User login
FDA: Gadolinium retention prompts new GBCA class warning, safety measures
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
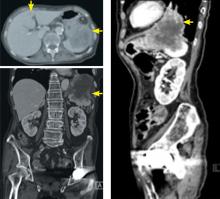
Emergency Imaging: Atraumatic Leg Pain
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
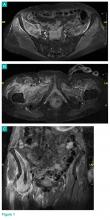
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
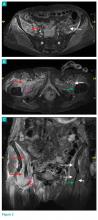
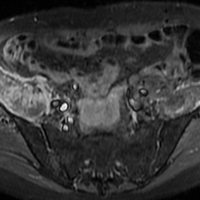
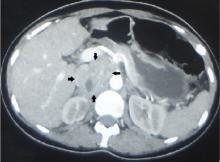
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
Case
A 96-year-old woman with a medical history of sciatica, vertigo, osteoporosis, and dementia presented with atraumatic right leg pain. She stated that the pain, which began 4 weeks prior to presentation, started in her right groin. The patient’s primary care physician diagnosed her with tendonitis, and prescribed acetaminophen/codeine and naproxen sodium for the pain. However, the patient’s pain progressively worsened to the point where she was no longer able to ambulate or bear weight on her right hip, prompting this visit to the ED.
On physical examination, the patient’s right hip was tender to palpation without any signs of physical deformity of the lower extremity. Upon hip flexion, she grimaced and communicated her pain.
Radiographs and computed tomography images taken of the right hip, femur, and pelvis demonstrated low-bone mineral density without fracture.
What is the diagnosis?
Answer
Axial and coronal edema-sensitive images of the pelvis demonstrated edema (increased signal) within the right psoas, iliacus, and iliopsoas muscles (red arrows, Figures 2a-2c), which were in contrast to the normal pelvic muscles on the left side (white arrows, Figures 2a-2c).
Iliopsoas Musculotendinous Unit
The iliopsoas musculotendinous unit consists of the psoas major, the psoas minor, and the iliacus, with the psoas minor absent in 40% to 50% of cases.1,2 The iliacus muscle arises from the iliac wing and inserts with the psoas tendon onto the lesser trochanter of the femur. These muscles function as primary flexors of the thigh and trunk, as well as lateral flexors of the lower vertebral column.2
Signs and Symptoms
In non-sports-related injuries, iliopsoas tendon tears typically occur in elderly female patients—even in the absence of any trauma or known predisposing factors. Patients with iliopsoas tears typically present with hip or groin pain, and weakness with hip flexion, which clinically may mimic hip or sacral fracture. An anterior thigh mass or ecchymosis may also be present. Complete tear of the iliopsoas tendon usually occurs at or near the distal insertion at the lesser trochanter, and is often associated with proximal retraction of the tendon to the level of the femoral head.1
Imaging Studies
Iliopsoas tendon injury is best evaluated with MRI, particularly with fluid-sensitive sequences. Patients with iliopsoas tendon tears have abnormal signal in the muscle belly, likely related to edema and hemorrhage, and hematoma or fluid around the torn tendon and at the site of retraction. In pediatric patients, iliopsoas injury is typically an avulsion of the lesser trochanter prior to fusion of the apophysis.3,4 In adult patients with avulsion of the lesser trochanter, this injury is regarded as a sign of metastatic disease until proven otherwise.5
Treatment
Patients with iliopsoas tendon rupture are treated conservatively with rest, ice, and physical therapy (PT). Preservation of the distal muscular insertion of the lateral portion of the iliacus muscle is thought to play a role in positive clinical outcomes.3
The patient in this case was admitted to the hospital and treated for pain with standing acetaminophen, tramadol as needed, and a lidocaine patch. After attending multiple inpatient PT sessions, she was discharged to a subacute rehabilitation facility.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
1. Bergman G. MRI Web clinic – October 2015: Iliopsoas tendinopathy. Radsource. http://radsource.us/iliopsoas-tendinopathy/. Accessed November 22, 2017.
2. Van Dyke JA, Holley HC, Anderson SD. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53-84. doi:10.1148/radiographics.7.1.3448631.
3. Lecouvet FE, Demondion X, Leemrijse T, Vande Berg BC, Devogelaer JP, Malghem J. Spontaneous rupture of the distal iliopsoas tendon: clinical and imaging findings, with anatomic correlations. Eur Radiol. 2005;15(11):2341-2346. doi:10.1007/s00330-005-2811-0.
4. Bui KL, Ilaslan H, Recht M, Sundaram M. Iliopsoas injury: an MRI study of patterns and prevalence correlated with clinical findings. Skeletal Radiol. 2008;37(3):245-249. doi:10.1007/s00256-007-0414-3.
5. James SL, Davies AM. Atraumatic avulsion of the lesser trochanter as an indicator of tumour infiltration. Eur Radiol. 2006;16(2):512-514.
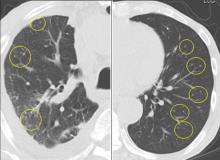
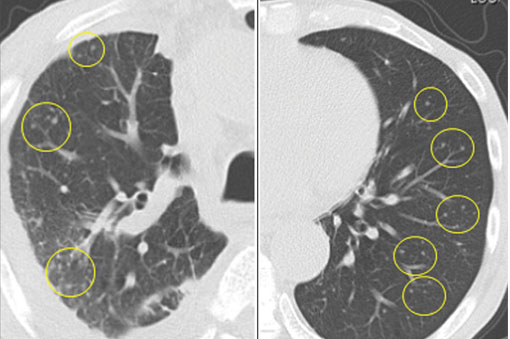
Drug reaction or metastatic lung cancer?
A 76-year-old man with ulcerative colitis presented with a 1-week history of low-grade fever and progressive dyspnea. He was taking infliximab for the ulcerative colitis. He was known to be negative for human immunodeficiency virus.
Since the M tuberculosis cultured from his lung proved to be sensitive to the antituberculosis drugs, we suspected that the nodules were a paradoxical reaction to the drug therapy, and thus we continued the treatment because of the continued low-grade fever. After 9 months of therapy, the fever had resolved and the nodules had disappeared, confirming our suspicion of a paradoxical reaction. The number of lymphocytes gradually increased during drug therapy.
Paradoxical reaction during tuberculosis treatment is defined as a worsening of pre-existing lesions or as the emergence of new lesions during appropriate therapy.1,2 The diagnosis is sometimes difficult, since new lesions can resemble other lung diseases. However, a paradoxical reaction involving randomly distributed nodules is rare and radiographically resembles metastatic lung cancer. Clinicians should be aware of this type of reaction in patients on tuberculosis therapy.
- Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2007; 11:1290–1295.
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998; 158:157–161.
A 76-year-old man with ulcerative colitis presented with a 1-week history of low-grade fever and progressive dyspnea. He was taking infliximab for the ulcerative colitis. He was known to be negative for human immunodeficiency virus.
Since the M tuberculosis cultured from his lung proved to be sensitive to the antituberculosis drugs, we suspected that the nodules were a paradoxical reaction to the drug therapy, and thus we continued the treatment because of the continued low-grade fever. After 9 months of therapy, the fever had resolved and the nodules had disappeared, confirming our suspicion of a paradoxical reaction. The number of lymphocytes gradually increased during drug therapy.
Paradoxical reaction during tuberculosis treatment is defined as a worsening of pre-existing lesions or as the emergence of new lesions during appropriate therapy.1,2 The diagnosis is sometimes difficult, since new lesions can resemble other lung diseases. However, a paradoxical reaction involving randomly distributed nodules is rare and radiographically resembles metastatic lung cancer. Clinicians should be aware of this type of reaction in patients on tuberculosis therapy.
A 76-year-old man with ulcerative colitis presented with a 1-week history of low-grade fever and progressive dyspnea. He was taking infliximab for the ulcerative colitis. He was known to be negative for human immunodeficiency virus.
Since the M tuberculosis cultured from his lung proved to be sensitive to the antituberculosis drugs, we suspected that the nodules were a paradoxical reaction to the drug therapy, and thus we continued the treatment because of the continued low-grade fever. After 9 months of therapy, the fever had resolved and the nodules had disappeared, confirming our suspicion of a paradoxical reaction. The number of lymphocytes gradually increased during drug therapy.
Paradoxical reaction during tuberculosis treatment is defined as a worsening of pre-existing lesions or as the emergence of new lesions during appropriate therapy.1,2 The diagnosis is sometimes difficult, since new lesions can resemble other lung diseases. However, a paradoxical reaction involving randomly distributed nodules is rare and radiographically resembles metastatic lung cancer. Clinicians should be aware of this type of reaction in patients on tuberculosis therapy.
- Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2007; 11:1290–1295.
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998; 158:157–161.
- Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2007; 11:1290–1295.
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998; 158:157–161.
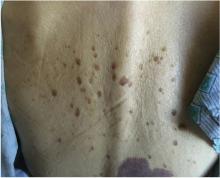
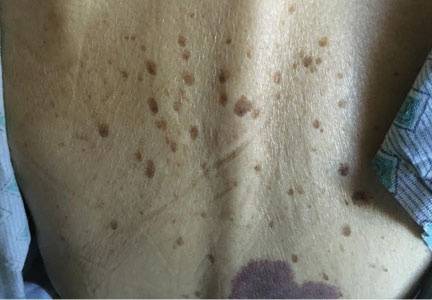
The Leser-Trélat sign
An 85-year-old woman presented with night sweats, dry cough, and an unintended 30-pound weight loss over the preceding 6 months. She also reported the sudden onset of “itchy moles” on her back.
KERATOSES AND MALIGNANCY
The Leser-Trélat sign is the sudden development of multiple pruritic seborrheic keratoses, often associated with malignancy.1–4 Roughly half of these associated malignancies are adenocarcinomas, most commonly of the stomach, breast, colon, or rectum. However, it can be seen in other malignancies, including lymphoma, leukemia, and squamous cell carcinoma, as in this case.
Eruption of seborrheic keratoses has also been observed with benign neoplasms, pregnancy, human immunodeficiency virus infections, and the use of adalimumab, which indicates that the Leser-Trélat sign is not very specific. Despite these concerns, the eruption of multiple seborrheic keratoses should continue to trigger the thought of an internal malignancy in the differential diagnosis.
- Ehst BD, Minzer-Conzetti K, Swerdlin A, Devere TS. Cutaneous manifestations of internal malignancy. Curr Probl Surg 2010; 47:384–445.
- Schwartz RA. Sign of Leser-Trélat. J Am Acad Dermatol 1996; 35:88–95.
- Ellis DL, Yates RA. Sign of Leser-Trélat. Clin Dermatol 1993; 11:141–148.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009; 59:73–98.
An 85-year-old woman presented with night sweats, dry cough, and an unintended 30-pound weight loss over the preceding 6 months. She also reported the sudden onset of “itchy moles” on her back.
KERATOSES AND MALIGNANCY
The Leser-Trélat sign is the sudden development of multiple pruritic seborrheic keratoses, often associated with malignancy.1–4 Roughly half of these associated malignancies are adenocarcinomas, most commonly of the stomach, breast, colon, or rectum. However, it can be seen in other malignancies, including lymphoma, leukemia, and squamous cell carcinoma, as in this case.
Eruption of seborrheic keratoses has also been observed with benign neoplasms, pregnancy, human immunodeficiency virus infections, and the use of adalimumab, which indicates that the Leser-Trélat sign is not very specific. Despite these concerns, the eruption of multiple seborrheic keratoses should continue to trigger the thought of an internal malignancy in the differential diagnosis.
An 85-year-old woman presented with night sweats, dry cough, and an unintended 30-pound weight loss over the preceding 6 months. She also reported the sudden onset of “itchy moles” on her back.
KERATOSES AND MALIGNANCY
The Leser-Trélat sign is the sudden development of multiple pruritic seborrheic keratoses, often associated with malignancy.1–4 Roughly half of these associated malignancies are adenocarcinomas, most commonly of the stomach, breast, colon, or rectum. However, it can be seen in other malignancies, including lymphoma, leukemia, and squamous cell carcinoma, as in this case.
Eruption of seborrheic keratoses has also been observed with benign neoplasms, pregnancy, human immunodeficiency virus infections, and the use of adalimumab, which indicates that the Leser-Trélat sign is not very specific. Despite these concerns, the eruption of multiple seborrheic keratoses should continue to trigger the thought of an internal malignancy in the differential diagnosis.
- Ehst BD, Minzer-Conzetti K, Swerdlin A, Devere TS. Cutaneous manifestations of internal malignancy. Curr Probl Surg 2010; 47:384–445.
- Schwartz RA. Sign of Leser-Trélat. J Am Acad Dermatol 1996; 35:88–95.
- Ellis DL, Yates RA. Sign of Leser-Trélat. Clin Dermatol 1993; 11:141–148.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009; 59:73–98.
- Ehst BD, Minzer-Conzetti K, Swerdlin A, Devere TS. Cutaneous manifestations of internal malignancy. Curr Probl Surg 2010; 47:384–445.
- Schwartz RA. Sign of Leser-Trélat. J Am Acad Dermatol 1996; 35:88–95.
- Ellis DL, Yates RA. Sign of Leser-Trélat. Clin Dermatol 1993; 11:141–148.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009; 59:73–98.
Bicarb, acetylcysteine during angiography don’t protect kidneys
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Periprocedural administration of intravenous sodium bicarbonate did not improve outcomes compared with standard sodium chloride in patients with impaired kidney function undergoing angiography, according to results of a randomized study of 5,177 patients.
In addition, there was no benefit for oral acetylcysteine administration over placebo for mitigating those same postangiography risks, Steven D. Weisbord, MD, said at the American Heart Association scientific sessions in Anaheim, Calif.
Hypothetically, both sodium bicarbonate and acetylcysteine could help prevent acute kidney injury associated with contrast material used during angiography, said Dr. Weisbord of the University of Pittsburgh.
However, multiple studies of the two agents have yielded “inconsistent results … consequently, equipoise exists regarding these interventions, despite their widespread use in clinical practice,” Dr. Weisbord said.
To provide more definitive evidence, Dr. Weisbord and his colleagues conducted PRESERVE, a multicenter, randomized, controlled trial comprising 5,177 patients scheduled for angiography who were at high risk of renal complications. Using a 2-by-2 factorial design, patients were randomized to receive intravenous 1.26% sodium bicarbonate or intravenous 0.9% sodium chloride, and to 5 days of oral acetylcysteine or oral placebo.
They found no significant differences between arms in the study’s composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine by 50% or more.
That composite endpoint occurred in 4.4% of patients receiving sodium bicarbonate, and similarly in 4.7% of patients receiving sodium chloride.
Likewise, the endpoint occurred in 4.6% of patients in the acetylcysteine group and 4.5% of the placebo group, Dr. Weisbord reported.
The investigators had planned to enroll 7,680 patients, but the sponsor of the trial stopped the study after enrollment of 5,177 based on the results showing no significant benefit of either treatment, he noted.
There are a few reasons why results of PRESERVE might show a lack of benefit for these agents, in contrast to some previous studies suggesting both the treatments might reduce risk of contrast-associated renal complications in high-risk patients.
Notably, “most of these interventions have been underpowered,” Dr. Weisbord noted.
Also, most previous trials used a primary endpoint of increase in blood creatinine level within days of the angiography. By contrast, the primary endpoint of the current study was a composite of serious adverse events “that are recognized sequelae of acute kidney injury,” he added.
Although subsequent investigations could shed new light on the controversy, the findings of PRESERVE support the “strong likelihood that these interventions are not clinically effective” in preventing acute kidney injury or longer-term adverse outcomes after angiography, he concluded.
The PRESERVE results were published simultaneously with Dr. Weisbord’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1710933).
The study was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The composite primary endpoint of death, need for dialysis, or persistent increase in serum creatinine was similar regardless of which treatments the patients received.
Data source: PRESERVE, a randomized study using a 2-by-2 factorial design to evaluate intravenous sodium bicarbonate versus sodium chloride and acetylcysteine versus placebo in 5,177 patients at high risk of renal complications.
Disclosures: PRESERVE was supported by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Dr. Weisbord reported receiving personal fees from Durect outside the submitted work.
Emergency Ultrasound: Pericardial Effusion and Tamponade: Making the Diagnosis at Bedside With Point-of-Care Echocardiography
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
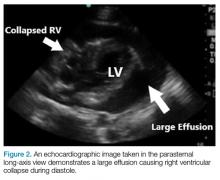
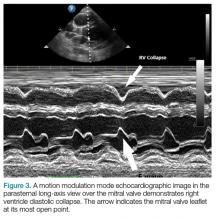
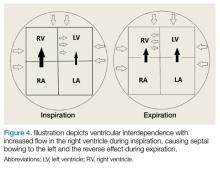
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
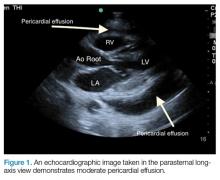
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
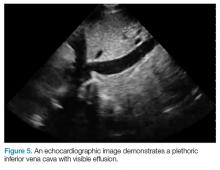
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
Background
Pericardial effusions are usually an incidental finding on bedside echocardiogram—unless a patient shows clinical signs of tamponade physiology and obstructive shock. Echocardiogram is both sensitive and specific to detecting pericardial effusions.1 Although there are many causes of pericardial effusions, the most common causes of symptomatic effusions in the Western World are due to neoplasm, pericarditis, traumatic pathology, or idiopathic etiology. In developing countries, however, pericardial effusions are predominantly due to tuberculosis in an area where it is endemic.2,3 The size of the effusion is classified based on measurements of a fluid pocket during diastole. Mild effusion is defined as less than 10 mm; moderate effusion, 10 to 20 mm; and large effusion, greater than 20 mm.3
It is important to note that the pericardial space contains up to 50 mL of physiological fluid which may be seen on echocardiography during systole. Small effusions usually contain less than 100 mL of fluid; moderate effusions contain 100 to 500 mL of fluid; and large effusions, over 500 mL of fluid. Fluid of smaller effusions typically layer posteriorly, while the fluid of some moderate and most large effusions may be seen circumferentially.
Clinical Signs and Symptoms
Patients with large chronic effusions are often asymptomatic, and clinical symptoms usually correlate to the acuity of pericardial accumulation. Patients with symptomatic effusions present with dyspnea on exertion that is followed by orthopnea, chest pain, and sometimes dysphagia, hoarseness, or hiccups due to irritation of surrounding structures until they exhibit tamponade physiology leading to hypotension secondary to obstructive shock. The most recognized signs of tamponade physiology on bedside echocardiography are early diastolic collapse of the right atrium and right ventricle, as well as ventricular interdependence.4
Imaging Technique
Pericardial effusion and cardiac tamponade can be detected in any of the standard echocardiographic views, with fluid usually appearing as an anechoic stripe. The fluid will first appear in the dependent portion of the pericardial space, but may become circumferential as it grows (Figure 1).
After evaluating for signs of tamponade on examination, the next step is to evaluate the inferior vena cava (IVC). A simple way to identify the IVC on echocardiography is to visualize the heart in the subxiphoid view, then rotate the probe so that the marker is pointing toward the patient’s head. As demonstrated in Figure 5, the IVC should be seen entering the right atrium; if the patient is truly in obstructive shock, the IVC should be plethoric with minimal respiratory variation.
Pericardiocentesis
In the event of obstructive shock or pulseless electric activity with visualized or suspected tamponade, pericardiocentesis is considered standard of care. There are many approaches to performing a pericardiocentesis, including the classically taught blind subxiphoid approach, which is associated with high rates of morbidity and mortality.5 More recent image-guided approaches employ echocardiography-guided techniques that identify the location and distribution of fluid, and perform pericardiocentesis closest to the area largest fluid accumulation.
Most of these guided techniques involve in-plane visualization of the needle in either a subxiphoid, apical, or parasternal approach. Studies have shown that the subxiphoid approach has a higher risk of injury to the liver, heart, and IVC, with complication rates up to 20% depending on the study.6
The apical approach involves locating the cardiac apex and inserting the needle 1 cm lateral to the apex, with the point directed toward the effusion and in-line with the ultrasound probe, taking care to avoid the lingula. Studies have shown that complication rates with this approach are around 3%.7
Recent studies also suggest that in-line medial-to-lateral parasternal approaches may have minimal complications. However, when employing this approach, care must be taken to avoid the internal mammary artery, which can be identified using color-flow Doppler echocardiology.6
Conclusion
In general, bedside ultrasound is a quick and useful tool to evaluate for pericardial effusion and signs of tamponade physiology. When present, tamponade, a clinical diagnosis, is the likely cause of shock in the hemodynamically unstable patient with circumferential pericardial effusion.
While most cases of pericardial effusion are found incidentally, a stepwise approach to evaluate for tamponade is to quickly look for signs of early right-sided diastolic collapse or ventricular interdependence, as well as a plethoric IVC. For patients who have tamponade requiring pericardiocentesis, the ultrasound-guided apical or parasternal approaches have been shown to have fewer complications compared to the subxiphoid approach.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
1. Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34(16):1186-1197. doi:10.1093/eurheartj/ehs372.
2. Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine. 2006;85(1):49-53. doi:10.1097/01.md.0000199556.69588.8e
3. Adler Y, Charron P, Imazio M, et al; European Society of Cardiology (ESC). 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318.
4. Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671-673. doi:10.1016/j.resuscitation.2011.02.004.
5. Kumar R, Sinha A, Lin MJ. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206-212. doi:10.4103/2229-5151.165007.
6. Osman A, Wan Chuan T, Ab Rahman J, Via G, Tavazzi G. Ultrasound-guided pericardiocentesis: a novel parasternal approach. Eur J Emerg Med. 2017;5. doi:10.1097/MEJ.0000000000000471.
7. Ozer HO, Davutoğlu V, Cakici M. Echocardiography-guided pericardiocentesis with the apical approach. Turk Kardiyol Dern Ars. 2009;37(3):177-181.
Developing machines that detect disease
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Rib fracture diagnosis in the panscan era
Clinical question: Do rib fractures observed on chest CT carry the same morbidity and mortality risk as those observed in chest radiograph?
Background: Traditionally studies have shown that first and second rib fractures on chest radiograph after blunt trauma are associated with substantial morbidity and mortality. With growing frequency of CT imaging in the “panscan” era, it is unknown whether similar rib fractures found on CT carry the same meaning.
Setting: 10 level I trauma centers.
Synopsis: Data from the National Emergency X-Radiography Utilization Study showed that, of the 8,661 patients who suffered blunt trauma and received both chest radiograph and chest CT, 23.9% had rib fractures. Rib fractures were observed in 66.1% of chest CT–only cases. Patients with rib fractures had a higher admission rate (88.7% versus 45.8%) and higher mortality (5.6% versus 2.7%) than patients without rib fractures. Mortality rate and great-vessel injury were higher in those with first or second rib fractures. The mortality of patients with rib fractures observed only on chest CT was not statistically different from those whose fractures were also seen in chest radiograph. The study included patients who were more severely injured and may have been more likely to receive a CT, which may have led to an overestimation of fractures found. The actual causes of admission and death were not reviewed.
Bottom line: CT in trauma-imaging protocol can identify patients with rib fractures well, compared with combined CT with chest radiograph. Rib fractures are associated with higher rates of admission and mortality risk than those without rib fractures. Specifically, first or second rib fractures are found to have greater risk for mortality and great-vessel injury.
Citation: Murphy CE 4th, Raja AS, Baumann BM, et al. Rib fracture diagnosis in the panscan era. Ann Emerg Med. 2017. doi: 10.1016/j.annemergmed.2017.04.011.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: Do rib fractures observed on chest CT carry the same morbidity and mortality risk as those observed in chest radiograph?
Background: Traditionally studies have shown that first and second rib fractures on chest radiograph after blunt trauma are associated with substantial morbidity and mortality. With growing frequency of CT imaging in the “panscan” era, it is unknown whether similar rib fractures found on CT carry the same meaning.
Setting: 10 level I trauma centers.
Synopsis: Data from the National Emergency X-Radiography Utilization Study showed that, of the 8,661 patients who suffered blunt trauma and received both chest radiograph and chest CT, 23.9% had rib fractures. Rib fractures were observed in 66.1% of chest CT–only cases. Patients with rib fractures had a higher admission rate (88.7% versus 45.8%) and higher mortality (5.6% versus 2.7%) than patients without rib fractures. Mortality rate and great-vessel injury were higher in those with first or second rib fractures. The mortality of patients with rib fractures observed only on chest CT was not statistically different from those whose fractures were also seen in chest radiograph. The study included patients who were more severely injured and may have been more likely to receive a CT, which may have led to an overestimation of fractures found. The actual causes of admission and death were not reviewed.
Bottom line: CT in trauma-imaging protocol can identify patients with rib fractures well, compared with combined CT with chest radiograph. Rib fractures are associated with higher rates of admission and mortality risk than those without rib fractures. Specifically, first or second rib fractures are found to have greater risk for mortality and great-vessel injury.
Citation: Murphy CE 4th, Raja AS, Baumann BM, et al. Rib fracture diagnosis in the panscan era. Ann Emerg Med. 2017. doi: 10.1016/j.annemergmed.2017.04.011.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: Do rib fractures observed on chest CT carry the same morbidity and mortality risk as those observed in chest radiograph?
Background: Traditionally studies have shown that first and second rib fractures on chest radiograph after blunt trauma are associated with substantial morbidity and mortality. With growing frequency of CT imaging in the “panscan” era, it is unknown whether similar rib fractures found on CT carry the same meaning.
Setting: 10 level I trauma centers.
Synopsis: Data from the National Emergency X-Radiography Utilization Study showed that, of the 8,661 patients who suffered blunt trauma and received both chest radiograph and chest CT, 23.9% had rib fractures. Rib fractures were observed in 66.1% of chest CT–only cases. Patients with rib fractures had a higher admission rate (88.7% versus 45.8%) and higher mortality (5.6% versus 2.7%) than patients without rib fractures. Mortality rate and great-vessel injury were higher in those with first or second rib fractures. The mortality of patients with rib fractures observed only on chest CT was not statistically different from those whose fractures were also seen in chest radiograph. The study included patients who were more severely injured and may have been more likely to receive a CT, which may have led to an overestimation of fractures found. The actual causes of admission and death were not reviewed.
Bottom line: CT in trauma-imaging protocol can identify patients with rib fractures well, compared with combined CT with chest radiograph. Rib fractures are associated with higher rates of admission and mortality risk than those without rib fractures. Specifically, first or second rib fractures are found to have greater risk for mortality and great-vessel injury.
Citation: Murphy CE 4th, Raja AS, Baumann BM, et al. Rib fracture diagnosis in the panscan era. Ann Emerg Med. 2017. doi: 10.1016/j.annemergmed.2017.04.011.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Scapular rash and endocrine neoplasia
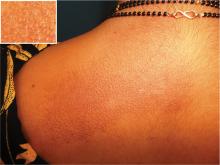
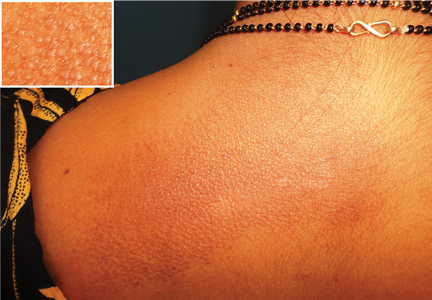
A woman in her 30s presented with an itchy skin-colored rash over her left scapular region that had first appeared 8 years earlier. It had started as itchy skin-colored papules that coalesced to a patch and later became hyperpigmented because of repeated scratching.
She had undergone total thyroidectomy for medullary thyroid carcinoma 1 year ago, and the rash had been diagnosed at that time as lichen planus. She was referred to us by her physician for histopathologic confirmation of the lesions. She denied any history of episodic headache or palpitation.
Her urine normetanephrine excretion was elevated at 1,425 μg/day (reference range 148–560), and her metanephrine excretion was also high at 2,024 μg/day (reference range 44–261).
At a 3-month follow-up visit, the woman’s skin lesions had improved with twice-a-day application of mometasone 0.1% cream; she was lost to follow-up after that visit.
MULTIPLE ENDOCRINE NEOPLASIA
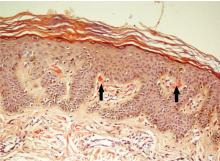
Our patient’s scapular lesions and first-degree family history of MEN type 2A confirmed the diagnosis of the newly recognized variant, MEN type 2A-related cutaneous lichen amyloidosis, in which the characteristic pigmented scapular rash typically predates the first diagnosis of neoplasia.1 The dermal amyloidosis is caused by deposition of keratinlike peptides rather than calcitoninlike peptides.2
A recent systematic review on MEN type 2A with cutaneous lichen amyloidosis showed a female preponderance and a high penetrance of cutaneous lichen amyloidosis, which was the second most frequent manifestation of the syndrome, preceded only by medullary thyroid carcinoma.1
As in our patient’s case, scapular rash and a history of medullary thyroid carcinoma should prompt an investigation for MEN type 2A. These patients should be closely followed for underlying MEN type 2A-related neoplasms.
The mucosal neuromas and skin lipomas seen in MEN type 1 and MEN type 2B are absent in MEN type 2A.3 Cutaneous lichen amyloidosis is the only dermatologic marker for MEN type 2A. Owing to a similar genetic background, cutaneous lichen amyloidosis is also associated with familial medullary thyroid carcinoma, another rare variant of MEN type 2A.4
DIFFERENTIAL DIAGNOSIS
Notalgia paresthetica is a unilateral chronic neuropathic pruritus on the back, mostly located between the shoulders and corresponding to the second and the sixth thoracic nerves. It is mostly attributed to compression of spinal nerves by an abnormality of the thoracic spine.5 In our patient, this was ruled out by the radiologic evaluation.
Before MEN type 2A with cutaneous lichen amyloidosis was recognized as a variant of MEN type 2A, lesions suggestive of notalgia paresthetica were reported with MEN type 2A.3 The classic infrascapular location, history of painful neck muscle spasms, touch hyperesthesia of the lesions, and absence of amyloid deposits on histopathologic study help to differentiate notalgia paresthetica from cutaneous lichen amyloidosis. However, later phases of notalgia paresthetica may show amyloid deposits on histopathologic study, while detection of a scant amount of amyloid is difficult in the early stages of cutaneous lichen amyloidosis.
TAKE-HOME POINT
Cutaneous lichen amyloidosis is usually seen on the extensor surfaces of the extremities. It is considered benign, caused by filamentous degeneration of keratinocytes from repeated scratching. But cutaneous lichen amyloidosis at an early age in the scapular area of women warrants a detailed family history for endocrine neoplasia, blood pressure monitoring, thyroid palpation, and blood testing for serum calcium, calcitonin, and parathyroid hormone.
- Scapineli JO, Ceolin L, Puñales MK, Dora JM, Maia AL. MEN 2A-related cutaneous lichen amyloidosis: report of three kindred and systematic literature review of clinical, biochemical and molecular characteristics. Fam Cancer 2016; 15:625–633.
- Donovan DT, Levy ML, Furst EJ, et al. Familial cutaneous lichen amyloidosis in association with multiple endocrine neoplasia type 2A: a new variant. Henry Ford Hosp Med J 1989; 37:147–150.
- Cox NH, Coulson IH. Systemic disease and the skin. In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook's Textbook of Dermatology. 8th ed. Chichester, UK: John Wiley and Sons Ltd; 2010:62.24.
- Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med 2011; 13:755–764.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
A woman in her 30s presented with an itchy skin-colored rash over her left scapular region that had first appeared 8 years earlier. It had started as itchy skin-colored papules that coalesced to a patch and later became hyperpigmented because of repeated scratching.
She had undergone total thyroidectomy for medullary thyroid carcinoma 1 year ago, and the rash had been diagnosed at that time as lichen planus. She was referred to us by her physician for histopathologic confirmation of the lesions. She denied any history of episodic headache or palpitation.
Her urine normetanephrine excretion was elevated at 1,425 μg/day (reference range 148–560), and her metanephrine excretion was also high at 2,024 μg/day (reference range 44–261).
At a 3-month follow-up visit, the woman’s skin lesions had improved with twice-a-day application of mometasone 0.1% cream; she was lost to follow-up after that visit.
MULTIPLE ENDOCRINE NEOPLASIA
Our patient’s scapular lesions and first-degree family history of MEN type 2A confirmed the diagnosis of the newly recognized variant, MEN type 2A-related cutaneous lichen amyloidosis, in which the characteristic pigmented scapular rash typically predates the first diagnosis of neoplasia.1 The dermal amyloidosis is caused by deposition of keratinlike peptides rather than calcitoninlike peptides.2
A recent systematic review on MEN type 2A with cutaneous lichen amyloidosis showed a female preponderance and a high penetrance of cutaneous lichen amyloidosis, which was the second most frequent manifestation of the syndrome, preceded only by medullary thyroid carcinoma.1
As in our patient’s case, scapular rash and a history of medullary thyroid carcinoma should prompt an investigation for MEN type 2A. These patients should be closely followed for underlying MEN type 2A-related neoplasms.
The mucosal neuromas and skin lipomas seen in MEN type 1 and MEN type 2B are absent in MEN type 2A.3 Cutaneous lichen amyloidosis is the only dermatologic marker for MEN type 2A. Owing to a similar genetic background, cutaneous lichen amyloidosis is also associated with familial medullary thyroid carcinoma, another rare variant of MEN type 2A.4
DIFFERENTIAL DIAGNOSIS
Notalgia paresthetica is a unilateral chronic neuropathic pruritus on the back, mostly located between the shoulders and corresponding to the second and the sixth thoracic nerves. It is mostly attributed to compression of spinal nerves by an abnormality of the thoracic spine.5 In our patient, this was ruled out by the radiologic evaluation.
Before MEN type 2A with cutaneous lichen amyloidosis was recognized as a variant of MEN type 2A, lesions suggestive of notalgia paresthetica were reported with MEN type 2A.3 The classic infrascapular location, history of painful neck muscle spasms, touch hyperesthesia of the lesions, and absence of amyloid deposits on histopathologic study help to differentiate notalgia paresthetica from cutaneous lichen amyloidosis. However, later phases of notalgia paresthetica may show amyloid deposits on histopathologic study, while detection of a scant amount of amyloid is difficult in the early stages of cutaneous lichen amyloidosis.
TAKE-HOME POINT
Cutaneous lichen amyloidosis is usually seen on the extensor surfaces of the extremities. It is considered benign, caused by filamentous degeneration of keratinocytes from repeated scratching. But cutaneous lichen amyloidosis at an early age in the scapular area of women warrants a detailed family history for endocrine neoplasia, blood pressure monitoring, thyroid palpation, and blood testing for serum calcium, calcitonin, and parathyroid hormone.
A woman in her 30s presented with an itchy skin-colored rash over her left scapular region that had first appeared 8 years earlier. It had started as itchy skin-colored papules that coalesced to a patch and later became hyperpigmented because of repeated scratching.
She had undergone total thyroidectomy for medullary thyroid carcinoma 1 year ago, and the rash had been diagnosed at that time as lichen planus. She was referred to us by her physician for histopathologic confirmation of the lesions. She denied any history of episodic headache or palpitation.
Her urine normetanephrine excretion was elevated at 1,425 μg/day (reference range 148–560), and her metanephrine excretion was also high at 2,024 μg/day (reference range 44–261).
At a 3-month follow-up visit, the woman’s skin lesions had improved with twice-a-day application of mometasone 0.1% cream; she was lost to follow-up after that visit.
MULTIPLE ENDOCRINE NEOPLASIA
Our patient’s scapular lesions and first-degree family history of MEN type 2A confirmed the diagnosis of the newly recognized variant, MEN type 2A-related cutaneous lichen amyloidosis, in which the characteristic pigmented scapular rash typically predates the first diagnosis of neoplasia.1 The dermal amyloidosis is caused by deposition of keratinlike peptides rather than calcitoninlike peptides.2
A recent systematic review on MEN type 2A with cutaneous lichen amyloidosis showed a female preponderance and a high penetrance of cutaneous lichen amyloidosis, which was the second most frequent manifestation of the syndrome, preceded only by medullary thyroid carcinoma.1
As in our patient’s case, scapular rash and a history of medullary thyroid carcinoma should prompt an investigation for MEN type 2A. These patients should be closely followed for underlying MEN type 2A-related neoplasms.
The mucosal neuromas and skin lipomas seen in MEN type 1 and MEN type 2B are absent in MEN type 2A.3 Cutaneous lichen amyloidosis is the only dermatologic marker for MEN type 2A. Owing to a similar genetic background, cutaneous lichen amyloidosis is also associated with familial medullary thyroid carcinoma, another rare variant of MEN type 2A.4
DIFFERENTIAL DIAGNOSIS
Notalgia paresthetica is a unilateral chronic neuropathic pruritus on the back, mostly located between the shoulders and corresponding to the second and the sixth thoracic nerves. It is mostly attributed to compression of spinal nerves by an abnormality of the thoracic spine.5 In our patient, this was ruled out by the radiologic evaluation.
Before MEN type 2A with cutaneous lichen amyloidosis was recognized as a variant of MEN type 2A, lesions suggestive of notalgia paresthetica were reported with MEN type 2A.3 The classic infrascapular location, history of painful neck muscle spasms, touch hyperesthesia of the lesions, and absence of amyloid deposits on histopathologic study help to differentiate notalgia paresthetica from cutaneous lichen amyloidosis. However, later phases of notalgia paresthetica may show amyloid deposits on histopathologic study, while detection of a scant amount of amyloid is difficult in the early stages of cutaneous lichen amyloidosis.
TAKE-HOME POINT
Cutaneous lichen amyloidosis is usually seen on the extensor surfaces of the extremities. It is considered benign, caused by filamentous degeneration of keratinocytes from repeated scratching. But cutaneous lichen amyloidosis at an early age in the scapular area of women warrants a detailed family history for endocrine neoplasia, blood pressure monitoring, thyroid palpation, and blood testing for serum calcium, calcitonin, and parathyroid hormone.
- Scapineli JO, Ceolin L, Puñales MK, Dora JM, Maia AL. MEN 2A-related cutaneous lichen amyloidosis: report of three kindred and systematic literature review of clinical, biochemical and molecular characteristics. Fam Cancer 2016; 15:625–633.
- Donovan DT, Levy ML, Furst EJ, et al. Familial cutaneous lichen amyloidosis in association with multiple endocrine neoplasia type 2A: a new variant. Henry Ford Hosp Med J 1989; 37:147–150.
- Cox NH, Coulson IH. Systemic disease and the skin. In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook's Textbook of Dermatology. 8th ed. Chichester, UK: John Wiley and Sons Ltd; 2010:62.24.
- Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med 2011; 13:755–764.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
- Scapineli JO, Ceolin L, Puñales MK, Dora JM, Maia AL. MEN 2A-related cutaneous lichen amyloidosis: report of three kindred and systematic literature review of clinical, biochemical and molecular characteristics. Fam Cancer 2016; 15:625–633.
- Donovan DT, Levy ML, Furst EJ, et al. Familial cutaneous lichen amyloidosis in association with multiple endocrine neoplasia type 2A: a new variant. Henry Ford Hosp Med J 1989; 37:147–150.
- Cox NH, Coulson IH. Systemic disease and the skin. In: Burns T, Breathnach S, Cox N, Griffiths C, eds. Rook's Textbook of Dermatology. 8th ed. Chichester, UK: John Wiley and Sons Ltd; 2010:62.24.
- Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med 2011; 13:755–764.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol 2005; 52:1085–1087.
Fever after recent travel
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
A 28-year-old man developed fever, night sweats, nausea, headache, reduced appetite, skin rash, and hemoptysis 2 weeks after returning to the United States from Mexico.
The patient had fistulizing Crohn disease and had been taking the tumor necrosis factor alpha (TNF-alpha) blocker adalimumab for the past 3 months. He had no risk factors for human immunodeficiency virus infection, and he had stopped smoking 1 year previously. Chest radiography and a tuberculin skin test before he started adalimumab therapy were negative. While in Mexico, he did not drink more than 1 alcoholic beverage a day.
He had presented recently to his local hospital with the same symptoms and had been prescribed ciprofloxacin, metronidazole, ceftriaxone, vancomycin, and ampicillin, which he was still taking but with no improvement of symptoms. Blood cultures drawn before the start of antibiotic therapy had been negative. Urinalysis, a screen for infectious mononucleosis, and lumbar puncture were also negative. Results of renal function testing were normal except for the anion gap, which was 20.8 mmol/L (reference range 10–20).
INITIAL EVALUATION
On presentation to this hospital, the patient was afebrile but continued to have temperature spikes up to 39.0°C (102.2°F). His heart rate was 90 per minute, blood pressure 104/61 mm Hg, respiratory rate 18 per minute, and oxygen saturation 95% on 2 L of oxygen via nasal cannula.
- White blood cell count 10.0 × 109/L (reference range 4.0–10.0 × 109/L)
- Lymphocyte count 6.1 × 109/L (1.2–3.4)
- Hemoglobin level 13.6 g/dL (14.0–18.0)
- Platelet count 87 × 109/L (150–400), reaching a nadir of 62 on hospital day 23
- Albumin 47 g/L (35–50)
- Total bilirubin 48 µmol/L (2–20)
- Alkaline phosphatase 137 U/L (40–135)
- Alanine aminotransferase 22 U/L (9–69)
- Aspartate aminotransferase 72 U/L (5–45).
He continued to have temperature spikes. His alkaline phosphatase level plateaued at 1,015 U/L on day 30, while his alanine aminotransferase and aspartate aminotransferase levels remained stable.
The patient’s ceftriaxone was continued, and the other antibiotics were replaced with doxycycline. Fluconazole was added when sputum culture grew Candida albicans. However, these drugs were later discontinued in view of worsening results on liver enzyme testing.
The evaluation continues
Sputum cultures were negative for acid-fast bacilli on 3 occasions.
Serologic testing was negative for:
- Hepatitis B surface antigen (but hepatitis B surface antibody was positive at > 1,000 IU/L)
- Hepatitis C virus antibody
- Cytomegalovirus immunoglobulin (Ig) G
- Toxoplasma gondii IgG
- Epstein-Barr virus viral capsid antigen IgM
- Rickettsia antibodies
- Antinuclear antibody
- Antineutrophil cytoplasmic antibody
- Antiglomerular basement membrane antibody.
Chest radiography showed blunting of both costophrenic angles and mild prominence of right perihilar interstitial markings and the right hilum.
Computed tomography of the chest, abdomen, and pelvis showed a subpleural density in the lower lobe of the right lung, small bilateral pleural effusions, right hilar lymphadenopathy, and splenomegaly with no specific hepatobiliary abnormality.
A white blood cell nuclear scan found no occult infection.
Abdominal ultrasonography showed a prominent liver and spleen. The liver parenchyma showed diffuse decreased echogenicity, suggestive of hepatitis.
Transesophageal echocardiography showed no vegetations or valvular abnormalities.
Bronchoscopy showed normal airways without evidence of pulmonary hemorrhage. No foci of infection were obtained. A focus of granuloma consisting of epithelioid histiocytes in tight clusters was seen on washings from the right lower lobe, but no malignant cells were seen.
Sections of pathologically enlarged right hilar and subcarinal lymph nodes obtained with transbronchial needle aspiration were sent for cytologic analysis and flow cytometry.
Cultures for tuberculous and fungal organisms were negative.
A clue. On further inquiry, the patient said he had gone swimming in the natural pool, or cenote, under a rock formation at Cenote Maya Park in Mexico.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is not in the differential diagnosis?
- Disseminated tuberculosis
- Coccidioidomycosis
- Subacute infective endocarditis
- Disseminated histoplasmosis
- Blastomycosis
Although the patient has a systemic disease, subacute infective endocarditis is not likely because of a lack of predisposing factors such as a history of endocarditis, abnormal or artificial heart valve, or intravenous drug abuse. Moreover, negative blood cultures and the absence of vegetations on echocardiography make endocarditis very unlikely.
Given that the patient is immunosuppressed, opportunistic infection must be at the top of the differential diagnosis. Histoplasmosis, coccidioidomycosis, and blastomycosis are endemic in Mexico. Disseminated histoplasmosis is the most likely diagnosis; coccidioidomycosis and blastomycosis are less likely, based on the history, signs, and symptoms. Disseminated tuberculosis must be excluded before other diagnostic possibilities are considered.
TUBERCULOSIS IN PATIENTS ON TNF-ALPHA ANTAGONISTS
Tuberculosis has been reported in patients taking TNF-alpha antagonists.1 The frequency of tuberculosis is much higher than that of other opportunistic infections, and over 50% of reported cases involve extrapulmonary tissues in patients treated with TNF-alpha antagonists.2
British Thoracic Society guidelines recommend screening for latent tuberculosis before starting treatment with a TNF-alpha antagonist; the screening should include a history of tuberculosis treatment, a clinical examination, chest radiography, and a tuberculin skin test.3 Patients found to have active tuberculosis should receive a minimum of 2 months of standard treatment before starting a TNF-alpha antagonist. Patients with evidence of past tuberculosis or a history of tuberculosis who received adequate treatment should be monitored regularly. Patients with prior tuberculosis not adequately treated should receive chemoprophylaxis before starting a TNF-alpha antagonist.
Fever, night sweats, and intrathoracic and intra-abdominal lymphadenopathy are common features of disseminated tuberculosis. Upper-lobe cavitary disease or miliary lesions may be seen on chest radiography, but atypical presentations with lower-lobe infiltrate are not uncommon in immunosuppressed patients.4
A negative tuberculin skin test and a normal chest radiograph 3 months ago, along with negative sputum and bronchial lavage fluid cultures and no history of tuberculosis contact, make tuberculosis unlikely in our patient.
COCCIDIOIDOMYCOSIS
Coccidioidomycosis (valley fever) is caused by the fungus Coccidioides immitis, which lives in the soil and is acquired by inhalation of airborne microscopic spores.
Fatigue, cough, fever, shortness of breath, headache, night sweats, muscle or joint pain, and a rash on the upper body or legs are common symptoms. It may cause a self-limiting flulike illness. From 5% to 10% of patients may develop serious long-term lung problems. In a small number of patients, the disease may progress beyond the lungs to involve the central nervous system, spinal cord, skin, bones, and joints.5
Serologic testing is highly useful for the diagnosis. Antigen testing has a sensitivity of 71% and a specificity of 98% for the diagnosis, but cross-reactivity occurs in 10% of patients with other types of mycosis. Respiratory secretions and tissue samples should undergo microscopic study and culture.
BLASTOMYCOSIS
Blastomycosis is caused by the fungus Blastomyces dermatitidis, which lives in soil and in association with decomposing organic matter such as wood and leaves. Inhalation of spores may cause a flulike illness or pneumonia. In serious cases, the disease can spread to skin and bone.
The diagnosis is established with fungal cultures of tissue samples or body fluids (bone marrow, liver tissue, skin, sputum, blood). Rapid diagnosis may be obtained by examination of the secretions under a microscope, where typical broad-based budding yeast can be seen in almost 90% of cases.6 Antigen may also be detected in urine and serum7; the sensitivity of antigen testing is 93% and the specificity is 98%. Serologic testing is not recommended for diagnosis of blastomycosis because of poor sensitivity and specificity.8
NARROWING THE DIFFERENTIAL
Both coccidioidomycosis and blastomycosis should be included in the differential diagnosis of a systemic disease with subacute onset and prominent lung involvement in a patient returning from travel to Mexico. The lack of involvement of the central nervous system, spinal cord, bones, or joints makes these infections less likely in our patient.
However, swimming in a cenote under a rock formation is an important clue to the diagnosis in our patient, as it puts him at risk of inhaling microconidia or hyphal elements of histoplasmosis. This, along with his immunocompromised status, fever, hemoptysis, night sweats, skin and lung features, and the generally subacute course of his illness, make disseminated histoplasmosis the most likely diagnosis.
Radiologic findings of pulmonary infiltrate with effusion and elevated lactate dehydrogenase, aminotransferases, and alkaline phosphatase increase the likelihood of disseminated histoplasmosis.
HISTOPLASMOSIS
Histoplasma capsulatum is a dimorphic fungus that thrives in the soil and caves of regions with moderate climate, especially in soil containing large amounts of bird excreta or bat guano.9 Bats are natural hosts of this organism, and it is endemic in North and Central America, including parts of Mexico. Air currents can carry the microconidia for miles, thus exposing people without direct contact with contaminated sites.
The infection is usually acquired by inhalation of microconidia or small hyphal elements or by reactivation of previously quiescent foci of infection in an immunosuppressed patient. Most patients exposed to H capsulatum remain asymptomatic or develop mild symptoms, which are self-limiting. A small number develop acute pulmonary histoplasmosis or chronic cavitary histoplasmosis. Disseminated disease usually occurs only in an immunosuppressed host.
Acute pulmonary histoplasmosis presents with fever, malaise, headache, weakness, substernal chest pain, and dry cough and may be associated with erythema nodosum, erythema multiforme, and arthralgias. It may be mistaken for sarcoidosis since enlarged hilar and mediastinal lymph nodes are often seen on chest radiography.10
Progressive disseminated histoplasmosis is defined as a clinical illness that does not improve after at least 3 weeks of observation and is associated with physical or radiographic findings with or without laboratory evidence of extrapulmonary involvement.11
Fever, malaise, anorexia, weight loss, night sweats, hepatosplenomegaly, and lymphadenopathy are features of progressive disseminated histoplasmosis.
Cutaneous manifestations of disseminated histoplasmosis occur in 10% to 25% of patients with acquired immunodeficiency syndrome and include papules, plaques with or without crust, pustules, nodules, lesions resembling molluscum contagiosum virus infection, acneiform eruptions, erythematous macules, and keratotic plaques.12
TESTING FOR HISTOPLASMOSIS
2. What investigation is least likely to help confirm the diagnosis of disseminated histoplasmosis?
- Polymerase chain reaction (PCR) testing of serum, cerebrospinal fluid, and bronchoalveolar lavage specimens
- Urinary Histoplasma antigen testing
- Serologic testing
- Blood and bronchoalveolar lavage cultures
Urinary Histoplasma antigen has a sensitivity of 90% for the diagnosis of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome.18 It is less useful for pulmonary forms of histoplasmosis: the sensitivity is 75% and may even be less in milder or chronic forms of pneumonia.19 False-positive reactions may occur in patients with other fungal infections such as coccidioidomycosis, blastomycosis, paracoccidioidomycosis and penicilliosis.20 Urine antigen levels can also be used to monitor therapy, since levels decrease during therapy and increase in 90% of those who have a relapse.21
Our patient’s urinary Histoplasma antigen level was greater than 23.0 ng/mL (positive is > 0.50).
Serologic testing. Immunodiffusion immunoglobulin G (IgG) testing for Histoplasma and Blastomyces was negative, as was an enzyme immunoassay for Coccidioides IgG and IgM. However, antibody tests are less useful in immunosuppressed patients,22 and thus a negative result does not rule out histoplasmosis. A fourfold rise in complement fixation antibody titer is diagnostic of acute histoplasmosis. A single complement fixation titer of 1:32 is suggestive but not diagnostic of histoplasmosis. Cross-reactions may occur with other fungal infections like blastomycosis. The immunodiffusion assay has a greater specificity but slightly less sensitivity than the complement fixation assay.19
Culture of H capsulatum is the definitive test to establish a diagnosis of histoplasmosis. Culture can be performed on samples taken from blood, bone marrow, sputum, and bronchoalveolar lavage fluid, or from lung, liver, or lymph node tissue. Cultures are positive in 74% to 82% of cases of progressive disseminated histoplasmosis.13 However, treatment should not await culture results since the fungus may take several weeks to grow.
Back to our patient
Although Histoplasma serologic studies and cultures were negative, the diagnosis of disseminated histoplasmosis was made on the basis of the patient’s immunosuppressed status, travel history, clinical features, and positivity for urine Histoplasma antigen. Though urine histoplama antigen may be falsely positive in other fungal infections such as coccidioidomycosis, paracoccidioidomycosis, and blastomycosis, clinical features and the absence of central nervous system, joint, and bone involvement suggested disseminated histoplasmosis.
TREATMENT
3. What is the appropriate treatment for this patient?
- Amphotericin B followed by oral itraconozole
- Oral fluconazole
- Oral itraconazole
Liposomal amphotericin B or amphotericin B deoxycholate is recommended as initial therapy for moderately severe to severe and progressive disseminated histoplasmosis. It should be continued for 1 to 2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days, then 200 mg 2 times daily for at least 12 months).
Monitoring itraconazole therapy through random serum levels is strongly recommended, and a random concentration of at least 1.0 mg/mL is recommended.23
Urine antigen levels should be measured before treatment is started, at 2 weeks, at 1 month, then every 3 months during therapy, continuing for 12 months after treatment is stopped.11
Lifelong suppressive therapy with itraconazole 200 mg daily may be required in immunosuppressed patients and patients who have a relapse despite appropriate therapy.11
While oral itraconazole is used as a sole agent for the treatment of mild to moderate acute pulmonary histoplasmosis and chronic cavitary pulmonary histoplasmosis, oral treatment alone with either fluconazole or itraconazole is not recommended for the treatment of progressive disseminated histoplasmosis.11
COMPLICATIONS OF HISTOPLASMOSIS
4. Which of the following is not a possible complication of histoplasmosis?
- Chronic cavitary pulmonary histoplasmosis
- Fibrosing mediastinitis
- Hypoadrenalism
- Hypothyroidism
Chronic cavitary pulmonary histoplasmosis usually develops in patients with underlying emphysema. Fatigue, night sweats, fever, anorexia, and weight loss are features of chronic cavitary pulmonary histoplasmosis. Progression of necrosis may lead to “marching cavity,” in which necrosis increases the size of the cavity and may consume an entire lobe.10
Fibrosing mediastinitis is an uncommon but often lethal complication of disseminated histoplasmosis. Increasing dyspnea, cough, hemoptysis, and signs of superior vena cava syndrome and right heart failure may develop. However, fibrosing mediastinitis is thought to be due to an exuberant immune response to past Histoplasma infection and would not be expected in an immunocompromised patient.17
Hypoadrenalism. Extensive destruction of the adrenal glands may lead to hypoadrenalism, manifesting as orthostatic hypotension, hyperkalemia, hyponatremia, and evidence of markedly enlarged adrenal glands with central necrosis on computed tomography.24
Hypothyroidism. Acute or disseminated histoplasmosis has not been reported to cause thyroid dysfunction.
CASE CONCLUSION
Our patient was treated with itraconazole 200 mg twice daily for 24 months. Although the literature supports lifelong itraconazole therapy in immunosuppressed patients, our patient was reluctant to do so. He agreed to close monitoring. If symptoms recur, itraconazole will be reinstituted and continued lifelong.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61:409–417.
- Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis 2003; 3:148–155.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60:800–805.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 38-1998. A 19-year-old man with the acquired immunodeficiency syndrome and persistent fever. N Engl J Med 1998; 339:1835–1843.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Lemos LB, Guo M, Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann Diagn Pathol 2000; 4:391–406.
- Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Micribiol 2004; 42:4873–4875.
- Wheat LJ. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 2009; 30:379–389.
- Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–798.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Chang P, Rodas C. Skin lesions in histoplasmosis. Clinics Dermatol 2012; 30:592–598.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 2012; 19:53–56.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am 2016; 30:247–264.
- Stockamp NW, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2016; 30:229–246.
- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am 2016; 30:207–227.
- Wheat LJ, Garringer T, Drizendine E, Connolly P. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 2002; 14:1389–1391.
- Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008; 21:421–425.
- Wheat LJ. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 2006; 6:1207–1221.
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother 2002; 46:248–250.
- Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol 2003; 11:488–494.
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35:461–473.
- Sarosi GA, Voth DW, Dahl BA, Doto IL, Tosh FE. Disseminated histoplasmosis: results of long-term follow-up. Ann Intern Med 1971; 75:511–516.