User login
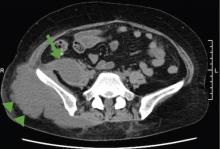
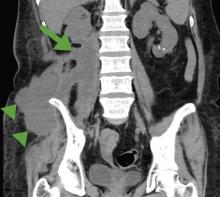
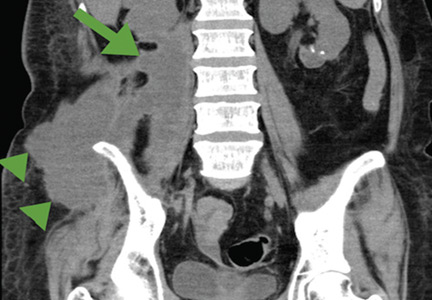
Iliopsoas abscess
A 52-year-old woman with diabetes mellitus presented with a 1-month history of pain in the right lower abdomen and right back. Although she had a fever when the pain started and her pain was aggravated by walking, her pain and fever had gotten better after taking antibiotics prescribed earlier.
The patient was admitted to the hospital for percutaneous drainage, which produced 26 mL of pus on the first day and 320 mL on the next day; culture was positive for Escherichia coli. Urine culture was also positive for E coli; blood culture was not. We concluded that these results were secondary to pyelonephritis.
We started intravenous piperacillin-tazobactam 2.25 g every 8 hours for empiric therapy. We changed this to oral ampicillin-cloxacillin 2 g/day after E coli was cultured and pyelonephritis was suspected. The patient was discharged after a 2-week hospital stay, with no significant complications.
ILIOPSOAS ABSCESS: DIAGNOSTIC CLUES
Iliopsoas abscess can occur at any age.1–3 Pain is the most common symptom, occurring in more than 90% of patients.1 Fever with temperatures over 38°C is less common at first, found in less than half of patients.1,2
Only 13% of patients with iliopsoas abscess may have a palpable mass on physical examination.1 The psoas sign—a worsening of lower abdominal pain on the affected side with passive extension of the thigh while supine—has a sensitivity of only 24% for iliopsoas abscess; it can also indicate inflammation to the iliopsoas muscle in other conditions such as retrocecal appendicitis.3
Hip flexion deformity can be a helpful diagnostic feature, as 96% of patients with iliopsoas abscess hold the hip in flexion to relieve pain.4 But pain on hip flexion can also occur in conditions such as septic arthritis.4
Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein may be elevated in all patients with iliopsoas abscess, so if those markers are not elevated, we may have to consider other conditions such as cancer.1 Computed tomography is nearly 100% sensitive for iliopsoas abscess and is the gold standard for diagnosis.3
TREATMENT
Inadequate treatment of iliopsoas abscess raises the risk of relapse and death.3 Drainage and appropriate antibiotic therapy have been shown to be effective.1,3
Iliopsoas abscess can also be secondary to a number of conditions, eg, Crohn disease, appendicitis, intra-abdominal infection, and cancer,5 and the primary condition needs to be addressed. In addition, culture of a secondary abscess is more likely to grow mixed organisms.5
The average size of the abscess is 6 cm. Percutaneous drainage is required if the mass is larger than 3.5 cm.1
TAKE-HOME MESSAGES
Iliopsoas abscess is difficult to diagnose because patients have few specific complaints. Checking for hip flexion deformity and inflammatory markers may help rule out the disease. When iliopsoas abscess is suspected, computed tomography is necessary to confirm the diagnosis. Drainage and appropriate antibiotics are effective treatment.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg 2012; 10:466–469.
- Huang JJ, Ruaan MK, Lan RR, Wang MC. Acute pyogenic iliopsoas abscess in Taiwan: clinical features, diagnosis, treatments and outcome. J Infect 2000; 40:248–255.
- Stefanich RJ, Moskowitz A. Hip flexion deformity secondary to acute pyogenic psoas abscess. Orthop Rev 1987; 16:67–77.
- Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg 1986; 10:834–843.
A 52-year-old woman with diabetes mellitus presented with a 1-month history of pain in the right lower abdomen and right back. Although she had a fever when the pain started and her pain was aggravated by walking, her pain and fever had gotten better after taking antibiotics prescribed earlier.
The patient was admitted to the hospital for percutaneous drainage, which produced 26 mL of pus on the first day and 320 mL on the next day; culture was positive for Escherichia coli. Urine culture was also positive for E coli; blood culture was not. We concluded that these results were secondary to pyelonephritis.
We started intravenous piperacillin-tazobactam 2.25 g every 8 hours for empiric therapy. We changed this to oral ampicillin-cloxacillin 2 g/day after E coli was cultured and pyelonephritis was suspected. The patient was discharged after a 2-week hospital stay, with no significant complications.
ILIOPSOAS ABSCESS: DIAGNOSTIC CLUES
Iliopsoas abscess can occur at any age.1–3 Pain is the most common symptom, occurring in more than 90% of patients.1 Fever with temperatures over 38°C is less common at first, found in less than half of patients.1,2
Only 13% of patients with iliopsoas abscess may have a palpable mass on physical examination.1 The psoas sign—a worsening of lower abdominal pain on the affected side with passive extension of the thigh while supine—has a sensitivity of only 24% for iliopsoas abscess; it can also indicate inflammation to the iliopsoas muscle in other conditions such as retrocecal appendicitis.3
Hip flexion deformity can be a helpful diagnostic feature, as 96% of patients with iliopsoas abscess hold the hip in flexion to relieve pain.4 But pain on hip flexion can also occur in conditions such as septic arthritis.4
Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein may be elevated in all patients with iliopsoas abscess, so if those markers are not elevated, we may have to consider other conditions such as cancer.1 Computed tomography is nearly 100% sensitive for iliopsoas abscess and is the gold standard for diagnosis.3
TREATMENT
Inadequate treatment of iliopsoas abscess raises the risk of relapse and death.3 Drainage and appropriate antibiotic therapy have been shown to be effective.1,3
Iliopsoas abscess can also be secondary to a number of conditions, eg, Crohn disease, appendicitis, intra-abdominal infection, and cancer,5 and the primary condition needs to be addressed. In addition, culture of a secondary abscess is more likely to grow mixed organisms.5
The average size of the abscess is 6 cm. Percutaneous drainage is required if the mass is larger than 3.5 cm.1
TAKE-HOME MESSAGES
Iliopsoas abscess is difficult to diagnose because patients have few specific complaints. Checking for hip flexion deformity and inflammatory markers may help rule out the disease. When iliopsoas abscess is suspected, computed tomography is necessary to confirm the diagnosis. Drainage and appropriate antibiotics are effective treatment.
A 52-year-old woman with diabetes mellitus presented with a 1-month history of pain in the right lower abdomen and right back. Although she had a fever when the pain started and her pain was aggravated by walking, her pain and fever had gotten better after taking antibiotics prescribed earlier.
The patient was admitted to the hospital for percutaneous drainage, which produced 26 mL of pus on the first day and 320 mL on the next day; culture was positive for Escherichia coli. Urine culture was also positive for E coli; blood culture was not. We concluded that these results were secondary to pyelonephritis.
We started intravenous piperacillin-tazobactam 2.25 g every 8 hours for empiric therapy. We changed this to oral ampicillin-cloxacillin 2 g/day after E coli was cultured and pyelonephritis was suspected. The patient was discharged after a 2-week hospital stay, with no significant complications.
ILIOPSOAS ABSCESS: DIAGNOSTIC CLUES
Iliopsoas abscess can occur at any age.1–3 Pain is the most common symptom, occurring in more than 90% of patients.1 Fever with temperatures over 38°C is less common at first, found in less than half of patients.1,2
Only 13% of patients with iliopsoas abscess may have a palpable mass on physical examination.1 The psoas sign—a worsening of lower abdominal pain on the affected side with passive extension of the thigh while supine—has a sensitivity of only 24% for iliopsoas abscess; it can also indicate inflammation to the iliopsoas muscle in other conditions such as retrocecal appendicitis.3
Hip flexion deformity can be a helpful diagnostic feature, as 96% of patients with iliopsoas abscess hold the hip in flexion to relieve pain.4 But pain on hip flexion can also occur in conditions such as septic arthritis.4
Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein may be elevated in all patients with iliopsoas abscess, so if those markers are not elevated, we may have to consider other conditions such as cancer.1 Computed tomography is nearly 100% sensitive for iliopsoas abscess and is the gold standard for diagnosis.3
TREATMENT
Inadequate treatment of iliopsoas abscess raises the risk of relapse and death.3 Drainage and appropriate antibiotic therapy have been shown to be effective.1,3
Iliopsoas abscess can also be secondary to a number of conditions, eg, Crohn disease, appendicitis, intra-abdominal infection, and cancer,5 and the primary condition needs to be addressed. In addition, culture of a secondary abscess is more likely to grow mixed organisms.5
The average size of the abscess is 6 cm. Percutaneous drainage is required if the mass is larger than 3.5 cm.1
TAKE-HOME MESSAGES
Iliopsoas abscess is difficult to diagnose because patients have few specific complaints. Checking for hip flexion deformity and inflammatory markers may help rule out the disease. When iliopsoas abscess is suspected, computed tomography is necessary to confirm the diagnosis. Drainage and appropriate antibiotics are effective treatment.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg 2012; 10:466–469.
- Huang JJ, Ruaan MK, Lan RR, Wang MC. Acute pyogenic iliopsoas abscess in Taiwan: clinical features, diagnosis, treatments and outcome. J Infect 2000; 40:248–255.
- Stefanich RJ, Moskowitz A. Hip flexion deformity secondary to acute pyogenic psoas abscess. Orthop Rev 1987; 16:67–77.
- Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg 1986; 10:834–843.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg 2012; 10:466–469.
- Huang JJ, Ruaan MK, Lan RR, Wang MC. Acute pyogenic iliopsoas abscess in Taiwan: clinical features, diagnosis, treatments and outcome. J Infect 2000; 40:248–255.
- Stefanich RJ, Moskowitz A. Hip flexion deformity secondary to acute pyogenic psoas abscess. Orthop Rev 1987; 16:67–77.
- Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg 1986; 10:834–843.
Emergency Imaging: Left Periorbital Swelling
Case
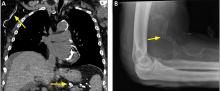
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
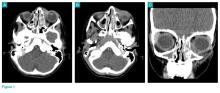
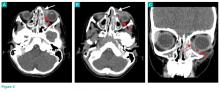
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
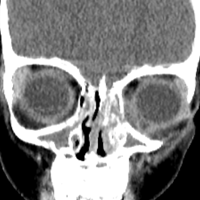
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
Case
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
Case
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
Sarcoidosis mimicking lytic osseous metastases
A 41-year-old woman presented with coughing, wheezing, and painful subcutaneous nodules on her legs. She had presented 6 years ago with similar nodules and enlarged retroauricular, occipital, and maxillary lymph nodes. At that time, biopsy study of submaxillary lymph nodes and skin showed nonnecrotizing granulomas, with negative microbiology studies. Chest radiography and spirometry were normal. A diagnosis of sarcoidosis was made. Treatment was offered but refused.
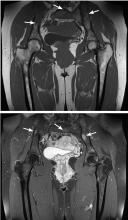
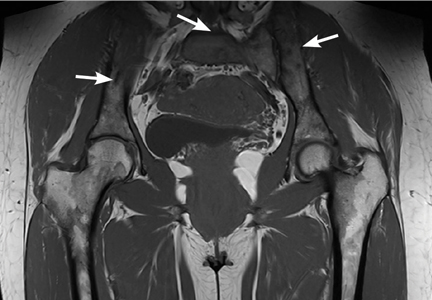
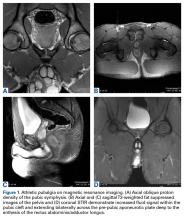
Based on this history, computed tomography of the thorax and abdomen was performed and showed mediastinal and hilar lymphadenopathy, small bilateral lung nodules, and osseous cystic areas in both iliac blades. Magnetic resonance imaging (MRI) showed numerous discrete lesions in both iliac bones (Figure 1). Biopsy study of iliac bone revealed preserved architecture with no evidence of malignancy or granulomas.
Radiography of the hands showed an osseous lytic lesion in the third proximal phalanx of the right hand. No other radiographic abnormalities were noted.
The clinical and radiographic features and the patient’s clinical course were consistent with osseous sarcoidosis. She was started on methotrexate and a low-dose corticosteroid and was symptom-free at 12-month follow-up. Follow-up MRI showed reduction in the lymphadenopathies and stabilization of the bone lesions.
SARCOIDOSIS AND BONE
Sarcoidosis is a systemic granulomatous disease that involves the lung in more than 90% of cases. Skeletal involvement has been reported in 1% to 14% of patients.1,2 Typical osseous involvement is cystic osteitis of the phalangeal bones of the hands and feet, but any part of the skeleton may be involved.3
Bone sarcoidosis is usually asymptomatic and is discovered incidentally. The diagnosis of sarcoidosis has usually been established clinically before bone lesions are detected on MRI. However, sarcoidosis-related bone lesions resembling bone metastases on MRI may be the initial presentation. The presence of intralesional fat has been described as a feature that excludes malignancy.
No treatment has been shown to be of benefit.4 Sarcoidosis is a diagnosis of exclusion and radiographic lytic bone features are not specific, so a neoplastic cause (such as primary osteoblastoma, metastasis, or multiple myeloma) must always be ruled out, as well as other bone conditions such as osteomyelitis or bone cyst.
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 2014; 383:1155–1167.
- James DG, Neville E, Carstairs LS. Bone and joint sarcoidosis. Semin Arthritis Rheum 1976; 6:53–81.
- Moore SL, Kransdorf MJ, Schweitzer ME, Murphey MD, Babb JS. Can sarcoidosis and metastatic bone lesions be reliably differentiated on routine MRI? AJR Am J Roentgenol 2012; 198:1387–1393.
- Hamoud S, Srour S, Fruchter O, Vlodavsky E, Hayek T. Lytic bone lesion: presenting finding of sarcoidosis. Isr Med Assoc J 2010; 12:59–60.
A 41-year-old woman presented with coughing, wheezing, and painful subcutaneous nodules on her legs. She had presented 6 years ago with similar nodules and enlarged retroauricular, occipital, and maxillary lymph nodes. At that time, biopsy study of submaxillary lymph nodes and skin showed nonnecrotizing granulomas, with negative microbiology studies. Chest radiography and spirometry were normal. A diagnosis of sarcoidosis was made. Treatment was offered but refused.
Based on this history, computed tomography of the thorax and abdomen was performed and showed mediastinal and hilar lymphadenopathy, small bilateral lung nodules, and osseous cystic areas in both iliac blades. Magnetic resonance imaging (MRI) showed numerous discrete lesions in both iliac bones (Figure 1). Biopsy study of iliac bone revealed preserved architecture with no evidence of malignancy or granulomas.
Radiography of the hands showed an osseous lytic lesion in the third proximal phalanx of the right hand. No other radiographic abnormalities were noted.
The clinical and radiographic features and the patient’s clinical course were consistent with osseous sarcoidosis. She was started on methotrexate and a low-dose corticosteroid and was symptom-free at 12-month follow-up. Follow-up MRI showed reduction in the lymphadenopathies and stabilization of the bone lesions.
SARCOIDOSIS AND BONE
Sarcoidosis is a systemic granulomatous disease that involves the lung in more than 90% of cases. Skeletal involvement has been reported in 1% to 14% of patients.1,2 Typical osseous involvement is cystic osteitis of the phalangeal bones of the hands and feet, but any part of the skeleton may be involved.3
Bone sarcoidosis is usually asymptomatic and is discovered incidentally. The diagnosis of sarcoidosis has usually been established clinically before bone lesions are detected on MRI. However, sarcoidosis-related bone lesions resembling bone metastases on MRI may be the initial presentation. The presence of intralesional fat has been described as a feature that excludes malignancy.
No treatment has been shown to be of benefit.4 Sarcoidosis is a diagnosis of exclusion and radiographic lytic bone features are not specific, so a neoplastic cause (such as primary osteoblastoma, metastasis, or multiple myeloma) must always be ruled out, as well as other bone conditions such as osteomyelitis or bone cyst.
A 41-year-old woman presented with coughing, wheezing, and painful subcutaneous nodules on her legs. She had presented 6 years ago with similar nodules and enlarged retroauricular, occipital, and maxillary lymph nodes. At that time, biopsy study of submaxillary lymph nodes and skin showed nonnecrotizing granulomas, with negative microbiology studies. Chest radiography and spirometry were normal. A diagnosis of sarcoidosis was made. Treatment was offered but refused.
Based on this history, computed tomography of the thorax and abdomen was performed and showed mediastinal and hilar lymphadenopathy, small bilateral lung nodules, and osseous cystic areas in both iliac blades. Magnetic resonance imaging (MRI) showed numerous discrete lesions in both iliac bones (Figure 1). Biopsy study of iliac bone revealed preserved architecture with no evidence of malignancy or granulomas.
Radiography of the hands showed an osseous lytic lesion in the third proximal phalanx of the right hand. No other radiographic abnormalities were noted.
The clinical and radiographic features and the patient’s clinical course were consistent with osseous sarcoidosis. She was started on methotrexate and a low-dose corticosteroid and was symptom-free at 12-month follow-up. Follow-up MRI showed reduction in the lymphadenopathies and stabilization of the bone lesions.
SARCOIDOSIS AND BONE
Sarcoidosis is a systemic granulomatous disease that involves the lung in more than 90% of cases. Skeletal involvement has been reported in 1% to 14% of patients.1,2 Typical osseous involvement is cystic osteitis of the phalangeal bones of the hands and feet, but any part of the skeleton may be involved.3
Bone sarcoidosis is usually asymptomatic and is discovered incidentally. The diagnosis of sarcoidosis has usually been established clinically before bone lesions are detected on MRI. However, sarcoidosis-related bone lesions resembling bone metastases on MRI may be the initial presentation. The presence of intralesional fat has been described as a feature that excludes malignancy.
No treatment has been shown to be of benefit.4 Sarcoidosis is a diagnosis of exclusion and radiographic lytic bone features are not specific, so a neoplastic cause (such as primary osteoblastoma, metastasis, or multiple myeloma) must always be ruled out, as well as other bone conditions such as osteomyelitis or bone cyst.
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 2014; 383:1155–1167.
- James DG, Neville E, Carstairs LS. Bone and joint sarcoidosis. Semin Arthritis Rheum 1976; 6:53–81.
- Moore SL, Kransdorf MJ, Schweitzer ME, Murphey MD, Babb JS. Can sarcoidosis and metastatic bone lesions be reliably differentiated on routine MRI? AJR Am J Roentgenol 2012; 198:1387–1393.
- Hamoud S, Srour S, Fruchter O, Vlodavsky E, Hayek T. Lytic bone lesion: presenting finding of sarcoidosis. Isr Med Assoc J 2010; 12:59–60.
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 2014; 383:1155–1167.
- James DG, Neville E, Carstairs LS. Bone and joint sarcoidosis. Semin Arthritis Rheum 1976; 6:53–81.
- Moore SL, Kransdorf MJ, Schweitzer ME, Murphey MD, Babb JS. Can sarcoidosis and metastatic bone lesions be reliably differentiated on routine MRI? AJR Am J Roentgenol 2012; 198:1387–1393.
- Hamoud S, Srour S, Fruchter O, Vlodavsky E, Hayek T. Lytic bone lesion: presenting finding of sarcoidosis. Isr Med Assoc J 2010; 12:59–60.
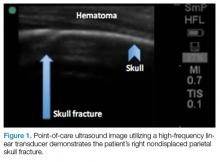
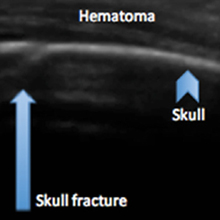
Identifying Pediatric Skull Fracture Using Point-of-Care Ultrasound
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
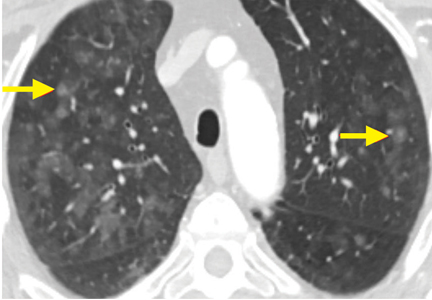
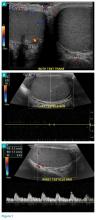
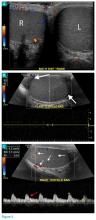
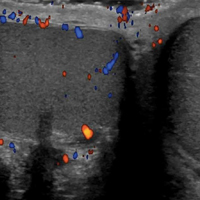
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
Another complication of cirrhosis
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
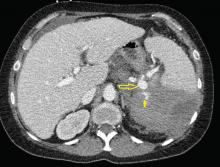
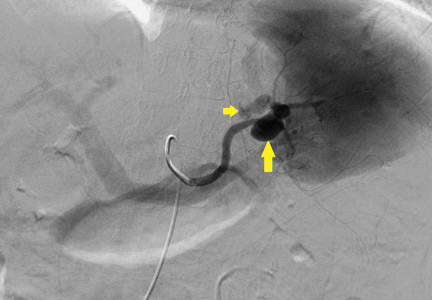
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
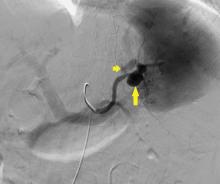
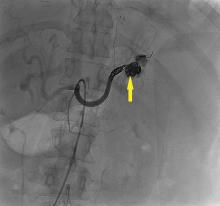
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
Metastatic pulmonary calcification and end-stage renal disease
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
Cardiac mass: Tumor or thrombus?
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
Referral Patterns for Chronic Groin Pain and Athletic Pubalgia/Sports Hernia: Magnetic Resonance Imaging Findings, Treatment, and Outcomes
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
Emergency Imaging: Severe Left Testicular Swelling
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
Concussion: Evaluation and management
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
Concussion, also known as mild traumatic brain injury, affects more than 600 adults per 100,000 each year and is commonly treated by nonneurologists.1 Public attention to concussion has been increasing, particularly to concussion sustained during sports. Coincident with this increased attention, the diagnosis of concussion continues to increase in the outpatient setting. Thus, a review of the topic is timely.
ACCELERATION OF THE BRAIN DUE TO TRAUMA
The definition of concussion has changed considerably over the years. It is currently defined as a pathophysiologic process that results from an acceleration or deceleration of the brain induced by trauma.2 It is largely a temporary, functional problem, as opposed to a gross structural injury.2–5
The acceleration of the brain that results in a concussion is usually initiated by a direct blow to the head, although direct impact is not required.6 As the brain rotates, different areas accelerate at different rates, resulting in a shear strain imparted to the parenchyma.
This shear strain causes deformation of axonal membranes and opening of membrane-associated sodium-potassium channels. This in turn leads to release of excitatory neurotransmitters, ultimately culminating in a wave of neuronal depolarization and a spreading depression-like phenomenon that may mediate the loss of consciousness, posttraumatic amnesia, confusion, and many of the other immediate signs and symptoms associated with concussion.
The sudden metabolic demand created by the massive excitatory phenomena triggers an increased utilization of glucose to restore cellular homeostasis. At the same time, cerebral blood flow decreases after concussion, which, in the setting of increased glucose demand, leads to an “energy crisis”: an increased need for adenosine triphosphate with a concomitant decreased delivery of glucose.7 This mismatch between energy demand and supply is thought to underlie the most common signs and symptoms of concussion.
ASSESSMENT
History
The history of present illness is essential to a diagnosis of concussion. In the classic scenario, an otherwise asymptomatic person sustains some trauma to the head that is followed immediately by the signs and symptoms of concussion.
Many of these signs and symptoms are nonspecific and may occur without concussion or other trauma.8,9 Thus, the diagnosis of concussion cannot be made on the basis of symptoms alone, but only in the overall context of history, physical examination, and, at times, additional clinical assessments.
The symptoms of concussion should gradually improve. While they may be exacerbated by certain activities or stimuli, the overall trend should be one of symptom improvement. If symptoms are worsening over time, alternative explanations for the patient’s symptoms should be considered.
Physical examination
A thorough neurologic examination should be conducted in all patients with suspected concussion and include the following.
A mental status examination should include assessment of attention, memory, and recall. Orientation is normal except in the most acute examinations.
Cranial nerve examination must include careful assessment of eye-movement control, including smooth pursuit and saccades. However, even in patients with prominent subjective dizziness, considerable experience may be needed to actually demonstrate abnormalities.
Balance testing. Balance demands careful assessment and, especially for young athletes, this testing should be more difficult than the tandem gait and eyes-closed, feet-together tests.
Standard strength, sensory, reflex, and coordination testing is usually normal.
Any focal neurologic findings should prompt consideration of other causes or of a more serious injury and should lead to further evaluation, including brain imaging.
Diagnostic tests
Current clinical brain imaging cannot diagnose a concussion. The purpose of neuroimaging is to assess for other etiologies or injuries, such as hemorrhage or contusion, that may cause similar symptoms but require different management.
Several guidelines are available to assess the need for imaging in the setting of recent trauma, of which 2 are typically used10–12:
The Canadian CT Head Rule10 states that computed tomography (CT) is indicated in any of the following situations:
- The patient fails to reach a Glasgow Coma Scale score of 15—on a scale of 3 (worst) to 15 (best)—within 2 hours
- There is a suspected open skull fracture
- There is any sign of basal skull fracture
- The patient has 2 or more episodes of vomiting
- The patient is 65 or older
- The patient has retrograde amnesia (ie, cannot remember events that occurred before the injury) for 30 minutes or more
- The mechanism of injury was dangerous (eg, a pedestrian was struck by a motor vehicle, or the patient fell from > 3 feet or > 5 stairs).
The New Orleans Criteria11 state that a patient warrants CT of the head if any of the following is present:
- Severe headache
- Vomiting
- Age over 60
- Drug or alcohol intoxication
- Deficit in short-term memory
- Physical evidence of trauma above the clavicles
- Seizure.
Caveats: these imaging guidelines apply to adults; those for pediatric patients differ.12 Also, because they were designed for use in an emergency department, their utility in clinical practice outside the emergency department is unclear.
Electroencephalography is not necessary in the evaluation of concussion unless a seizure disorder is believed to be the cause of the injury.
Concussion in athletes
Athletes who participate in contact and collision sports are at higher risk of concussion than the nonathletic population. Therefore, specific assessments of symptoms, balance, oculomotor function, cognitive function, and reaction time have been developed for athletes.
Ideally, these measures are taken at preseason baseline, so that they are available for comparison with postinjury assessments after a known or suspected concussion. These assessments can be used to help make the diagnosis of concussion in cases that are unclear and to help monitor recovery. Objective measures of injury are especially useful for athletes who may be reluctant to report symptoms in order to return to play.
Like most medical tests, these assessments need to be properly interpreted in the overall context of the medical history and physical examination by those who know how to administer them. It is important to remember that the natural history of concussion recovery differs between sport-related concussion and concussion that occurs outside of sports.8
MANAGEMENT
The symptoms and signs after concussion are so variable and multidimensional that they make a generally applicable treatment hard to define.
Rest: Physical and cognitive
Treatment depends on the specifics of the injury, but there are common recommendations for the acute days after injury. Lacking hard data, the consensus among experts is that patients should undergo a period of physical and cognitive rest.13,14 Exactly what “rest” means and how long it should last are unknown, leading to a wide variation in its application.
Rest aids recovery but also may have adverse effects: fatigue, diurnal sleep disruption, reactive depression, anxiety, and physiologic deconditioning.15,16 Many guidelines recommend physical and cognitive rest until symptoms resolve,14 but this is likely too cautious. Even without a concussion, inactivity is associated with many of the nonspecific symptoms also associated with concussion. As recovery progresses, the somatic symptoms of concussion improve, while emotional symptoms worsen, likely in part due to prolonged rest.17
We recommend a period of rest lasting 3 to 5 days after injury, followed by a gradual resumption of both physical and cognitive activities as tolerated, remaining below the level at which symptoms are exacerbated.
Not surprisingly, many guidelines for returning to physical activity are focused on athletes. Yet the same principles apply to management of concussion in the general population who exercise: light physical activity (typically walking or stationary bicycling), followed by more vigorous aerobic activity, followed by some resistance activities. Mild aerobic exercise (to below the threshold of symptoms) may speed recovery from refractive postconcussion syndrome, even in those who did not exercise before the injury.18
Athletes require specific and strict instructions to avoid increased trauma to the head during the gradual increase of physical activities. The National Collegiate Athletic Association has published an algorithm for a gradual return to sport-specific training that is echoed in recent consensus statements on concussion.19 Once aerobic reconditioning produces no symptoms, then noncontact, sport-specific activities are begun, followed by contact activities. We have patients return to the clinic once they are symptom-free for repeat evaluation before clearing them for high-risk activities (eg, skiing, bicycling) or contact sports (eg, basketball, soccer, football, ice hockey).
Cognitive rest
While physical rest is fairly straightforward, cognitive rest is more challenging. The concept of cognitive rest is hard to define and even harder to enforce. Patients are often told to minimize any activities that require attention or concentration. This often includes, but is not limited to, avoiding reading, texting, playing video games, and using computers.13
In the modern world, full avoidance of these activities is difficult and can be profoundly socially isolating. Further, complete cognitive rest may be associated with symptoms of its own.15,16,20 Still, some reasonable limitation of cognitive activities, at least initially, is likely beneficial.21 For patients engaged in school or academic work, often the daily schedule needs to be adjusted and accommodations made to help them return to a full academic schedule and level of activity. It is reasonable to have patients return gradually to work or school rather than attempt to immediately return to their preinjury level.
With these interventions, most patients have full resolution of their symptoms and return to preinjury levels of performance.
TREATING SOMATIC SYMPTOMS
Posttraumatic headache
Posttraumatic headache is the most common sequela of concussion.22 Surprisingly, it is more common after concussion than after moderate or severe traumatic brain injury.23 A prior history of headache, particularly migraine, is a known risk factor for development of posttraumatic headache.24
Posttraumatic headache is usually further defined by headache type using the International Classification of Headache Disorders criteria (www.ichd-3.org). Migraine or probable migraine is the most common type of posttraumatic headache; tension headache is less common.25
Analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) are often used initially by patients to treat posttraumatic headache. One study found that 70% of patients used acetaminophen or an NSAID.26
Treating early with effective therapy is the most important tenet of posttraumatic headache treatment, since 80% of those who self-treat have incomplete relief, and almost all of them are using over-the-counter products.27 Overuse of over-the-counter abortive medications can lead to medication overuse headache, also known as rebound headache, thus complicating the treatment of posttraumatic headache.26
Earlier treatment with a preventive medication can often limit the need for and overuse of over-the-counter analgesics and can minimize the occurrence of subsequent medication overuse headache. However, in pediatric populations, nonpharmacologic interventions such as rest and sleep hygiene are typically used first, then medications after 4 to 6 weeks if this is ineffective.
A number of medications have been studied for prophylactic treatment of posttraumatic headache, including topiramate, amitriptyline, and divalproex sodium,28–30 but there is little compelling evidence for use of one over the other. If posttraumatic headache is migrainous, beta-blockers, calcium-channel blockers, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibtors, and gabapentin are other prophylactic medication options under the appropriate circumstances.27,31,32 In adults, we have clinically had success with nortriptyline 20 mg or gabapentin 300 mg at night as an initial prophylactic headache medication, increasing as tolerated or until pain is controlled, though there are no high-quality data to guide this decision.
The ideal prophylactic medication depends on headache type, patient tolerance, comorbidities, allergies, and medication sensitivities. Gabapentin, amitriptyline, and nortriptyline can produce sedation, which can help those suffering from sleep disturbance.
If a provider is not comfortable prescribing these medications or doesn’t prescribe them regularly, the patient should be referred to a concussion or headache specialist more familiar with their use.
In some patients, even some athletes, headache may be related to a cervical strain injury—whiplash—that should be treated with an NSAID (or acetaminophen), perhaps with a short course of a muscle relaxant in adults, and with physical therapy.32
Some patients have chronic headache despite oral medications.26 Therefore, alternatives to oral medications and complementary therapies should be considered. Especially for protracted cases requiring more complicated headache management or injectable treatments, patients should be referred to a pain clinic, headache specialist, or concussion specialist.
Dizziness
Dizziness is also common after concussion. But what the patient means by dizziness requires a little probing. Some have paroxysms of vertigo. This typically represents a peripheral vestibular injury, usually benign paroxysmal positional vertigo. The latter can be elicited with a Hallpike maneuver and treated in the office with the Epley maneuver.33
Usually, dizziness is a subjective sense of poor coordination, gait instability, or dysequilibrium. Patients may also complain of associated nausea and motion sensitivity. This may all be secondary to a mechanism in the middle or inner ear or the brain. Patients should be encouraged to begin movement—gradually and safely—to help the vestibular system accommodate, which it will do with gradual stimulation. It usually resolves spontaneously.
Specific treatment is unfortunately limited. There is no established benefit from vestibular suppressants such as meclizine. Vestibular rehabilitation may accelerate improvement and decrease symptoms.33 Referral for a comprehensive balance assessment or to vestibular therapy (a subset of physical therapy) should be considered and is something we typically undertake in our clinic if there is no recovery from dizziness 4 to 6 weeks after the concussion.
Visual symptoms can contribute to dizziness. Convergence spasm or convergence insufficiency (both related to muscle spasm of the eye) can occur after concussion, with some studies estimating that up to 69% of patients have these symptoms.34 This can interfere with visual tracking and contribute to a feeling of dysequilibrium.34 Referral to a concussion specialist or vestibular rehabilitation physical therapist can be helpful in treating this issue if it does not resolve spontaneously.
Orthostasis and lightheadedness also contribute to dizziness and are associated with cerebrovascular autoregulation. Available data suggest that dysregulation of neurovascular coupling, cerebral vasoreactivity, and cerebral autoregulation contribute to some of the chronic symptoms of concussion, including dizziness. A gradual return to exercise may help regulate cerebral blood flow and improve this type of dizziness.35
Sleep disturbance
Sleep disturbance is common after concussion, but the form is variable: insomnia, excessive daytime somnolence, and alteration of the sleep-wake cycle are all seen and may themselves affect recovery.36
Sleep hygiene education should be the first intervention for postconcussive sleep issues. For example, the patient should be encouraged to do the following:
- Minimize “screen time” an hour before going to bed: cell phone, tablet, and computer screens emit a wavelength of light that suppresses endogenous melatonin release37,38
- Go to bed and wake up at the same time each day
- Minimize or avoid caffeine, nicotine, and alcohol
- Avoid naps.39
Melatonin is a safe and effective treatment that could be added.40 In addition, some studies suggest that melatonin may improve recovery from traumatic brain injury.41,42
Mild exercise (to below the threshold of causing or exacerbating symptoms) may also improve sleep quality.
Amitriptyline or nortriptyline may reduce headache frequency and intensity and also help treat insomnia.
Trazodone is recommended by some as a first-line agent,39 but we usually reserve it for protracted insomnia refractory to the above treatments.
Benzodiazepines should be avoided, as they reduce arousal, impair cognition, and exacerbate motor impairments.43
Emotional symptoms
Acute-onset anxiety or depression often occurs after concussion.44,45 There is abundant evidence that emotional effects of injury may be the most significant factor in recovery.46 A preinjury history of anxiety may be a prognostic factor.9 Patients with a history of anxiety or depression are more likely to develop emotional symptoms after a concussion, but emotional problems may develop in any patient after a concussion.47,48
The circumstances under which an injury is sustained may be traumatic (eg, car accident, assault), leading to an acute stress reaction or disorder and, if untreated, may result in a more chronic condition—posttraumatic stress disorder. Moreover, the injury and subsequent symptoms may have repercussions in many aspects of the patient’s life, leading to further psychologic stress (eg, loss of wages or the inability to handle normal work, school, and family responsibilities).
Referral to a therapist trained in skills-based psychotherapy (eg, cognitive-behavioral therapy, exposure-based treatment) is often helpful.
Pharmacologic treatment can be a useful adjunct. Several studies have shown that selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants may improve depression after concussion.49 The prescription of antidepressants, however, is best left to providers with experience in treating anxiety and depression.
As with sleep disorders after concussion, benzodiazepines should be avoided, as they can impair cognition.43
Cognitive problems
Cognitive problems are also common after concussion. Patients complain about everyday experiences of forgetfulness, distractibility, loss of concentration, and mental fatigue. Although patients often subjectively perceive these symptoms as quite limiting, the impairments can be difficult to demonstrate in office testing.
A program of gradual increase in mental activity, parallel to recovery of physical capacity, should be undertaken. Most patients make a gradual recovery within a few weeks.50
When cognitive symptoms cause significant school or vocational problems or become persistent, patients should be referred to a specialty clinic. As with most of the consequences of concussion, there are few established treatments. When cognitive difficulties persist, it is important to consider the complications of concussion mentioned above: headache, pain, sleep disturbance, and anxiety, all of which may cause subjective cognitive problems and are treatable.
If cognitive symptoms are prolonged despite improvement of other issues like headache and sleep disturbance, a low-dose stimulant medication such as amphetamine salts or methylphenidate may be useful for symptoms of poor attention.49 They should be only a temporary measure after concussion to carry the patient through a cognitively challenging period, unless there was a history of attention-deficit disorder before the injury. A variety of other agents, including amantadine,51 have been proposed based on limited studies; all are off-label uses. Before considering these types of interventions, referral to a specialist or a specialty program would be appropriate.
IF SYMPTOMS PERSIST
With the interventions suggested above, most patients with concussion have a resolution of symptoms and can return to preinjury levels of performance. But some have prolonged symptoms and sequelae. Approximately 10% of athletes have persistent signs and symptoms of concussion beyond 2 weeks. If concussion is not sport-related, most patients recover completely within the first 3 months, but up to 33% may have symptoms beyond that.52
Four types of patients have persistent symptoms:
Patients who sustained a high-force mechanism of injury. These patients simply need more time and accommodation.
Patients who sustained multiple concussions. These patients may also need more time and accommodation.
Patients with an underlying neurologic condition, recognized prior to injury or not, may have delayed or incomplete recovery. Even aging may be an “underlying condition” in concussion.
Patients whose symptoms from an apparently single mild concussion do not resolve despite appropriate treatments may have identifiable factors, but intractable pain (usually headache) or significant emotional disturbance or both are common. Once established and persistent, this is difficult to treat. Referral to a specialty practice is appropriate, but even in that setting effective treatment may be elusive.
PATIENT EDUCATION
Most important for patient education is reassurance. Ultimately, concussion is a self-limited phenomenon, and reinforcing this is helpful for patients. If concussion is not sport-related, most patients recover completely within 3 months.
The next important tenet in patient education is that they should rest for 3 to 5 days, then resume gradual physical and cognitive activities. If resuming activities too soon results in symptoms, then they should rest for a day and gradually resume activity. If their recovery is prolonged (ie, longer than 6 weeks), they likely need to be referred to a concussion specialist.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
- Cassidy JD, Carroll LJ, Peloso PM, et al; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004; (suppl):28–60.
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 2002; 67:281–344.
- Denny-Brown DE, Russell WR. Experimental concussion: (section of neurology). Proc R Soc Med 1941; 34:691–692.
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain 1974; 97:633–654.
- Houlburn AHS, Edin MA. Mechanics of head injuries. Lancet 1943; 242:438–441.
- Gennarelli TA, Adams JH, Graham DI. Acceleration induced head injury in the monkey. I. The model, its mechanical and physiological correlates. Acta Neuropathol Suppl 1981; 7:23–25.
- Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235.
- Meehan WP 3rd, Bachur RG. Sport-related concussion. Pediatrics 2009; 123:114–123.
- Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 2015; 169:1132–1140.
- Stiell IG, Wells GA, Vandemheen K. et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357:1391–1396.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PMC. Indications for computed tomography in patients with minor head injury. N Engl J Med 2000; 343:100–105.
- Kuppermann N, Holmes JF, Dayan PS, et al; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically important brain injuries after head trauma: a prospective cohort study. Lancet 2009; 374:1160–1170.
- McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med 2009; 43(suppl 1):i76–i90.
- DeMatteo C, Stazyk K, Singh SK, et al; Ontario Neurotrauma Foundation. Development of a conservative protocol to return children and youth to activity following concussive injury. Clin Pediatr (Phila) 2015; 54:152–163.
- Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006; 8:415–426.
- DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016; 55:443–451.
- Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223.
- Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20:21–27.
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47:250–258.
- Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241.
- Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP 3rd. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304.
- Packard RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil 1999; 14:9–21.
- Couch JR, Bearss C. Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 2001; 41:559–564.
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34:93–102.
- Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012; 32:600–606.
- DiTommaso C, Hoffman JM, Lucas S, Dikmen S, Temkin N, Bell KR. Medication usage patterns for headache treatment after mild traumatic brain injury. Headache 2014; 54:511–519.
- Lucas S. Characterization and management of headache after mild traumatic brain injury. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Franis Group; 2015:145–154.
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache 2011; 51:932–944.
- Tyler GS, McNeely HE, Dick ML. Treatment of post-traumatic headache with amitriptyline. Headache 1980; 20:213–216.
- Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache 2000; 40:736–739.
- Kacperski J, Arthur T. Management of post-traumatic headaches in children and adolescents. Headache 2016; 56:36–48.
- Lenaerts ME, Couch JR, Couch JR. Posttraumatic headache. Curr Treat Options Neurol 2004; 6:507–517.
- Valovich McLeod TC, Hale TD. Vestibular and balance issues following sport-related concussion. Brain Inj 2015; 29:175–184.
- Master CL, Cheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016; 55:260–267.
- Tan CO, Meehan WP 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise and mild traumatic brain injury. Neurology 2014; 83:1665–1672.
- Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil 2004; 19:378–390.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science 1980; 210:1267–1269.
- Figueiro MG, Wood B, Plitnick B, Rea MS. The impact of light from computer monitors on melatonin levels in college students. Neuro Endocrinol Lett 2011; 32:158–163.
- Rao V, Rollings P. Sleep disturbances following traumatic brain injury. Curr Treat Options Neurol 2002; 4:77–87.
- Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 2009; 47:134–142.
- Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015; 91:46–54.
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, et al. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci 2015; 18:867–872.
- Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327.
- O’Donnell ML, Creamer M, Pattison P, Atkin C. Psychiatric morbidity following injury. Am J Psychiatry 2004; 161:507–514.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004; 85:1457–1464.
- Massey JS, Meares S, Batchelor J, Bryant RA. An exploratory study of the association of acute posttraumatic stress, depression, and pain to cognitive functioning in mild traumatic brain injury. Neuropsychology 2015; 29:530–542.
- Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 2011; 25:454–465.
- Solomon GS, Kuhn AW, Zuckerman SL. Depression as a modifying factor in sport-related concussion: a critical review of the literature. Phys Sportsmed 2016; 44:14–19.
- Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma 2006; 23:1468–1501.
- Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232.
- Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil 2013; 28:260–265.
- Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012; 4:147–154.
KEY POINTS
- Concussion results from a traumatic acceleration of the brain that leads to a metabolic mismatch, with an increased demand for adenosine triphosphate but decreased blood flow to the brain. This “energy crisis” results in variable signs and symptoms, most commonly headache, dizziness, sleep disturbance, cognitive problems, and emotional difficulties.
- Initial therapy involves several days of cognitive and physical rest, followed by a gradual return to physical and cognitive activities.
- There is no direct treatment for the physiology of concussion, but early treatment of symptoms and education about recovery and accommodations aids functional recovery.