful to clinicians in deciding who needs a CT scan and who does not, but a larger study is still needed.
Serum markers seem to be most useful in patients with moderate to severe head trauma. The typical sports concussion (mild traumatic brain injury) does not cause enough damage to raise serum markers, and so far studies of markers in that group have shown that they are not helpful in determining severity of the injury.
Dr. Gregory L. Landry is affiliated with the University of Wisconsin–Madison, specializing in pediatric and adolescent primary care sports medicine. These comments were taken from an interview with Dr. Landry, who said he had no relevant financial disclosures.
Out of the 99 markers studied previously, glial fibrillary acidic protein appears to be the most promising in adults. The authors wanted to examine its usefulness in children.

|
Dr. Gregory L. Landry |
This serum marker may be helpful to clinicians in deciding who needs a CT scan and who does not, but a larger study is still needed.
Serum markers seem to be most useful in patients with moderate to severe head trauma. The typical sports concussion (mild traumatic brain injury) does not cause enough damage to raise serum markers, and so far studies of markers in that group have shown that they are not helpful in determining severity of the injury.
Dr. Gregory L. Landry is affiliated with the University of Wisconsin–Madison, specializing in pediatric and adolescent primary care sports medicine. These comments were taken from an interview with Dr. Landry, who said he had no relevant financial disclosures.
Glial fibrillary acidic protein appears to be a candidate biomarker for detecting traumatic intracranial lesions on head CT after mild to moderate head trauma in youth, a study showed.
Previous studies have found that head CT scans in children less than 5 years old may contribute to the risk for brain cancer and leukemia because children are more sensitive to ionizing radiation. Ninety-nine different pediatric biomarkers have been researched for traumatic brain injury (TBI); some studies have indicated glial fibrillary acidic protein (GFAP) may be a promising biomarker for mild to moderate TBI in adults.
Dr. Linda Papa of the department of emergency medicine at Orlando Regional Medical Center and colleagues compared the GFAP level in the serum of children and youth evaluated for mild to moderate TBI with pediatric trauma patients without brain injury to see how these levels were related to evidence of traumatic lesions on head CT. Their results were published in Academic Emergency Medicine (2015 Nov;22[11]:1274-82. doi: 10.1111/acem.12795).
They conducted a prospective cohort study of 197 children and youth who presented with a Glasgow Coma Scale (GCS) score of 9-15 after blunt head trauma. The 60 control patients included those without head trauma and a GSC score of 15. A head CT scan was obtained in 152 patients, with 11% demonstrating traumatic intracranial lesions. Serum samples were drawn within 6 hours of injury, at a mean 3.3 hours in those with head injury and 4.1 hours in those without head injury.
Children with traumatic intracranial lesions on CT scan had higher median GFAP levels (1.01, interquartile range = 0.59-1.48), compared with those without lesions on CT (0.18, IQR = 0.06-0.47).
When GFAP was used to detect traumatic lesions on head CT, the area under the receiver operating characteristic curve (AUC) was 0.82 (95% confidence interval, 0.71-0.93); it was 0.80 (95% CI, 0.68-0.92) for those with a GCS of 15, and 0.83 (95% CI, 0.56-1.00) in those younger than 5 years old.
Using a cutoff level of 0.15 ng/mL for GFAP, Dr. Papa and colleagues noted a negative predictive value of 98%, a specificity of 47%, and a sensitivity of 94% for detecting intracranial lesions.
Several limitations to the study included not having research assistants available to enroll participants 24/7, a lack of long-term outcome data, and a small cohort without any participants requiring neurosurgical intervention.
The next steps would involve clinical validation with a large, multicenter study.
This study was supported by an award from the National Institute of Neurological Disorders and Stroke. Dr. Papa reported consulting for Banyan Biomarkers.
Glial fibrillary acidic protein appears to be a candidate biomarker for detecting traumatic intracranial lesions on head CT after mild to moderate head trauma in youth, a study showed.
Previous studies have found that head CT scans in children less than 5 years old may contribute to the risk for brain cancer and leukemia because children are more sensitive to ionizing radiation. Ninety-nine different pediatric biomarkers have been researched for traumatic brain injury (TBI); some studies have indicated glial fibrillary acidic protein (GFAP) may be a promising biomarker for mild to moderate TBI in adults.
Dr. Linda Papa of the department of emergency medicine at Orlando Regional Medical Center and colleagues compared the GFAP level in the serum of children and youth evaluated for mild to moderate TBI with pediatric trauma patients without brain injury to see how these levels were related to evidence of traumatic lesions on head CT. Their results were published in Academic Emergency Medicine (2015 Nov;22[11]:1274-82. doi: 10.1111/acem.12795).
They conducted a prospective cohort study of 197 children and youth who presented with a Glasgow Coma Scale (GCS) score of 9-15 after blunt head trauma. The 60 control patients included those without head trauma and a GSC score of 15. A head CT scan was obtained in 152 patients, with 11% demonstrating traumatic intracranial lesions. Serum samples were drawn within 6 hours of injury, at a mean 3.3 hours in those with head injury and 4.1 hours in those without head injury.
Children with traumatic intracranial lesions on CT scan had higher median GFAP levels (1.01, interquartile range = 0.59-1.48), compared with those without lesions on CT (0.18, IQR = 0.06-0.47).
When GFAP was used to detect traumatic lesions on head CT, the area under the receiver operating characteristic curve (AUC) was 0.82 (95% confidence interval, 0.71-0.93); it was 0.80 (95% CI, 0.68-0.92) for those with a GCS of 15, and 0.83 (95% CI, 0.56-1.00) in those younger than 5 years old.
Using a cutoff level of 0.15 ng/mL for GFAP, Dr. Papa and colleagues noted a negative predictive value of 98%, a specificity of 47%, and a sensitivity of 94% for detecting intracranial lesions.
Several limitations to the study included not having research assistants available to enroll participants 24/7, a lack of long-term outcome data, and a small cohort without any participants requiring neurosurgical intervention.
The next steps would involve clinical validation with a large, multicenter study.
This study was supported by an award from the National Institute of Neurological Disorders and Stroke. Dr. Papa reported consulting for Banyan Biomarkers.
FROM ACADEMIC EMERGENCY MEDICINE
Key clinical point: GFAP appears to be associated with severity of injury and identification of lesions on head CT scan after head trauma in youth.
Major finding: The negative predictive value was 98%, the specificity was 47%, and the sensitivity was 94% for detecting intracranial lesions when a GFAP cutoff level of 0.15 ng/mL was used.
Data source: A prospective controlled cohort study of children and youth who presented with a Glasgow Coma Scale score of 9-15 after blunt head trauma.
Disclosures: This study was supported by an award from the National Institute of Neurological Disorders and Stroke. Dr. Papa reported consulting for Banyan Biomarkers.
In angiography, intracoronary contrast damaged kidneys more than IV contrast
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Patients undergoing angiography with intracoronary contrast agent instead of IV contrast agent may be at greater risk of kidney injury.
Major finding: Kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration in patients undergoing angiography for suspected heart disease.
Data source: A randomized study of 326 patients with atypical angina pectoris who were scheduled for angiography.
Disclosures: The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
Radiation exposure exceeds 50 mSv in 2% of ICU patients
MONTREAL – Some of the sickest patients treated at U.S. hospitals receive high levels of radiation exposure, based on a review of more than 4,000 medical ICU patients treated recently at one U.S. quaternary-care center.
During 2013, 98 patients admitted to the medical ICU at the Cleveland Clinic – 2% of the 4,155 patients who passed through the medical ICU that year – had cumulative radiation exposure of at least 50 mSv while in the ICU, thereby exceeding the U.S. standard for maximum annual workplace exposure, Dr. Sudhir Krishnan said at the annual meeting of the American College of Chest Physicians. The finding raises questions of whether all these exposures are appropriate and whether they reflect overuse of certain imaging modalities.
Dr. Krishnan and his associates ran a retrospective review of case records for the medical ICU–admitted patients at the Cleveland Clinic during 2013 (Chest. 2015 Oct 25. doi: 10.1378/chest.2278486). During their ICU stay, 3,490 patients (84%) received some amount of radiation exposure. Exposure averaged 7 mSv, with a median of 1.5 mSv. The radiation exposure came primarily from imaging and more specifically from CT examinations, which produced more than half of all radiation-exposure episodes. Other sources included x-rays, nuclear scans, and interventional procedures.
Based on typical radiation dosages received during each type of procedure, the researchers calculated an estimated total radiation dosage received by each patient during their ICU stay. Nearly two-thirds of patients had an exposure of less than 3 mSv, the average annual exposure a person receives from ambient radiation. A quarter of the patients had an exposure of 3-14 mSv, 11% had an exposure of 15-49 mSv, and 2% – 98 patients – had exposure during their ICU stay that ran to 50 mSv or greater, exceeding the U.S. workplace annual maximum . Thirteen patients had an exposure level during their ICU stay that reached 100 mSv or higher; the maximum exposure level was in a patient with cumulative exposure of 176 mSv, said Dr. Krishnan, a critical-care medicine specialist at the Cleveland Clinic.
He and his coworkers did a multivariate analysis to identify factors that linked with a higher likelihood of having high radiation exposure. Patients at greatest risk for high exposure levels were sicker patients with higher APACHE 3 scores, longer stays in the ICU, and the presence of cirrhosis, but those most at risk also tended to be younger. Rates of both ICU deaths and deaths during the entire hospitalization were significantly higher among those with radiation exposure that was 50 mSv or greater.
Dr. Krishnan cautioned that he has not run any analysis that assessed the appropriateness of the imaging that the ICU patients received, nor did he have any data documenting the clinical consequences to the patients who had higher radiation exposure. Despite that uncertainty, he suggested that efforts focus on avoiding unnecessary radiation exposure to patients.
On Twitter @mitchelzoler
MONTREAL – Some of the sickest patients treated at U.S. hospitals receive high levels of radiation exposure, based on a review of more than 4,000 medical ICU patients treated recently at one U.S. quaternary-care center.
During 2013, 98 patients admitted to the medical ICU at the Cleveland Clinic – 2% of the 4,155 patients who passed through the medical ICU that year – had cumulative radiation exposure of at least 50 mSv while in the ICU, thereby exceeding the U.S. standard for maximum annual workplace exposure, Dr. Sudhir Krishnan said at the annual meeting of the American College of Chest Physicians. The finding raises questions of whether all these exposures are appropriate and whether they reflect overuse of certain imaging modalities.
Dr. Krishnan and his associates ran a retrospective review of case records for the medical ICU–admitted patients at the Cleveland Clinic during 2013 (Chest. 2015 Oct 25. doi: 10.1378/chest.2278486). During their ICU stay, 3,490 patients (84%) received some amount of radiation exposure. Exposure averaged 7 mSv, with a median of 1.5 mSv. The radiation exposure came primarily from imaging and more specifically from CT examinations, which produced more than half of all radiation-exposure episodes. Other sources included x-rays, nuclear scans, and interventional procedures.
Based on typical radiation dosages received during each type of procedure, the researchers calculated an estimated total radiation dosage received by each patient during their ICU stay. Nearly two-thirds of patients had an exposure of less than 3 mSv, the average annual exposure a person receives from ambient radiation. A quarter of the patients had an exposure of 3-14 mSv, 11% had an exposure of 15-49 mSv, and 2% – 98 patients – had exposure during their ICU stay that ran to 50 mSv or greater, exceeding the U.S. workplace annual maximum . Thirteen patients had an exposure level during their ICU stay that reached 100 mSv or higher; the maximum exposure level was in a patient with cumulative exposure of 176 mSv, said Dr. Krishnan, a critical-care medicine specialist at the Cleveland Clinic.
He and his coworkers did a multivariate analysis to identify factors that linked with a higher likelihood of having high radiation exposure. Patients at greatest risk for high exposure levels were sicker patients with higher APACHE 3 scores, longer stays in the ICU, and the presence of cirrhosis, but those most at risk also tended to be younger. Rates of both ICU deaths and deaths during the entire hospitalization were significantly higher among those with radiation exposure that was 50 mSv or greater.
Dr. Krishnan cautioned that he has not run any analysis that assessed the appropriateness of the imaging that the ICU patients received, nor did he have any data documenting the clinical consequences to the patients who had higher radiation exposure. Despite that uncertainty, he suggested that efforts focus on avoiding unnecessary radiation exposure to patients.
On Twitter @mitchelzoler
MONTREAL – Some of the sickest patients treated at U.S. hospitals receive high levels of radiation exposure, based on a review of more than 4,000 medical ICU patients treated recently at one U.S. quaternary-care center.
During 2013, 98 patients admitted to the medical ICU at the Cleveland Clinic – 2% of the 4,155 patients who passed through the medical ICU that year – had cumulative radiation exposure of at least 50 mSv while in the ICU, thereby exceeding the U.S. standard for maximum annual workplace exposure, Dr. Sudhir Krishnan said at the annual meeting of the American College of Chest Physicians. The finding raises questions of whether all these exposures are appropriate and whether they reflect overuse of certain imaging modalities.
Dr. Krishnan and his associates ran a retrospective review of case records for the medical ICU–admitted patients at the Cleveland Clinic during 2013 (Chest. 2015 Oct 25. doi: 10.1378/chest.2278486). During their ICU stay, 3,490 patients (84%) received some amount of radiation exposure. Exposure averaged 7 mSv, with a median of 1.5 mSv. The radiation exposure came primarily from imaging and more specifically from CT examinations, which produced more than half of all radiation-exposure episodes. Other sources included x-rays, nuclear scans, and interventional procedures.
Based on typical radiation dosages received during each type of procedure, the researchers calculated an estimated total radiation dosage received by each patient during their ICU stay. Nearly two-thirds of patients had an exposure of less than 3 mSv, the average annual exposure a person receives from ambient radiation. A quarter of the patients had an exposure of 3-14 mSv, 11% had an exposure of 15-49 mSv, and 2% – 98 patients – had exposure during their ICU stay that ran to 50 mSv or greater, exceeding the U.S. workplace annual maximum . Thirteen patients had an exposure level during their ICU stay that reached 100 mSv or higher; the maximum exposure level was in a patient with cumulative exposure of 176 mSv, said Dr. Krishnan, a critical-care medicine specialist at the Cleveland Clinic.
He and his coworkers did a multivariate analysis to identify factors that linked with a higher likelihood of having high radiation exposure. Patients at greatest risk for high exposure levels were sicker patients with higher APACHE 3 scores, longer stays in the ICU, and the presence of cirrhosis, but those most at risk also tended to be younger. Rates of both ICU deaths and deaths during the entire hospitalization were significantly higher among those with radiation exposure that was 50 mSv or greater.
Dr. Krishnan cautioned that he has not run any analysis that assessed the appropriateness of the imaging that the ICU patients received, nor did he have any data documenting the clinical consequences to the patients who had higher radiation exposure. Despite that uncertainty, he suggested that efforts focus on avoiding unnecessary radiation exposure to patients.
On Twitter @mitchelzoler
AT CHEST 2015
Key clinical point: A small but significant percentage of medical ICU patients receive cumulative radiation doses that exceed federal standards for annual workplace exposure.
Major finding: Two percent of medical ICU patients received at least 50 mSv of radiation exposure during their ICU stay.
Data source: Single-center, retrospective study with 4,155 consecutive medical ICU patients during 2013.
Disclosures: Dr. Krishnan had no disclosures.
Malpractice Counsel: Aneurysm, Falls
Sued If You Do, Sued If You Don’t
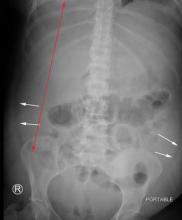
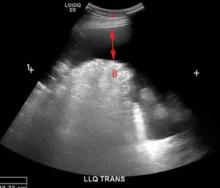
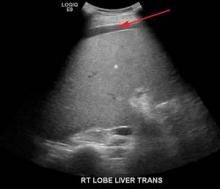
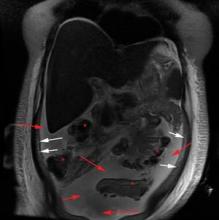
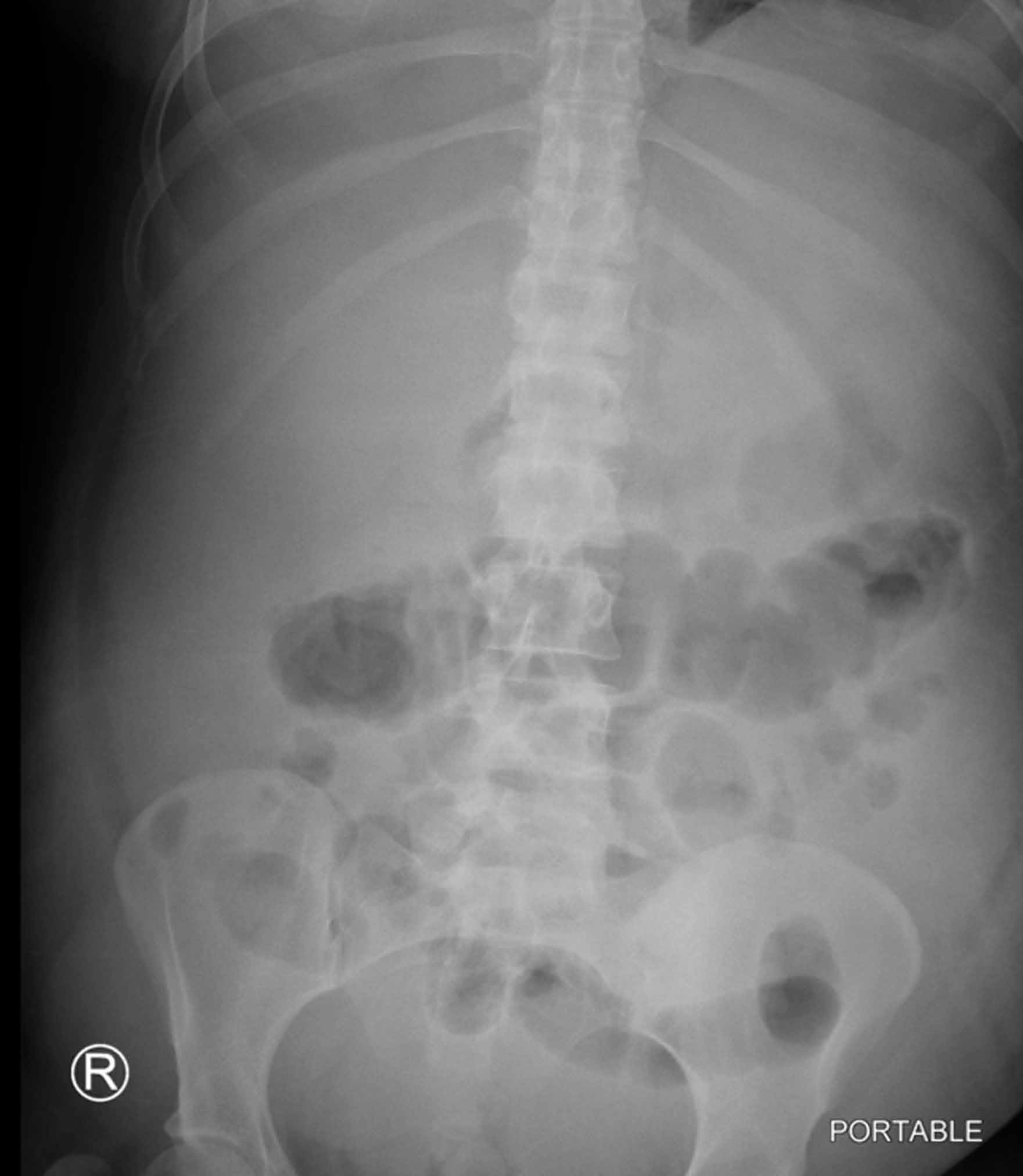
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.
Sued If You Do, Sued If You Don’t
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
Sued If You Do, Sued If You Don’t
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.
Case Report: Not Just Another Kidney Stone
Case
A 36-year-old woman with a 2-week history of left flank pain presented to the ED via emergency medical services. The patient, who had a history of nephrolithiasis, assumed her pain was due to another kidney stone. She stated that while waiting for the presumed stone to pass, the pain in her left flank worsened and she felt lightheaded and weak.
The patient’s vital signs at presentation were: heart rate, 96 beats/minute; blood pressure, 133/76 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.9˚F. Oxygen saturation was 98% on room air. On physical examination, the patient had left lower quadrant pain and left costovertebral angle tenderness. Laboratory studies were remarkable for a negative urine pregnancy test, a hemoglobin level of 6.8 g/dL, and a hematocrit of 21.1%. Based on the patient’s history and symptoms, axial and coronal computed tomography (CT) scans were ordered, revealing a ruptured left renal calyx with hemorrhage from ureterolithiasis (Figures 1a and 1b).
Rupture of renal calyx and extravasation of blood or urine is a potential complication of nephrolithiasis. Stone size, degree of obstruction, and length of symptomatic presentation presumably contribute to complications from nephrolithiasis. Stones that are symptomatic for more than 4 weeks are estimated to have an increased complication rate of up to 20%.1
Calyx or fornix rupture results from increased intraluminal pressure. Rupture of these structures is thought to be a type of “safety-valve” function to relieve obstructive uropathy.2
Obstructions from small leaks to large urinomas can cause extravasation of urine. In most cases, urinary extravasation is confined to the subcapsular space or perirenal space within the Gerota’s fascia;3 however, as seen in this patient, mixed hematoma/urinomas can form.
Causes
In cases of nontraumatic calyx rupture, the cause of the obstruction is most often a distal obstructing ureteral stone.4 Other causes of rupture include extrinsic compression from malignant and benign masses, ureteric junction obstructions, or iatrogenic causes.4 Interestingly, in one small study, the median size of the obstructing stone was only 4 mm. The same study also noted that proximal ureteral obstruction occurred when larger stones where present.4
Conservative Versus Nonconservative Management
Potential complications of urinomas include abscess formation, sepsis, hydronephrosis, and paralytic ileus.3 Despite possible adverse sequelae, uncomplicated urinomas may be managed conservatively with supportive care. According to a study by Chapman et al,5 about 40% of patients managed conservatively recover without complications. In addition, in a retrospective study by Doehn et al6 involving 160 cases of fornix rupture treated with endoscopic therapy or nephrostomy tube supplemented with antibiotics, no instances of perinephric abscess or other complications requiring a second procedure were noted.
Management of suspected ureterolithiasis in the ED is focused on analgesia and supportive care. Acute analgesia is often provided parenterally with opioids alone or with an opioid/nonsteroidal anti-inflammatory drug (NSAID) combination.7 Frequent reassessment of the patient is required to ensure adequate pain control and to prevent sedation. Other symptoms, such as nausea, vomiting, and dehydration, may be treated with intravenous (IV) fluids and antiemetic medications. Further radiographic evaluation is needed once analgesia is achieved.7,8
Imaging Studies
Radiological evaluation of patients with suspected ureterolithiasis may involve several imaging modalities. Noncontrast helical CT scan is the standard for rapid and efficient identification of ureteral stones while allowing visualization of other potential pathology (eg, urinoma).7-9 Other modalities, such as ultrasonography; radiography of the kidneys, ureters, and bladder; and an IV pyelogram with contrasted CT, may be ordered if noncontrast helical CT scan is not available on-site or if there are comorbidities. In addition to imaging studies, basic laboratory studies (eg, serum creatinine and blood urea nitrogen testing) are indicated to assess overall renal function and direct the choice of radiological study.7
Disposition
Clinical decision-making is key when recommending inpatient versus outpatient treatment in patients with ureterolithiasis. Patients with uncontrolled pain or vomiting may require inpatient admission for supportive care, while those demonstrating acute renal failure, pyuria with bacteriuria, complete bilateral ureteral obstruction, urinoma, or signs of sepsis demand emergent urology consultation. Specifically, patients with urinoma require ureteroscopy versus nephrostomy6,10 to allow drainage while carefully monitoring for development of subsequent bleeding and infection.
When discharging patients from the ED, expulsive therapy using tamsulosin9 and analgesia with combination of oral opioids and NSAIDs are most commonly effective.11 Outpatient urology referrals are recommended for ureteral stones greater than 5 mm in size or if the stones have been present in the ureter for greater than 4 weeks.1 Proper evaluation and management of ureterolithiasis in the ED is crucial for positive outcomes and to reduce long-term complications.
Case Conclusion
Computed tomography revealed a ruptured renal calyx on the left side with free fluid in the abdomen. Urology services were consulted and the patient was taken to the operating room for cystoscopy, ureteral stent placement, and laser lithotripsy. Following surgery, she subsequently developed urosepsis for which she was successfully treated with IV antibiotics and discharged on hospital day 15.
Mr Eisenstat is a fourth-year medical student at the University of South Carolina School of Medicine, Greenville. Dr Fabiano is an emergency physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina. Dr Collins is family medicine physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina.
- Hübner WA, Irby P, Stoller M. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24(2):172-176.
- Lin DY, Fang YC, Huang DY, Lin SP. Spontaneous rupture of the ureter secondary to urolithiasis and extravasation of calyceal fornix due to acute urinary bladder distension: four case reports. Chin J Radiology. 2004;29:269-275.
- Behzad-Noori M, Blandon JA, Negrin Exposito JE, Sarmiento JL, Dias AL, Hernandez GT. Urinoma: a rare complication from being between a rock and soft organ. El Paso Physician. 2010;33(6):5-6.
- Gershman B, Kulkarni N, Sahani DV, Eisner BH. Causes of renal forniceal rupture. BJU Int. 2011;108(11):1909-1911.
- Chapman JP, Gonzalez J, Diokno AC. Significance of urinary extravasation during renal colic. Urology. 1987;30(6):541-545.
- Doehn C, Fiola L, Peter M, Jocham D. Outcome analysis of fornix ruptures in 162 consecutive patients. J Endourol. 2010;24(11):1869-1873
- Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338
- Smith RC, Verga M, Dalrymple N, McCarthy S, Rosenfield AT. Acute ureteral obstruction: value of secondary signs of helical unenhanced CT. AJR Am J Roentgenol. 1996;167(5):1109-1113.Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131-140.
- Ha M, MacDonald RD. Impact of CT scan in patients with first episode of suspected nephrolithiasis. J Emerg Med. 2004;27(3):225-231.
- Tawfiek ER, Bagley DH. Management of upper urinary tract calculi with ureteroscopic techniques. Urology. 1999;53(1):25-31.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D'Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
Case
A 36-year-old woman with a 2-week history of left flank pain presented to the ED via emergency medical services. The patient, who had a history of nephrolithiasis, assumed her pain was due to another kidney stone. She stated that while waiting for the presumed stone to pass, the pain in her left flank worsened and she felt lightheaded and weak.
The patient’s vital signs at presentation were: heart rate, 96 beats/minute; blood pressure, 133/76 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.9˚F. Oxygen saturation was 98% on room air. On physical examination, the patient had left lower quadrant pain and left costovertebral angle tenderness. Laboratory studies were remarkable for a negative urine pregnancy test, a hemoglobin level of 6.8 g/dL, and a hematocrit of 21.1%. Based on the patient’s history and symptoms, axial and coronal computed tomography (CT) scans were ordered, revealing a ruptured left renal calyx with hemorrhage from ureterolithiasis (Figures 1a and 1b).
Rupture of renal calyx and extravasation of blood or urine is a potential complication of nephrolithiasis. Stone size, degree of obstruction, and length of symptomatic presentation presumably contribute to complications from nephrolithiasis. Stones that are symptomatic for more than 4 weeks are estimated to have an increased complication rate of up to 20%.1
Calyx or fornix rupture results from increased intraluminal pressure. Rupture of these structures is thought to be a type of “safety-valve” function to relieve obstructive uropathy.2
Obstructions from small leaks to large urinomas can cause extravasation of urine. In most cases, urinary extravasation is confined to the subcapsular space or perirenal space within the Gerota’s fascia;3 however, as seen in this patient, mixed hematoma/urinomas can form.
Causes
In cases of nontraumatic calyx rupture, the cause of the obstruction is most often a distal obstructing ureteral stone.4 Other causes of rupture include extrinsic compression from malignant and benign masses, ureteric junction obstructions, or iatrogenic causes.4 Interestingly, in one small study, the median size of the obstructing stone was only 4 mm. The same study also noted that proximal ureteral obstruction occurred when larger stones where present.4
Conservative Versus Nonconservative Management
Potential complications of urinomas include abscess formation, sepsis, hydronephrosis, and paralytic ileus.3 Despite possible adverse sequelae, uncomplicated urinomas may be managed conservatively with supportive care. According to a study by Chapman et al,5 about 40% of patients managed conservatively recover without complications. In addition, in a retrospective study by Doehn et al6 involving 160 cases of fornix rupture treated with endoscopic therapy or nephrostomy tube supplemented with antibiotics, no instances of perinephric abscess or other complications requiring a second procedure were noted.
Management of suspected ureterolithiasis in the ED is focused on analgesia and supportive care. Acute analgesia is often provided parenterally with opioids alone or with an opioid/nonsteroidal anti-inflammatory drug (NSAID) combination.7 Frequent reassessment of the patient is required to ensure adequate pain control and to prevent sedation. Other symptoms, such as nausea, vomiting, and dehydration, may be treated with intravenous (IV) fluids and antiemetic medications. Further radiographic evaluation is needed once analgesia is achieved.7,8
Imaging Studies
Radiological evaluation of patients with suspected ureterolithiasis may involve several imaging modalities. Noncontrast helical CT scan is the standard for rapid and efficient identification of ureteral stones while allowing visualization of other potential pathology (eg, urinoma).7-9 Other modalities, such as ultrasonography; radiography of the kidneys, ureters, and bladder; and an IV pyelogram with contrasted CT, may be ordered if noncontrast helical CT scan is not available on-site or if there are comorbidities. In addition to imaging studies, basic laboratory studies (eg, serum creatinine and blood urea nitrogen testing) are indicated to assess overall renal function and direct the choice of radiological study.7
Disposition
Clinical decision-making is key when recommending inpatient versus outpatient treatment in patients with ureterolithiasis. Patients with uncontrolled pain or vomiting may require inpatient admission for supportive care, while those demonstrating acute renal failure, pyuria with bacteriuria, complete bilateral ureteral obstruction, urinoma, or signs of sepsis demand emergent urology consultation. Specifically, patients with urinoma require ureteroscopy versus nephrostomy6,10 to allow drainage while carefully monitoring for development of subsequent bleeding and infection.
When discharging patients from the ED, expulsive therapy using tamsulosin9 and analgesia with combination of oral opioids and NSAIDs are most commonly effective.11 Outpatient urology referrals are recommended for ureteral stones greater than 5 mm in size or if the stones have been present in the ureter for greater than 4 weeks.1 Proper evaluation and management of ureterolithiasis in the ED is crucial for positive outcomes and to reduce long-term complications.
Case Conclusion
Computed tomography revealed a ruptured renal calyx on the left side with free fluid in the abdomen. Urology services were consulted and the patient was taken to the operating room for cystoscopy, ureteral stent placement, and laser lithotripsy. Following surgery, she subsequently developed urosepsis for which she was successfully treated with IV antibiotics and discharged on hospital day 15.
Mr Eisenstat is a fourth-year medical student at the University of South Carolina School of Medicine, Greenville. Dr Fabiano is an emergency physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina. Dr Collins is family medicine physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina.
Case
A 36-year-old woman with a 2-week history of left flank pain presented to the ED via emergency medical services. The patient, who had a history of nephrolithiasis, assumed her pain was due to another kidney stone. She stated that while waiting for the presumed stone to pass, the pain in her left flank worsened and she felt lightheaded and weak.
The patient’s vital signs at presentation were: heart rate, 96 beats/minute; blood pressure, 133/76 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.9˚F. Oxygen saturation was 98% on room air. On physical examination, the patient had left lower quadrant pain and left costovertebral angle tenderness. Laboratory studies were remarkable for a negative urine pregnancy test, a hemoglobin level of 6.8 g/dL, and a hematocrit of 21.1%. Based on the patient’s history and symptoms, axial and coronal computed tomography (CT) scans were ordered, revealing a ruptured left renal calyx with hemorrhage from ureterolithiasis (Figures 1a and 1b).
Rupture of renal calyx and extravasation of blood or urine is a potential complication of nephrolithiasis. Stone size, degree of obstruction, and length of symptomatic presentation presumably contribute to complications from nephrolithiasis. Stones that are symptomatic for more than 4 weeks are estimated to have an increased complication rate of up to 20%.1
Calyx or fornix rupture results from increased intraluminal pressure. Rupture of these structures is thought to be a type of “safety-valve” function to relieve obstructive uropathy.2
Obstructions from small leaks to large urinomas can cause extravasation of urine. In most cases, urinary extravasation is confined to the subcapsular space or perirenal space within the Gerota’s fascia;3 however, as seen in this patient, mixed hematoma/urinomas can form.
Causes
In cases of nontraumatic calyx rupture, the cause of the obstruction is most often a distal obstructing ureteral stone.4 Other causes of rupture include extrinsic compression from malignant and benign masses, ureteric junction obstructions, or iatrogenic causes.4 Interestingly, in one small study, the median size of the obstructing stone was only 4 mm. The same study also noted that proximal ureteral obstruction occurred when larger stones where present.4
Conservative Versus Nonconservative Management
Potential complications of urinomas include abscess formation, sepsis, hydronephrosis, and paralytic ileus.3 Despite possible adverse sequelae, uncomplicated urinomas may be managed conservatively with supportive care. According to a study by Chapman et al,5 about 40% of patients managed conservatively recover without complications. In addition, in a retrospective study by Doehn et al6 involving 160 cases of fornix rupture treated with endoscopic therapy or nephrostomy tube supplemented with antibiotics, no instances of perinephric abscess or other complications requiring a second procedure were noted.
Management of suspected ureterolithiasis in the ED is focused on analgesia and supportive care. Acute analgesia is often provided parenterally with opioids alone or with an opioid/nonsteroidal anti-inflammatory drug (NSAID) combination.7 Frequent reassessment of the patient is required to ensure adequate pain control and to prevent sedation. Other symptoms, such as nausea, vomiting, and dehydration, may be treated with intravenous (IV) fluids and antiemetic medications. Further radiographic evaluation is needed once analgesia is achieved.7,8
Imaging Studies
Radiological evaluation of patients with suspected ureterolithiasis may involve several imaging modalities. Noncontrast helical CT scan is the standard for rapid and efficient identification of ureteral stones while allowing visualization of other potential pathology (eg, urinoma).7-9 Other modalities, such as ultrasonography; radiography of the kidneys, ureters, and bladder; and an IV pyelogram with contrasted CT, may be ordered if noncontrast helical CT scan is not available on-site or if there are comorbidities. In addition to imaging studies, basic laboratory studies (eg, serum creatinine and blood urea nitrogen testing) are indicated to assess overall renal function and direct the choice of radiological study.7
Disposition
Clinical decision-making is key when recommending inpatient versus outpatient treatment in patients with ureterolithiasis. Patients with uncontrolled pain or vomiting may require inpatient admission for supportive care, while those demonstrating acute renal failure, pyuria with bacteriuria, complete bilateral ureteral obstruction, urinoma, or signs of sepsis demand emergent urology consultation. Specifically, patients with urinoma require ureteroscopy versus nephrostomy6,10 to allow drainage while carefully monitoring for development of subsequent bleeding and infection.
When discharging patients from the ED, expulsive therapy using tamsulosin9 and analgesia with combination of oral opioids and NSAIDs are most commonly effective.11 Outpatient urology referrals are recommended for ureteral stones greater than 5 mm in size or if the stones have been present in the ureter for greater than 4 weeks.1 Proper evaluation and management of ureterolithiasis in the ED is crucial for positive outcomes and to reduce long-term complications.
Case Conclusion
Computed tomography revealed a ruptured renal calyx on the left side with free fluid in the abdomen. Urology services were consulted and the patient was taken to the operating room for cystoscopy, ureteral stent placement, and laser lithotripsy. Following surgery, she subsequently developed urosepsis for which she was successfully treated with IV antibiotics and discharged on hospital day 15.
Mr Eisenstat is a fourth-year medical student at the University of South Carolina School of Medicine, Greenville. Dr Fabiano is an emergency physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina. Dr Collins is family medicine physician, department of emergency medicine, Greenville Health Systems, Greenville, South Carolina.
- Hübner WA, Irby P, Stoller M. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24(2):172-176.
- Lin DY, Fang YC, Huang DY, Lin SP. Spontaneous rupture of the ureter secondary to urolithiasis and extravasation of calyceal fornix due to acute urinary bladder distension: four case reports. Chin J Radiology. 2004;29:269-275.
- Behzad-Noori M, Blandon JA, Negrin Exposito JE, Sarmiento JL, Dias AL, Hernandez GT. Urinoma: a rare complication from being between a rock and soft organ. El Paso Physician. 2010;33(6):5-6.
- Gershman B, Kulkarni N, Sahani DV, Eisner BH. Causes of renal forniceal rupture. BJU Int. 2011;108(11):1909-1911.
- Chapman JP, Gonzalez J, Diokno AC. Significance of urinary extravasation during renal colic. Urology. 1987;30(6):541-545.
- Doehn C, Fiola L, Peter M, Jocham D. Outcome analysis of fornix ruptures in 162 consecutive patients. J Endourol. 2010;24(11):1869-1873
- Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338
- Smith RC, Verga M, Dalrymple N, McCarthy S, Rosenfield AT. Acute ureteral obstruction: value of secondary signs of helical unenhanced CT. AJR Am J Roentgenol. 1996;167(5):1109-1113.Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131-140.
- Ha M, MacDonald RD. Impact of CT scan in patients with first episode of suspected nephrolithiasis. J Emerg Med. 2004;27(3):225-231.
- Tawfiek ER, Bagley DH. Management of upper urinary tract calculi with ureteroscopic techniques. Urology. 1999;53(1):25-31.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D'Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
- Hübner WA, Irby P, Stoller M. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24(2):172-176.
- Lin DY, Fang YC, Huang DY, Lin SP. Spontaneous rupture of the ureter secondary to urolithiasis and extravasation of calyceal fornix due to acute urinary bladder distension: four case reports. Chin J Radiology. 2004;29:269-275.
- Behzad-Noori M, Blandon JA, Negrin Exposito JE, Sarmiento JL, Dias AL, Hernandez GT. Urinoma: a rare complication from being between a rock and soft organ. El Paso Physician. 2010;33(6):5-6.
- Gershman B, Kulkarni N, Sahani DV, Eisner BH. Causes of renal forniceal rupture. BJU Int. 2011;108(11):1909-1911.
- Chapman JP, Gonzalez J, Diokno AC. Significance of urinary extravasation during renal colic. Urology. 1987;30(6):541-545.
- Doehn C, Fiola L, Peter M, Jocham D. Outcome analysis of fornix ruptures in 162 consecutive patients. J Endourol. 2010;24(11):1869-1873
- Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338
- Smith RC, Verga M, Dalrymple N, McCarthy S, Rosenfield AT. Acute ureteral obstruction: value of secondary signs of helical unenhanced CT. AJR Am J Roentgenol. 1996;167(5):1109-1113.Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131-140.
- Ha M, MacDonald RD. Impact of CT scan in patients with first episode of suspected nephrolithiasis. J Emerg Med. 2004;27(3):225-231.
- Tawfiek ER, Bagley DH. Management of upper urinary tract calculi with ureteroscopic techniques. Urology. 1999;53(1):25-31.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D'Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
Emergency Imaging
30-year-old woman with a history of alcoholism and anxiety presented to the ED for evaluation of abdominal distension and pain. She reported new social stressors at home and increased alcohol consumption. She also noted gradually increasing abdominal distension over the past several weeks. As patient’s liver function tests were elevated, supine radiographs of the abdomen were acquired for further evaluation; a representative image is presented below (Figure 1a).
What is the suspected diagnosis?
Is any additional imaging required?
Answer
Centralization of bowel is suggestive of abdominopelvic ascites.1 Additional radiographic findings that may be seen with ascites include diffusely increased density of the abdomen and bulging of the peritoneal fat stripes/flanks. In the average adult, a minimum of approximately 800 mL of ascitic fluid is required to medially displace bowel loops or abdominal viscera.2 While there are many causes of ascites, the radiograph in this case suggests the underlying etiology of cirrhosis. Although the top of the liver is not visualized on the radiograph, the liver contour appears enlarged (red arrow, Figure 1b).
In the ED, ultrasound is the imaging modality of choice to confirm the presence of ascites as it is widely available, quick, relatively inexpensive, and does not require ionizing radiation. Ultrasound is also useful when examining the abdominal viscera since it may detect the underlying cause of ascites.3 The ultrasound image of the abdominal viscera in this case demonstrated a hypoechoic (dark) signal (red arrow, Figure 1c) between the abdominal wall (red asterisk, Figure 1c) and bowel loops (letter ‘B,” Figure 1c). This finding confirmed the presence of ascites because simple fluid does not reflect the ultrasound beam, but instead results in the hypoechoic signal seen in Figure 1c. An additional sonographic image confirmed the presence of an enlarged liver (white asterisk, Figure 1d) and ascites in the right upper quadrant (red arrow, Figure 1d).
The patient was admitted for further workup and treatment of alcoholic cirrhosis. A
Dr Lyons is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-
- Meschan I. Analysis of Roentgen Signs in General Radiology. Philadelphia, PA: Saunders; 1973:1231.
- Keeffee EJ, Gagliardi RA, Pfister RC. The roentgenographic evaluation of ascites. Am J Roentgenol. 1967;101(2):388-396.
- Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22.
30-year-old woman with a history of alcoholism and anxiety presented to the ED for evaluation of abdominal distension and pain. She reported new social stressors at home and increased alcohol consumption. She also noted gradually increasing abdominal distension over the past several weeks. As patient’s liver function tests were elevated, supine radiographs of the abdomen were acquired for further evaluation; a representative image is presented below (Figure 1a).
What is the suspected diagnosis?
Is any additional imaging required?
Answer
Centralization of bowel is suggestive of abdominopelvic ascites.1 Additional radiographic findings that may be seen with ascites include diffusely increased density of the abdomen and bulging of the peritoneal fat stripes/flanks. In the average adult, a minimum of approximately 800 mL of ascitic fluid is required to medially displace bowel loops or abdominal viscera.2 While there are many causes of ascites, the radiograph in this case suggests the underlying etiology of cirrhosis. Although the top of the liver is not visualized on the radiograph, the liver contour appears enlarged (red arrow, Figure 1b).
In the ED, ultrasound is the imaging modality of choice to confirm the presence of ascites as it is widely available, quick, relatively inexpensive, and does not require ionizing radiation. Ultrasound is also useful when examining the abdominal viscera since it may detect the underlying cause of ascites.3 The ultrasound image of the abdominal viscera in this case demonstrated a hypoechoic (dark) signal (red arrow, Figure 1c) between the abdominal wall (red asterisk, Figure 1c) and bowel loops (letter ‘B,” Figure 1c). This finding confirmed the presence of ascites because simple fluid does not reflect the ultrasound beam, but instead results in the hypoechoic signal seen in Figure 1c. An additional sonographic image confirmed the presence of an enlarged liver (white asterisk, Figure 1d) and ascites in the right upper quadrant (red arrow, Figure 1d).
The patient was admitted for further workup and treatment of alcoholic cirrhosis. A
Dr Lyons is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-
30-year-old woman with a history of alcoholism and anxiety presented to the ED for evaluation of abdominal distension and pain. She reported new social stressors at home and increased alcohol consumption. She also noted gradually increasing abdominal distension over the past several weeks. As patient’s liver function tests were elevated, supine radiographs of the abdomen were acquired for further evaluation; a representative image is presented below (Figure 1a).
What is the suspected diagnosis?
Is any additional imaging required?
Answer
Centralization of bowel is suggestive of abdominopelvic ascites.1 Additional radiographic findings that may be seen with ascites include diffusely increased density of the abdomen and bulging of the peritoneal fat stripes/flanks. In the average adult, a minimum of approximately 800 mL of ascitic fluid is required to medially displace bowel loops or abdominal viscera.2 While there are many causes of ascites, the radiograph in this case suggests the underlying etiology of cirrhosis. Although the top of the liver is not visualized on the radiograph, the liver contour appears enlarged (red arrow, Figure 1b).
In the ED, ultrasound is the imaging modality of choice to confirm the presence of ascites as it is widely available, quick, relatively inexpensive, and does not require ionizing radiation. Ultrasound is also useful when examining the abdominal viscera since it may detect the underlying cause of ascites.3 The ultrasound image of the abdominal viscera in this case demonstrated a hypoechoic (dark) signal (red arrow, Figure 1c) between the abdominal wall (red asterisk, Figure 1c) and bowel loops (letter ‘B,” Figure 1c). This finding confirmed the presence of ascites because simple fluid does not reflect the ultrasound beam, but instead results in the hypoechoic signal seen in Figure 1c. An additional sonographic image confirmed the presence of an enlarged liver (white asterisk, Figure 1d) and ascites in the right upper quadrant (red arrow, Figure 1d).
The patient was admitted for further workup and treatment of alcoholic cirrhosis. A
Dr Lyons is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-
- Meschan I. Analysis of Roentgen Signs in General Radiology. Philadelphia, PA: Saunders; 1973:1231.
- Keeffee EJ, Gagliardi RA, Pfister RC. The roentgenographic evaluation of ascites. Am J Roentgenol. 1967;101(2):388-396.
- Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22.
- Meschan I. Analysis of Roentgen Signs in General Radiology. Philadelphia, PA: Saunders; 1973:1231.
- Keeffee EJ, Gagliardi RA, Pfister RC. The roentgenographic evaluation of ascites. Am J Roentgenol. 1967;101(2):388-396.
- Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22.
Surgical Management of Gorham-Stout Disease of the Pelvis Refractory to Medical and Radiation Therapy
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Coracoid Fracture After Reverse Total Shoulder Arthroplasty: A Report of 2 Cases
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.