User login
Understanding the bell-ringing of concussion
We well recall, back in the day, getting our “bell rung” from some form of sports-related head contact. If we could count the coach’s fingers clearly, run fast and straight, and know the plays, we could happily go back into the game. There was little additional thought given to short-term or lasting effects. I recall hearing tales from my grandfather, a boxing enthusiast, of retired punch-drunk fighters working as bouncers and greeters at sports-focused restaurants and clubs. I certainly didn’t draw any link to a few episodes of personally feeling spacey or dizzy after playing football.
But now, as parents, we are all highly tuned in to the issue of wrongly minimized “minor” head contact and concussion in our children playing sports. There is a growing research-based understanding of the mechanisms of concussion, which remains a clinical syndrome diagnosed on the basis of symptoms and sometimes subtle objective findings that occur in the appropriate environmental context. Intracranial brain impact sets the stage for locally spreading firing of neurons outside their usual pattern. This can result in a diffuse jamming of some normal electrochemical pathways of cognitive function, as well as create additional mismatch between neuronal metabolic needs and the local blood flow providing oxygen and nutrients. This disruption in autoregulation of blood flow sets the stage for enhanced brain sensitivity to any second injurious event, even a minimal one. Hence the aggressive implementation of enforced rest and recovery time for athletes and others with concussion.
It is critical to realize that the patient may not have had a loss of consciousness. Equally important is to consider the need for imaging and protection of patients who are not recovering as expected in 7 to 10 days, as well as for initial imaging of those with severe head impact or baseline neurologic disease, the aged, and those on anticoagulation.
We well recall, back in the day, getting our “bell rung” from some form of sports-related head contact. If we could count the coach’s fingers clearly, run fast and straight, and know the plays, we could happily go back into the game. There was little additional thought given to short-term or lasting effects. I recall hearing tales from my grandfather, a boxing enthusiast, of retired punch-drunk fighters working as bouncers and greeters at sports-focused restaurants and clubs. I certainly didn’t draw any link to a few episodes of personally feeling spacey or dizzy after playing football.
But now, as parents, we are all highly tuned in to the issue of wrongly minimized “minor” head contact and concussion in our children playing sports. There is a growing research-based understanding of the mechanisms of concussion, which remains a clinical syndrome diagnosed on the basis of symptoms and sometimes subtle objective findings that occur in the appropriate environmental context. Intracranial brain impact sets the stage for locally spreading firing of neurons outside their usual pattern. This can result in a diffuse jamming of some normal electrochemical pathways of cognitive function, as well as create additional mismatch between neuronal metabolic needs and the local blood flow providing oxygen and nutrients. This disruption in autoregulation of blood flow sets the stage for enhanced brain sensitivity to any second injurious event, even a minimal one. Hence the aggressive implementation of enforced rest and recovery time for athletes and others with concussion.
It is critical to realize that the patient may not have had a loss of consciousness. Equally important is to consider the need for imaging and protection of patients who are not recovering as expected in 7 to 10 days, as well as for initial imaging of those with severe head impact or baseline neurologic disease, the aged, and those on anticoagulation.
We well recall, back in the day, getting our “bell rung” from some form of sports-related head contact. If we could count the coach’s fingers clearly, run fast and straight, and know the plays, we could happily go back into the game. There was little additional thought given to short-term or lasting effects. I recall hearing tales from my grandfather, a boxing enthusiast, of retired punch-drunk fighters working as bouncers and greeters at sports-focused restaurants and clubs. I certainly didn’t draw any link to a few episodes of personally feeling spacey or dizzy after playing football.
But now, as parents, we are all highly tuned in to the issue of wrongly minimized “minor” head contact and concussion in our children playing sports. There is a growing research-based understanding of the mechanisms of concussion, which remains a clinical syndrome diagnosed on the basis of symptoms and sometimes subtle objective findings that occur in the appropriate environmental context. Intracranial brain impact sets the stage for locally spreading firing of neurons outside their usual pattern. This can result in a diffuse jamming of some normal electrochemical pathways of cognitive function, as well as create additional mismatch between neuronal metabolic needs and the local blood flow providing oxygen and nutrients. This disruption in autoregulation of blood flow sets the stage for enhanced brain sensitivity to any second injurious event, even a minimal one. Hence the aggressive implementation of enforced rest and recovery time for athletes and others with concussion.
It is critical to realize that the patient may not have had a loss of consciousness. Equally important is to consider the need for imaging and protection of patients who are not recovering as expected in 7 to 10 days, as well as for initial imaging of those with severe head impact or baseline neurologic disease, the aged, and those on anticoagulation.
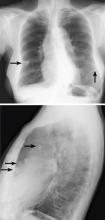
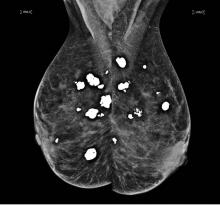
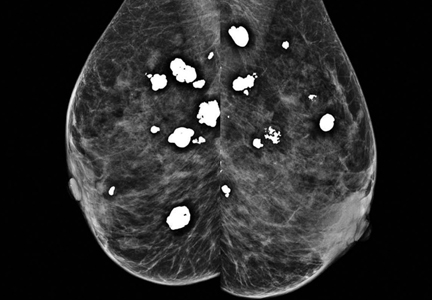
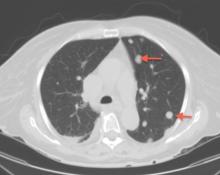
Breast calcifications mimicking pulmonary nodules
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
Necrotizing pancreatitis: Diagnose, treat, consult
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
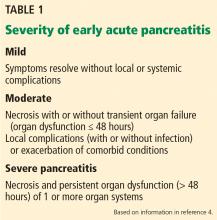
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
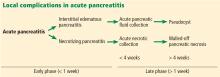
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
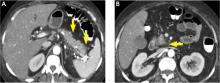
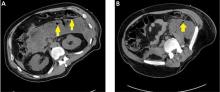
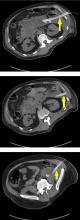
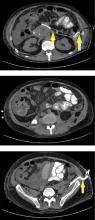
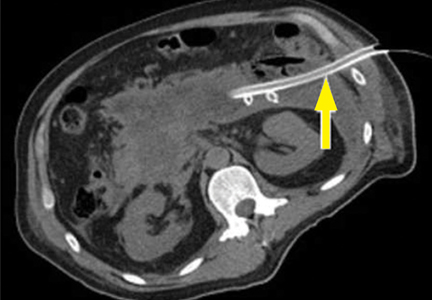
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Selective and appropriate timing of radiologic imaging is vital in managing necrotizing pancreatitis. Protocols are valuable tools.
- While the primary indication for debridement and drainage in necrotizing pancreatitis is infection, other indications are symptomatic walled-off pancreatic necrosis, intractable abdominal pain, bowel obstruction, and failure to thrive.
- Open surgical necrosectomy remains an important treatment for infected pancreatic necrosis or intractable symptoms.
- A “step-up” approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
High Value Care curriculum reduced echocardiogram ordering
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.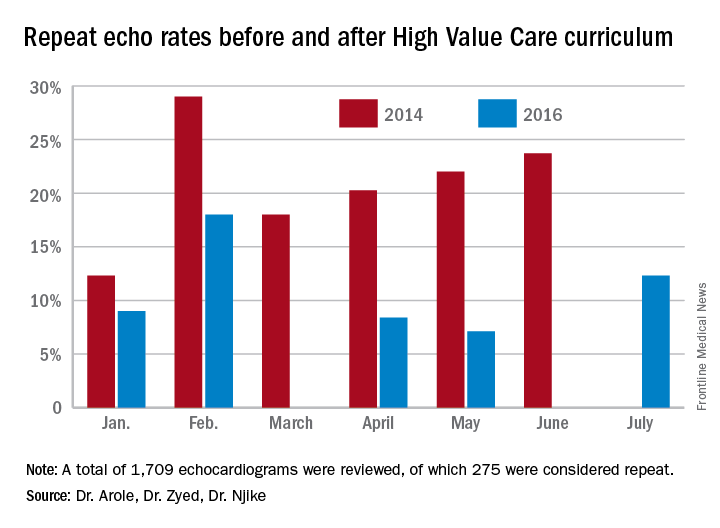
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Emergency Ultrasound: Ultrasound-Guided Arthrocentesis of the Ankle
Ankle effusions can be quite debilitating, causing band-like swelling and stiffness to the anterior aspect of ankle at the tibiotalar joint. Significant swelling can impair ankle dorsiflexion and plantar flexion. The differential diagnosis for joint effusions is wide, and includes traumatic effusion; gout; osteoarthritis; rheumatoid arthritis; and septic arthritis, which is one of the most important diagnoses for the emergency physician (EP) to identify and initiate prompt treatment to reduce the risk of serious morbidity and mortality. Differentiating these conditions requires joint aspiration and synovial fluid analysis. While a large effusion will be palpable and likely ballotable, smaller effusions are more challenging clinically. In such cases, point-of-care (POC) ultrasound can be a valuable tool in confirming a joint effusion.
Identifying Landmarks and Tibiotalar Joint
To access the tibiotalar joint space, it is important to identify useful landmarks.1 This is best accomplished by having the patient in the supine position, with the affected knee flexed approximately 90° and plantar surface of the foot lying flat on the bed (Figure 1).
Performing the Arthrocentesis
The arthrocentesis is performed under sterile conditions using the high-frequency linear probe. A sterile probe cover is highly recommended if the operator will be using ultrasound to guide the procedure in real time.2 Using the palpable landmarks as a guide, the clinician should align the probe just medial to the tibialis anterior tendon with the probe marker oriented cephalad; scanning should begin superior to the ankle joint. The tibia will appear as a hyperechoic stripe just under a thin soft tissue layer. When the tibia is visible, the clinician should then slide the probe distally. The joint space will demonstrated by visualization of the distal tibia and talus bone (Figure 3).
Pearls and Pitfalls
Point-of-care ultrasound is not only useful to guide arthrocentesis of joint effusions, but also to confirm the presence of an effusion prior to aspiration. At our institution, we have had many cases in which POC ultrasound demonstrated an absence of effusion, and we were able to avoid an unnecessary joint aspiration. Moreover, when an effusion is present, POC ultrasound-guided aspiration avoids complications. The use of POC ultrasound can also increase the confidence of the provider performing arthrocentesis of joints less commonly aspirated.
Summary
Joint aspiration is an important procedural tool for EPs, especially when used to rule out life-threatening conditions such as septic arthritis. Deeper joints and small fluid collections, however, can be difficult to access without image guidance. In the ED setting, POC ultrasound provides a widely available, easy-to-use, low-cost tool to increase the likelihood of success while minimizing damage to adjacent structures.
1. Nagdev A. Ultrasound-guided ankle arthrocentesis. Highland General Hospital Emergency Medicine Ultrasound Web site. http://highlandultrasound.com/ankle-arthrocentesis. Accessed June 8, 2017.
2. Reichman EF, Simon RR. Arthrocentesis. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. 2nd ed. McGraw Hill Education: New York, NY; 2013.
Ankle effusions can be quite debilitating, causing band-like swelling and stiffness to the anterior aspect of ankle at the tibiotalar joint. Significant swelling can impair ankle dorsiflexion and plantar flexion. The differential diagnosis for joint effusions is wide, and includes traumatic effusion; gout; osteoarthritis; rheumatoid arthritis; and septic arthritis, which is one of the most important diagnoses for the emergency physician (EP) to identify and initiate prompt treatment to reduce the risk of serious morbidity and mortality. Differentiating these conditions requires joint aspiration and synovial fluid analysis. While a large effusion will be palpable and likely ballotable, smaller effusions are more challenging clinically. In such cases, point-of-care (POC) ultrasound can be a valuable tool in confirming a joint effusion.
Identifying Landmarks and Tibiotalar Joint
To access the tibiotalar joint space, it is important to identify useful landmarks.1 This is best accomplished by having the patient in the supine position, with the affected knee flexed approximately 90° and plantar surface of the foot lying flat on the bed (Figure 1).
Performing the Arthrocentesis
The arthrocentesis is performed under sterile conditions using the high-frequency linear probe. A sterile probe cover is highly recommended if the operator will be using ultrasound to guide the procedure in real time.2 Using the palpable landmarks as a guide, the clinician should align the probe just medial to the tibialis anterior tendon with the probe marker oriented cephalad; scanning should begin superior to the ankle joint. The tibia will appear as a hyperechoic stripe just under a thin soft tissue layer. When the tibia is visible, the clinician should then slide the probe distally. The joint space will demonstrated by visualization of the distal tibia and talus bone (Figure 3).
Pearls and Pitfalls
Point-of-care ultrasound is not only useful to guide arthrocentesis of joint effusions, but also to confirm the presence of an effusion prior to aspiration. At our institution, we have had many cases in which POC ultrasound demonstrated an absence of effusion, and we were able to avoid an unnecessary joint aspiration. Moreover, when an effusion is present, POC ultrasound-guided aspiration avoids complications. The use of POC ultrasound can also increase the confidence of the provider performing arthrocentesis of joints less commonly aspirated.
Summary
Joint aspiration is an important procedural tool for EPs, especially when used to rule out life-threatening conditions such as septic arthritis. Deeper joints and small fluid collections, however, can be difficult to access without image guidance. In the ED setting, POC ultrasound provides a widely available, easy-to-use, low-cost tool to increase the likelihood of success while minimizing damage to adjacent structures.
Ankle effusions can be quite debilitating, causing band-like swelling and stiffness to the anterior aspect of ankle at the tibiotalar joint. Significant swelling can impair ankle dorsiflexion and plantar flexion. The differential diagnosis for joint effusions is wide, and includes traumatic effusion; gout; osteoarthritis; rheumatoid arthritis; and septic arthritis, which is one of the most important diagnoses for the emergency physician (EP) to identify and initiate prompt treatment to reduce the risk of serious morbidity and mortality. Differentiating these conditions requires joint aspiration and synovial fluid analysis. While a large effusion will be palpable and likely ballotable, smaller effusions are more challenging clinically. In such cases, point-of-care (POC) ultrasound can be a valuable tool in confirming a joint effusion.
Identifying Landmarks and Tibiotalar Joint
To access the tibiotalar joint space, it is important to identify useful landmarks.1 This is best accomplished by having the patient in the supine position, with the affected knee flexed approximately 90° and plantar surface of the foot lying flat on the bed (Figure 1).
Performing the Arthrocentesis
The arthrocentesis is performed under sterile conditions using the high-frequency linear probe. A sterile probe cover is highly recommended if the operator will be using ultrasound to guide the procedure in real time.2 Using the palpable landmarks as a guide, the clinician should align the probe just medial to the tibialis anterior tendon with the probe marker oriented cephalad; scanning should begin superior to the ankle joint. The tibia will appear as a hyperechoic stripe just under a thin soft tissue layer. When the tibia is visible, the clinician should then slide the probe distally. The joint space will demonstrated by visualization of the distal tibia and talus bone (Figure 3).
Pearls and Pitfalls
Point-of-care ultrasound is not only useful to guide arthrocentesis of joint effusions, but also to confirm the presence of an effusion prior to aspiration. At our institution, we have had many cases in which POC ultrasound demonstrated an absence of effusion, and we were able to avoid an unnecessary joint aspiration. Moreover, when an effusion is present, POC ultrasound-guided aspiration avoids complications. The use of POC ultrasound can also increase the confidence of the provider performing arthrocentesis of joints less commonly aspirated.
Summary
Joint aspiration is an important procedural tool for EPs, especially when used to rule out life-threatening conditions such as septic arthritis. Deeper joints and small fluid collections, however, can be difficult to access without image guidance. In the ED setting, POC ultrasound provides a widely available, easy-to-use, low-cost tool to increase the likelihood of success while minimizing damage to adjacent structures.
1. Nagdev A. Ultrasound-guided ankle arthrocentesis. Highland General Hospital Emergency Medicine Ultrasound Web site. http://highlandultrasound.com/ankle-arthrocentesis. Accessed June 8, 2017.
2. Reichman EF, Simon RR. Arthrocentesis. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. 2nd ed. McGraw Hill Education: New York, NY; 2013.
1. Nagdev A. Ultrasound-guided ankle arthrocentesis. Highland General Hospital Emergency Medicine Ultrasound Web site. http://highlandultrasound.com/ankle-arthrocentesis. Accessed June 8, 2017.
2. Reichman EF, Simon RR. Arthrocentesis. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. 2nd ed. McGraw Hill Education: New York, NY; 2013.
Foreign Body Insertions: A Review
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
Anorectal and urethral foreign body insertions (polyembolokoilamania) are not infrequent presentations to the ED. The motivations behind these insertions vary, ranging from autoeroticism to reckless behavior. These insertions can lead to major complications and even death. Though ED staff members are used to the unpredictability of human behavior, foreign body insertions bring a mixture of responses from the staff, ranging from awe and incredulousness to anger and frustration. A knowledge and comfort in managing these cases includes a nonjudgmental triage assessment, collective professionalism, and self-awareness of the staff’s reaction.
Case 1
A 58-year-old man presented to the ED for evaluation of a foreign body in his rectum. He admitted to placing a beer bottle in his rectum, but was unable to remove it at home. The staff reported that the patient was previously seen in the ED for removal of a vibrator from his rectum.
Radiographic evaluation in the form of an acute abdominal series was obtained and confirmed a beer bottle in the rectum (Figures 1 and 2).
Case 2
A 55-year-old man presented to the ED after he inserted a pen cap into his urethra to aid in obtaining an erection. A pelvic X-ray was obtained and showed a radiolucent structure in the penis (Figure 3).
The patient was admitted to the hospital and taken to the OR by the consulting urologist. Using a rigid cystoscope and flexible graspers, the pen cap was removed from the proximal urethra under monitored anesthesia control. The procedure went without any complications.
A psychiatrist was consulted, and during the encounter, the patient admitted that his behavior was pathological. He revealed that he was a victim of child abuse and reported he had been having mixed emotions of anxiety, guilt, and embarrassment because of his behavior.
Discussion
Foreign body insertions are seen in patients with a wide variety of backgrounds, ages, and lifestyles. Approximately 80,000 cases of foreign body ingestion are seen annually in children under age 20 years. Young males have a higher predilection of swallowing foreign bodies when compared to young females,1 and rectal foreign body insertions are seen more commonly in males than in females.2 In this age group, intentional foreign body insertion may be an initial manifestation of psychiatric illness.
Rectal Insertions
The earliest published report of a rectal foreign body insertion was in 1919 by Smiley.6 The typical age at presentation ranges from 20 to 90 years old, with a mean age of 44 years old.2 Household objects such as bottles and glasses are the most commonly seen, but a long list of other items have also been reported in the literature, including toothbrushes, knives, deodorant bottles, food articles, sports equipment, cell phones, flashlights, wooden rods, broomsticks, sex toys, light bulbs, construction tools, nails, ornaments, aerosol canisters, cocaine packets, jewelry, batteries, guitar picks, and many other items.1,2,7
In nearly half of the reported cases, the reasons for rectal insertion was for sexual arousal/stimulation.1,7 Other reasons include nonsuicidal injurious behavior (eg, borderline personality disorder); suicide attempt; psychosis; depression; factitious disorder; malingering; cognitive disorders, including dementia and delirium; treatment of constipation and hemorrhoids; concealment; attention-seeking behavior; “accidental”; assault; and the consequences of drunken wagers.1,2 Additionally, abuse should be considered, especially in patients with developmental delay and/or psychiatric illness.
Close to 20% of all traumatic rectal injuries are due to foreign body insertions. In most cases, foreign bodies fail to cause significant anorectal injuries. Complications, however, can result from the process of insertion, removal, or from the contents introduced into the orifice.1 Any rectal examination should be preceded by an anatomical survey utilizing radiographic modalities to evaluate the integrity and orientation of the object in question. Any sharp object can injure the examining physician if this is not done prior. All examinations should be chaperoned.2,7 The most obvious and dangerous complication is perforation, and the patient’s care should proceed in the same manner as any other trauma patient. Additionally, resulting sepsis should be managed with the same standards as any other septic patient.7
Treatment. The method of object removal is determined by the presence or absence of a surgical abdomen and the need for general anesthesia. The location and shape of the object, however, may not equate with successful retrieval. Objects placed in the sigmoid colon are more than twice as likely to require surgical intervention compared to items placed distally.2 Once it is determined that the patient is clinically stable and does not have an acute abdomen, attempts in removing the rectal foreign object can be done in the ED or, if anesthesia is needed, in the OR. Any attempts at transanal removal require optimal patient relaxation, which can be achieved via procedural sedation. The patient should be placed in a lithotomy or left lateral decubitus position to allow palpation of the object in the lower gastrointestinal tract. From here, several methods of removal can be employed. Blunt objects can be grasped and removed by a gloved hand or with a clamp. A Foley catheter can also be passed alongside the object and the balloon inflated above the foreign body to aid in extraction as the Foley is pulled out slowly. Sengstaken-Blakemore tubes, obstetric forceps, and vacuum extractors have also been utilized.7
While bedside extraction is advocated by many authors, Cawich et al8 recently reported that transanal extraction in the ED failed in 89% of cases. Additionally, these researchers reported that in 63% of the failed extractions, the objects were inadvertently pushed higher into the rectosigmoid region, and therefore recommended early mobilization of the OR team so that exploration under anesthesia can be performed under optimal conditions.8
Once the foreign body is successfully removed, follow-up imaging or postextraction endoscopy is warranted. Close observation in the hospital is recommended to facilitate serial abdominal examination.7
Urethral Insertions
Sexual exploration, efforts at contraception, transport of illicit drugs, assault or sexual violence, and accidental insertion have all been described as reasons for genitourinary (GU) insertion.1 The motives, however, mirror those who insert foreign bodies rectally.
Most presentations are due to pain or inability to void. Aggressive treatment should be undertaken because even when the penis appears dark or necrotic, salvage rates have been high. Complications include urinary tract infections, hematuria, urinary retention, urethral tears, abscess, ascending GU infections, and diverticula and fistula formations.1,3 In women, vaginal insertions can lead to pelvic pain and septic shock.1 Foreign bodies can also lead to a condition first described in the ancient literature as strangury—the process of slow and painful discharge of urine due to a significant inflammatory component or stricture. The term strangury has been replaced with the more general term bladder spasms.9
Treatment. Removal of urethral foreign bodies typically is done in conjunction with a urologist. A cystoscopic procedure is usually successful in removing the foreign body and is an effective method to minimize urethral and bladder injuries. However, more invasive surgical options, including perineal urethrotomy, suprapubic cystostomy, cystolithotomy, and external urethrotomy, have been used in more complicated cases or when the foreign body prevents urethral access of an endoscopic instrument.10
Patient and Staff Reactions
When patients realize they are unable to remove the inserted object, some present immediately to the ED for evaluation. Interestingly, others may wait up to 2 weeks after insertion before seeking help.2 Patients report feelings of being ashamed and report a feeling of being despised, frowned upon, and being talked about during the course of their ED evaluation. As a consequence, these patients may not readily come to the ED or if they do come, may not be open to conversation and hide the true reason of why they came in the first place.1,4 The amplified paranoia and perceived prejudice may delay diagnosis and lifesaving measures, or worse, lead patients to leave prior to a medical screening examination. Therefore, creating a nonjudgmental environment is essential, even when the presenting story appears to be fabricated.2
Once the patient’s foreign body is removed, and complications are excluded or properly managed, the goal is to understand the motivation behind the insertion, mitigate the consequences of the behavior, and prevent future recurrence. A psychiatric evaluation should be obtained in the ED, or if the patient is admitted, during hospitalization. Psychiatric behavior leading to insertions can be unmasked, treated, and harm-reduction strategies can be taught and instituted.1,3
Experienced ED staff members are used to the unpredictability of human behavior. However, patients who present with foreign body insertions can elicit a mixture of responses, ranging from awe and incredulousness to anger and frustration. It is not unusual for staff members to not understand or recognize their own reactions. The unique nature of the presentation, along with the astonishing radiographic images, can lead to a breach of privacy and dissemination of the digital photographs by cell phones and into social media sites.1 Staff members should be encouraged to foster open-mindedness and indifference. Ensuring privacy, professionalism, and empathy can go a long way to helping these patients. Moreover, ED staff members should be educated about countertransference reactions,1 as these actions are necessary to ensure the singular purpose of optimum patient-staff relationship.
Conclusion
Patients with foreign body insertions challenge the ED staff, as the presenting complaint not only tests the collective technical know-how of the staff, but also their emotional competencies. A nonjudgmental and open-minded approach is crucial, with the tone set during triage. Coordination with surgical specialties should be done early to ensure safe removal and to identify and manage complications. Psychiatric evaluation should be strongly considered prior to disposition in an attempt to prevent future recurrences.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
1. Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): underpinnings and management strategies. Prim Care Companion CNS Disord. 2012;14(1). doi:10.4088/PCC.11f01192.
2. Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25(4):214-218. doi:10.1055/s-0032-1329392.3. Naidu K, Chung A, Mulcahy M. An unusual urethral foreign body. Int J Surg Case Rep. 2013;4(11):1052-1054. doi:10.1016/j.ijscr.2013.07.017.4. Rahman NU, Elliott SP, McAninch JW. Self-inflicted male urethral foreign body insertion: endoscopic management and complications. BJU Int. 2004;94(7):1051-1053. 10.1111/j.1464-410X.2004.05103.x.
5. SaiSwaroop Y, Darakh P, Amlani D. Self insertion of urethral foreign body in a child: a rare case report. Int J Curr Med Appl Sci. 2015;9(1):22-24.
6. Smiley O. A glass tumbler in the rectum. JAMA. 1919;72:1285.
7. Coskun A, Erkan N, Yakan S, Yıldirim M, Cengiz F. Management of rectal foreign bodies. World J Emerg Surg. 2013;8(1):11. doi:10.1186/1749-7922-8-11.
8. Cawich SO, Thomas DA, Mohammed F, Bobb NJ, Williams D, Naraynsingh V. A management algorithm for retained rectal foreign bodies. Am J Mens Health. 2017;11(3):684-692. doi:10.1177/1557988316680929.
9. Wright B, Husbands E. Strangury: the case of a symptom with ancient origins. BMJ Support Palliat Care. 2011;1(1):49-50. doi:10.1136/bmjspcare-2011-000030.
10. Moon SJ, Kim DH, Chung JH, et al. Unusual foreign bodies in the urinary bladder and urethra due to autoerotism. Int Neurourol J. 2010;14(3):186-189. doi:10.5213/inj.2010.14.3.186.
A large mass in the right ventricle: Tumor or thrombus?
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
Breast cancer screening: Does tomosynthesis augment mammography?
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
KEY POINTS
- DBT creates 3-dimensional images of the breast that the radiologist can view slice by slice, as in other cross-sectional imaging examinations.
- Initial studies suggest that, when used in conjunction with standard 2-dimensional digital mammography as a screening test, DBT can reduce recall rates and increase cancer detection rates, but its impact on breast cancer mortality rates and cancer stage at diagnosis is not known.
- Drawbacks of DBT: it exposes the patient to more radiation, takes the radiologist longer to interpret, and costs more than standard digital mammography alone.
- Not all insurance companies cover DBT for breast cancer screening.
- Dr. Lee has received research grant funding from GE Healthcare. Dr. Lee’s time is supported in part by the American Cancer Society (126947-MRSG-14-160-01-CPHPS).
- The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the University of Washington, Seattle.
Swelling of both arms and chest after push-ups
A healthy 16-year-old boy presented with muscle pain and weakness in the chest and both arms after performing 50 push-ups daily for 3 days, and the symptoms did not seem to improve after 3 days.
EXERCISE-INDUCED RHABDOMYOLYSIS
Approximately 50% of patients with rhabdomyolysis present with the characteristic triad of myalgia (84%), muscle weakness (73%), and dark urine (80%), and 8.1% to 52% present with muscle swelling.1 Rhabdomyolysis may be caused by exercise,2 and risk factors include physical deconditioning, high ambient temperature, high humidity, impaired sweating (due to anticholinergic drugs), sickle cell trait, and hypokalemia from sweating.2 Pain and swelling of the affected focal muscles is the chief complaint.3
Although acute renal failure in exercise-induced rhabdomyolysis is rare, failure to recognize rhabdomyolysis can cause diagnostic delay and inappropriate treatment.4
In healthy people, exercise-induced muscle damage begins to resolve within 1 to 3 days.5,6 Physicians should suspect exercise-induced rhabdomyolysis in patients with prolonged muscle swelling and tenderness in affected muscles that lasts longer than expected.7
- Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 2015; 51:793–810.
- Sayers SP, Clarkson PM. Exercise-induced rhabdomyolysis. Curr Sports Med Rep 2002; 1:59–60.
- Have L, Drouet A. Isolated exercise-induced rhabdomyolysis of brachialis and brachioradialis muscles: an atypical clinical case. Ann Phys Rehabil Med 2011; 54:525–529.
- Keah SH, Chng K. Exercise-induced rhabdomyolysis with acute renal failure after strenuous push-ups. Malays Fam Physician 2009; 4:37–39.
- Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 1996; 28:953–961.
- Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005; 11:64–85.
- Lee G. Exercise-induced rhabdomyolysis. R I Med J (2013) 2014; 97:22–24.
A healthy 16-year-old boy presented with muscle pain and weakness in the chest and both arms after performing 50 push-ups daily for 3 days, and the symptoms did not seem to improve after 3 days.
EXERCISE-INDUCED RHABDOMYOLYSIS
Approximately 50% of patients with rhabdomyolysis present with the characteristic triad of myalgia (84%), muscle weakness (73%), and dark urine (80%), and 8.1% to 52% present with muscle swelling.1 Rhabdomyolysis may be caused by exercise,2 and risk factors include physical deconditioning, high ambient temperature, high humidity, impaired sweating (due to anticholinergic drugs), sickle cell trait, and hypokalemia from sweating.2 Pain and swelling of the affected focal muscles is the chief complaint.3
Although acute renal failure in exercise-induced rhabdomyolysis is rare, failure to recognize rhabdomyolysis can cause diagnostic delay and inappropriate treatment.4
In healthy people, exercise-induced muscle damage begins to resolve within 1 to 3 days.5,6 Physicians should suspect exercise-induced rhabdomyolysis in patients with prolonged muscle swelling and tenderness in affected muscles that lasts longer than expected.7
A healthy 16-year-old boy presented with muscle pain and weakness in the chest and both arms after performing 50 push-ups daily for 3 days, and the symptoms did not seem to improve after 3 days.
EXERCISE-INDUCED RHABDOMYOLYSIS
Approximately 50% of patients with rhabdomyolysis present with the characteristic triad of myalgia (84%), muscle weakness (73%), and dark urine (80%), and 8.1% to 52% present with muscle swelling.1 Rhabdomyolysis may be caused by exercise,2 and risk factors include physical deconditioning, high ambient temperature, high humidity, impaired sweating (due to anticholinergic drugs), sickle cell trait, and hypokalemia from sweating.2 Pain and swelling of the affected focal muscles is the chief complaint.3
Although acute renal failure in exercise-induced rhabdomyolysis is rare, failure to recognize rhabdomyolysis can cause diagnostic delay and inappropriate treatment.4
In healthy people, exercise-induced muscle damage begins to resolve within 1 to 3 days.5,6 Physicians should suspect exercise-induced rhabdomyolysis in patients with prolonged muscle swelling and tenderness in affected muscles that lasts longer than expected.7
- Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 2015; 51:793–810.
- Sayers SP, Clarkson PM. Exercise-induced rhabdomyolysis. Curr Sports Med Rep 2002; 1:59–60.
- Have L, Drouet A. Isolated exercise-induced rhabdomyolysis of brachialis and brachioradialis muscles: an atypical clinical case. Ann Phys Rehabil Med 2011; 54:525–529.
- Keah SH, Chng K. Exercise-induced rhabdomyolysis with acute renal failure after strenuous push-ups. Malays Fam Physician 2009; 4:37–39.
- Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 1996; 28:953–961.
- Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005; 11:64–85.
- Lee G. Exercise-induced rhabdomyolysis. R I Med J (2013) 2014; 97:22–24.
- Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 2015; 51:793–810.
- Sayers SP, Clarkson PM. Exercise-induced rhabdomyolysis. Curr Sports Med Rep 2002; 1:59–60.
- Have L, Drouet A. Isolated exercise-induced rhabdomyolysis of brachialis and brachioradialis muscles: an atypical clinical case. Ann Phys Rehabil Med 2011; 54:525–529.
- Keah SH, Chng K. Exercise-induced rhabdomyolysis with acute renal failure after strenuous push-ups. Malays Fam Physician 2009; 4:37–39.
- Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 1996; 28:953–961.
- Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 2005; 11:64–85.
- Lee G. Exercise-induced rhabdomyolysis. R I Med J (2013) 2014; 97:22–24.
Multimodality Approach to a Stener Lesion: Radiographic, Ultrasound, Magnetic Resonance Imaging, and Surgical Correlation
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Torn, displaced, and entrapped UCL is a Stener lesion.
- Hyperabduction injury with pain and joint laxity on examination.
- MRI and ultrasound are useful in evaluating UCL tears.
- Ultrasound offers dynamic evaluation.
- Must be treated appropriately to avoid pain, instability, and osteoarthritis.
In the literature, hyperabduction injuries to the thumb metacarpophalangeal (MCP) joint have been referred to interchangeably as gamekeeper’s thumb and skier’s thumb. Historically, though, gamekeeper’s thumb was initially described in hunters with chronic injury to the ulnar collateral ligament (UCL),1 and skier’s thumb typically has been described as an acute hyperabduction injury of the UCL.2-5 The proximal portion of a torn UCL may retract with further abduction and displace dorsally, becoming entrapped by the adductor pollicis aponeurosis insertion, known as a Stener lesion.6
The first MCP joint is stabilized by static and dynamic structures that contribute in varying degrees in flexion and extension of the joint. The static stabilizers include the proper and accessory radial and UCLs, the palmar plate, and the dorsal capsule. The UCL originates at the dorsal ulnar aspect of the first metacarpal head at the metacarpal tubercle about 5 mm proximal to the articular surface. The UCL courses distally in the palmar direction to insert volar and proximal to the medial tubercle of the proximal phalanx about 3 mm distal to the articular surface.7 In flexion, the proper collateral ligament is taut and is the primary static stabilizer. In extension, the accessory collateral ligament, which inserts on the palmar plate, is taut and is the primary static stabilizer.8-11
The dynamic stabilizers include the extrinsic muscles (flexor pollicis longus, extensor pollicis longus and brevis) and the intrinsic muscles (abductor pollicis brevis, adductor pollicis, flexor pollicis brevis) inserting on the thumb at the distal phalanx and proximal phalanx and at the base of the first metacarpal.8-10
We report the case of an acute hyperabduction injury of the thumb MCP joint with radiographic, ultrasound, and magnetic resonance imaging (MRI) findings consistent with a Stener lesion and subsequently confirmed with intraoperative photographs. The patient provided written informed consent for print and electronic publication of this case report.
Clinical Findings
A 33-year-old healthy man had persistent left hand pain and grip weakness after performing a handstand. He presented to the orthopedic hand clinic 20 days after injury, having failed nonoperative management (use of nonsteroidal anti-inflammatory drugs and soft thumb spica splint). Physical examination revealed soft-tissue swelling and focal tenderness to palpation at the ulnar aspect of the thumb MCP joint. Despite bilateral first MCP joint laxity on varus and valgus stress without identification of a firm endpoint, pain was elicited only on valgus stress of the left first MCP joint. Given the laxity and the left thumb soft-tissue swelling with pain, plain radiographs, ultrasound, and MRI were used to evaluate for severity of presumed left thumb UCL injury.
Imaging Findings
Plain radiographs showed normal bony anatomy without fracture, normal joint space, and mild soft-tissue swelling at the left thumb MCP level (Figures 3A, 3B).
Surgical Findings
Given laxity with pain at the UCL on stress testing, MRI and ultrasound findings, and continued pain and instability of the thumb with pinching and grasping during activities of daily living, the patient and orthopedic hand surgeon proceeded with surgical intervention. Preoperative examination under anesthesia confirmed significant laxity on valgus stress without a palpable endpoint (Figures 5A, 5B).
Discussion
Hyperabduction injuries to the thumb may rupture the UCL of the MCP joint of the thumb or cause a bony avulsion of the base of the proximal phalanx. Injury to the UCL, most often at its distal portion,4,14,15 may result in a sprain or full-thickness tear of the ligament.
It is vital for the radiologist to identify a Stener lesion because a nondisplaced tear of the UCL is often treated nonsurgically, but UCL tears displaced more than 3 mm and Stener lesions usually must be operated on to avoid chronic instability, pain, and osteoarthritis.2-5,8,12-23 Sensitivity and specificity of MRI in evaluating UCL injuries are reported to be almost 100%, with resolution of 1 mm using current surface coils.23 There are various UCL injury patterns, including partial tears, displaced and nondisplaced complete tears, and even complex injuries, such as an incomplete tear with the torn portion retracted as a Stener lesion.22 MRI is needed to establish the extent of injury, as 90% of complete tears that are displaced at least 3 mm, and all tears with retraction proximal and superficial to the aponeurosis (true Stener lesions), failed immobilization and required surgical treatment.23Although they vary in the literature, mean sensitivity and specificity of ultrasound in detecting UCL tears in level I studies have been reported as 76% and 81%, respectively.24 When Melville and colleagues21 applied their ultrasound criteria—including absence of normal UCL fibers traversing the first MCP joint as well as heterogeneous masslike tissue at least partially proximal to the apex of the metacarpal lateral tubercle—they were able to distinguish displaced full-thickness tears from nondisplaced full-thickness tears with 100% accuracy. Hergan and colleagues25 found that the diagnostic accuracy of MRI was superior to that of ultrasound; while MRI accuracy was perfect, 12% of patients were incorrectly diagnosed with ultrasound, with false-positive or false-negative tendon-edge displacement. In our experience, ultrasound is uniquely useful in its ability to characterize the real-time dynamic interaction of the UCL with the adductor aponeurosis. It has been observed that passive flexion of the first interphalangeal joint moves the adductor aponeurosis in isolation, allowing differentiation from the subjacent UCL.21 Had a partial tear been in the differential diagnosis of our patient’s Stener lesion, such a maneuver under ultrasound visualization would have solved the dilemma. In addition, ultrasound allows for comparison with the contralateral ligament at the time of examination should a diagnostic dilemma arise.
As many have reported both bony avulsion of the base of the proximal phalanx and concomitant injury to the UCL, identification of a bony avulsion does not exclude a ligamentous injury and the possibility of a Stener lesion (Figure 7).16,19
Conclusion
A Stener lesion—retraction of a completely torn UCL becoming entrapped dorsally and proximally to the adductor insertion—can cause pain, instability, and ultimately osteoarthritis if not treated appropriately. The orthopedic surgeon should have a high index of suspicion for a Stener lesion in the appropriate clinical scenario and consider all imaging modalities for diagnosis. Likewise, it is of utmost importance for the radiologist to identify imaging findings of a Stener lesion, as physical examination alone may be limited in its ability to characterize injury severity. Both MRI and ultrasound are useful in evaluating UCL tears, and ultrasound provides the additional benefit of dynamic visualization and comparison with the contralateral side.
Am J Orthop. 2017;46(3):E195-E199. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Anderson D. Skier’s thumb. Aust Family Physician. 2010;39(8):575-577.
3. Heim D. The skier’s thumb. Acta Orthop Belg. 1999;65(4):440-446.
4. Lohman M, Vasenius J, Kivisaari A, Kivisaari L. MR imaging in chronic rupture of the ulnar collateral ligament of the thumb. Acta Radiol. 2001;42(1):10-14.
5. Kundu N, Asfaw S, Polster J, Lohman R. The Stener lesion. Eplasty. 2012;12:ic11.
6. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Br. 1962;44:869-879.
7. Carlson MG, Warner KK, Meyers KN, Hearns KA, Kok PL. Anatomy of the thumb metacarpophalangeal ulnar and radial collateral ligaments. J Hand Surg Am. 2012;37(10):2021-2026.
8. Heyman P. Injuries to the ulnar collateral ligament of the thumb metacarpophalangeal joint. J Am Acad Orthop Surg. 1997;5(4):224-229.
9. Minami A, An KN, Cooney WP 3rd, Linscheid RL, Chao EY. Ligamentous structures of the metacarpophalangeal joint: a quantitative anatomic study. J Orthop Res. 1984;1(4):361-368.
10. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
11. Patel S, Potty A, Taylor EJ, Sorene ED. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: a treatment algorithm. Strategies Trauma Limb Reconstr. 2010;5(1):1-10.
12. O’Callaghan BI, Kohut G, Hoogewoud HM. Gamekeeper thumb: identification of the Stener lesion with US. Radiology. 1994;192(2):477-480.
13. Ebrahim FS, De Maeseneer M, Jager T, Marcelis S, Jamadar DA, Jacobson JA. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007-1020.
14. Haramati N, Hiller N, Dowdle J, et al. MRI of the Stener lesion. Skeletal Radiol. 1995;24(7):515-518.
15. Shinohara T, Horii E, Majima M, et al. Sonographic diagnosis of acute injuries of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Clin Ultrasound. 2007;35(2):73-77.
16. Giele H, Martin J. The two-level ulnar collateral ligament injury of the metacarpophalangeal joint of the thumb. J Hand Surg Br. 2003;28(1):92-93.
17. Kaplan SJ. The Stener lesion revisited: a case report. J Hand Surg Am. 1998;23(5):833-836.
18. Thirkannad S, Wolff TW. The “two fleck sign” for an occult Stener lesion. J Hand Surg Eur Vol. 2008;33(2):208-211.
19. Badawi RA, Hussain S, Compson JP. Two in one: a variant of the Stener lesion. Injury. 2002;33(4):379-380.
20. McKeon KE, Gelberman RH, Calfee RP. Ulnar collateral ligament injuries of the thumb: phalangeal translation during valgus stress in human cadavera. J Bone Joint Surg Am. 2013;95(10):881-887.
21. Melville D, Jacobson JA, Haase S, Brandon C, Brigido MK, Fessell D. Ultrasound of displaced ulnar collateral ligament tears of the thumb: the Stener lesion revisited. Skeletal Radiol. 2013;42(5):667-673.
22. Romano WM, Garvin G, Bhayana D, Chaudhary O. The spectrum of ulnar collateral ligament injuries as viewed on magnetic resonance imaging of the metacarpophalangeal joint of the thumb. Can Assoc Radiol J. 2003;54(4):243-248.
23. Milner CS, Manon-Matos Y, Thirkannad SM. Gamekeeper’s thumb—a treatment-oriented magnetic resonance imaging classification. J Hand Surg Am. 2015;40(1):90-95.
24. Papandrea RF, Fowler T. Injury at the thumb UCL: is there a Stener lesion? J Hand Surg Am. 2008;33(10):1882-1884.
25. Hergan K, Mittler C, Oser W. Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US and MR imaging. Radiology. 1995;194(1):65-71.