User login
Managing malignant pleural effusion
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
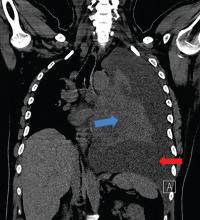
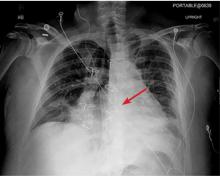
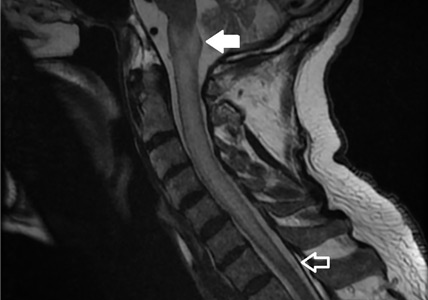
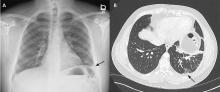
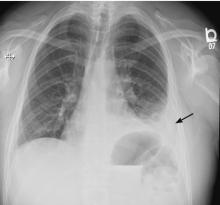
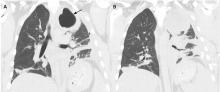
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
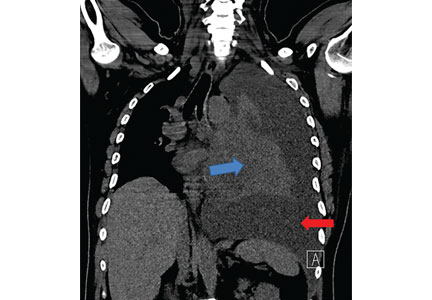
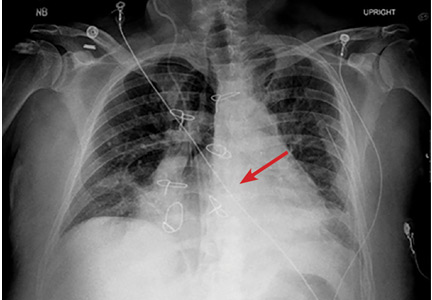
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
KEY POINTS
- Asymptomatic pleural effusion in patients currently on chemotherapy does not require treatment but should be monitored for progression.
- Indwelling pleural catheters are best used to treat effusion with lung collapse and are increasingly used as first-line therapy in other settings.
- Chemical or mechanical pleurodesis results in filling the pleural space to prevent further fluid accumulation and can be accomplished by one of several methods.
- For patients near the end of life, simple thoracentesis, repeated as needed, is a reasonable strategy.
Dancing sternal wires: A radiologic sign of sternal dehiscence
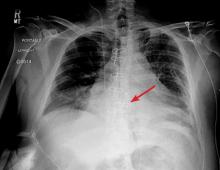
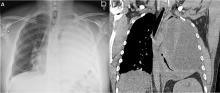
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
CTPA may not rule out VTE in high-risk patients
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does a negative computed tomography pulmonary angiography rule out venous thromboembolism (VTE)?
Background: Computed tomography pulmonary angiography (CTPA) is the most common diagnostic modality used to diagnose pulmonary embolism (PE) and has a high negative predictive value in patients with a low 3-month risk of VTE. In patients with higher pretest probability of PE, it is unknown whether CTPA is sufficient to rule out VTE.
Study design: Meta-analysis.
Setting: Published prospective outcome studies of patients with suspected PE using CTPA as a diagnostic strategy.
Synopsis: The authors reviewed 3,143 publications from MEDLINE, EMBASE, and the Cochrane Library and identified 22 prospective outcome studies to include in their meta-analysis. A VTE was diagnosed in 3,923 out of 11,872 participants (33%) using CTPA. Of the 7,863 patients with a negative CTPA, 148 patients had an acute VTE confirmed by venous ultrasound, ventilation/perfusion scan, or angiography, and 74 patients experienced VTE during a 3-month follow-up period, yielding an overall proportion of 2.4% of patients (95% confidence interval, 1.3%-3.8%).
Subgroup analysis showed that cumulative occurrence of VTE was related to pretest prevalence. In the subgroup of patients with a VTE prevalence greater than 40%, VTE was observed in 8.1% of patients with a negative CTPA (95% CI, 3.4%-14.5%).
Bottom line: CTPA may be insufficient to rule out VTE in patients with a high pretest probability of PE.
Citation: Belzile D et al. Outcomes following a negative computed tomography pulmonary angiography according to pulmonary embolism prevalence: a meta-analysisof the management outcome studies. J Thromb Haemost. 2018 Jun;16(6):1107-20.
Dr. Jenkins is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Appropriate use criteria for imaging in nonvalvular heart disease released
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Common benign breast concerns for the primary care physician
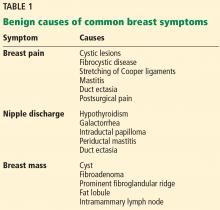
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
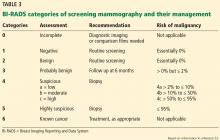
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
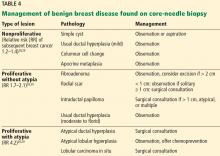
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
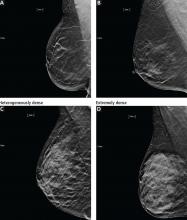
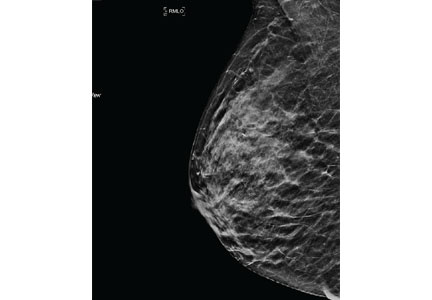
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
KEY POINTS
- The two most common breast symptoms are lumps and pain.
- Most breast problems are not caused by cancer.
- Evaluation of any breast problem begins with a focused history followed by a breast examination and, when necessary, imaging.
- If the results of the breast examination and imaging suggest a benign cause, no further follow-up is necessary.
- If there is discordance between imaging and breast examination results, or if there is a high clinical suspicion of cancer, then consider serial follow-up examinations at short intervals, referral to a breast surgeon for excision, or both.
Acute-onset quadriplegia with hyperreflexia
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
Emphysematous cystitis
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
Rapidly progressive pleural effusion
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
Delirious after undergoing workup for stroke
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.
Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of
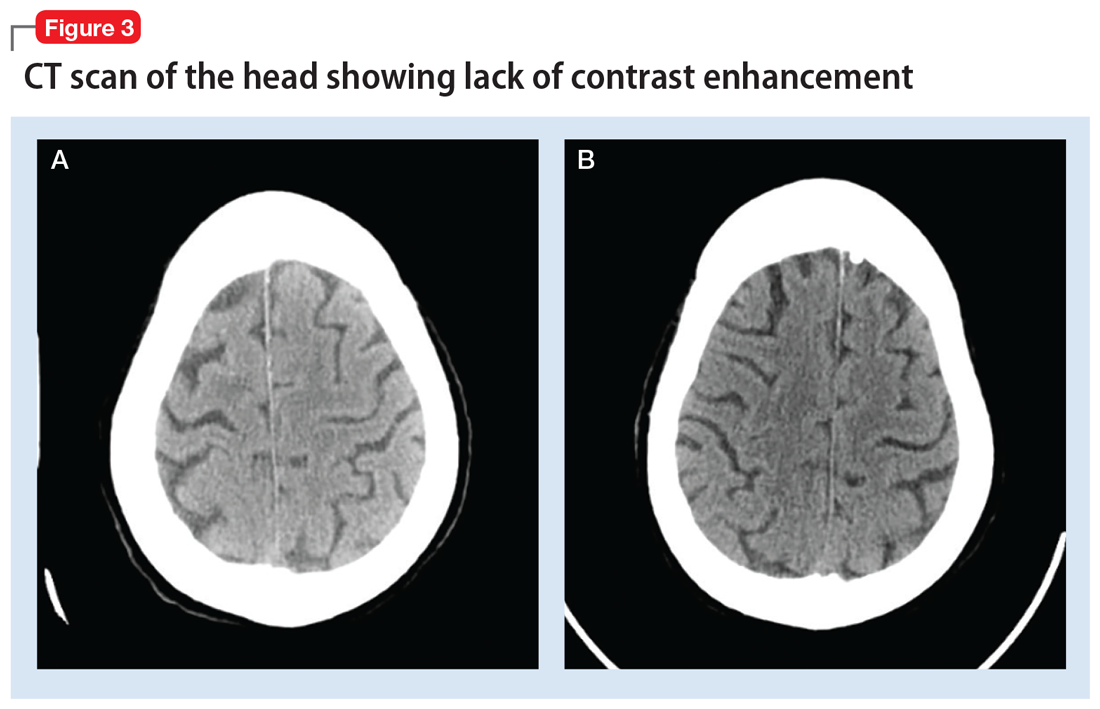
Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.

Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of

Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
CASE Altered mental status after stroke workup
Ms. L, age 91, is admitted to the hospital for a neurologic evaluation of a recent episode of left-sided weakness that occurred 1 week ago. This left-sided weakness resolved without intervention within 2 hours while at home. This presentation is typical of a transient ischemic attack (TIA). She has a history of hypertension, bradycardia, and pacemaker implantation. On initial evaluation, her memory is intact, and she is able to walk normally. Her score on the St. Louis University Mental Status (SLUMS) exam is 25, which suggests normal cognitive functioning for her academic background. A CT scan of the head reveals a subacute stroke of the right posterior limb of the internal capsule consistent with recent TIA.
Ms. L is admitted for a routine stroke workup and prepares to undergo a CT angiogram (CTA) with the use of the iodinated agent iopamidol (100 mL, 76%) to evaluate patency of cerebral vessels. Her baseline blood urea nitrogen (BUN) and creatinine levels are within normal limits.
A day after undergoing CTA, Ms. L starts mumbling to herself, has unpredictable mood outbursts, and is not oriented to time, place, or person.
[polldaddy:10199351]
The authors’ observations
Due to her acute altered mental status (AMS), Ms. L underwent an emergent CT scan of the head to rule out any acute intracranial hemorrhages or thromboembolic events. The results of this test were negative. Urinalysis, BUN, creatinine, basic chemistry, and complete blood count panels were unrevealing. On a repeat SLUMS exam, Ms. L scored 9, indicating cognitive impairment.
Ms. L also underwent a comprehensive metabolic profile, which excluded any electrolyte abnormalities, or any hepatic or renal causes of AMS. There was no sign of dehydration, acidosis, hypoglycemia, hypoxemia, hypotension, or bradycardia/tachycardia. A urinalysis, chest X-ray, complete blood count, and 2 blood cultures conducted 24 hours apart did not reveal any signs of infection. There were no recent changes in her medications and she was not taking any sleep medications or other psychiatric medications that might precipitate a withdrawal syndrome.
There have been multiple reports of contrast-induced nephropathy (CIN), which may be evidenced by high BUN-to-creatinine ratios and could cause AMS in geriatric patients. However, CIN was ruled out as a potential cause in our patient because her BUN-to-creatinine was unremarkable.
Continue to: Routine EEG was clinically...
Routine EEG was clinically inconclusive. Diffusion-weighted MRI may have been helpful to identify ischemic strokes that a CT scan of the head might miss,1 but we were unable to conduct this test because Ms. L had a pacemaker. Barber et al2 suggested that in the setting of acute stroke, the use of MRI may not have an added advantage over the CT scan of the head.
[polldaddy:10199352]
TREATMENT Rapid improvement with supportive therapy
Intravenous fluids are administered as supportive therapy to Ms. L for suspected contrast-induced encephalopathy (CIE). The next day, Ms. L experiences a notable improvement in cognition, beyond that attributed to IV hydration. By 3 days post-contrast injection, her SLUMS score increases to 15. By 72 hours after contrast administration, Ms. L’s cognition returns to baseline. She is monitored for 24 hours after returning to baseline cognitive functioning. After observing her to be in no physical or medical distress and at baseline functioning, she is discharged home under the care of her son with outpatient follow-up and rehab services.
The authors’ observations
For Ms. L, the differential diagnosis included post-ictal phenomenon, new-onset ischemic or hemorrhagic changes, hyperperfusion syndrome, and CIE.
Seizures were ruled out because EEG was inconclusive, and Ms. L did not have the clinical features one would expect in an ictal episode. Transient ischemic attack is, by definition, an ischemic event with clinical return to baseline within 24 hours. Although a CT scan of the head may not be the most sensitive way to detect early ischemic changes and small ischemic zones, the self-limiting course and complete resolution of Ms. L’s symptoms with return to baseline is indicative of a more benign pathology, such as CIE. New hemorrhagic conversions have a dramatic presentation on radiologic studies. Historically, CIE presentations on imaging have been closely associated with the hyperattentuation seen in subarachnoid hemorrhage (SAH). The absence of typical radiologic and clinical findings in our case ruled out SAH.
Continue to: Typical CT scan findings in CIE include...
Typical CT scan findings in CIE include abnormal cortical contrast enhancement and edema, subarachnoid contrast enhancement, and striatal contrast enhancement (Figure 1, Figure 2, and Figure 3). Since the first clinical description, reports of 39 CT-/MRI-confirmed cases of CIE have been published in English language medical literature, with documented clinical follow-up3 and a median recovery time of 2.5 days. In a case report by Ito et al,4 there were no supportive radiographic findings. Ours is the second documented case that showed no radiologic signs of CIE. With a paucity of other etiologic evidence, negative lab tests for other causes of delirium, and the rapid resolution of Ms. L’s AMS after providing IV fluids as supportive treatment, a temporal correlation can be deduced, which implicates iodine-based contrast as the inciting factor.

Iodine-based contrast agents have been used since the 1920s. Today, >75 million procedures requiring iodine dyes are performed annually worldwide.5 This level of routine iodine contrast usage compels a mention of risk factors and complications from using such dyes. As a general rule, contrast agent reactions can be categorized as immediate (<1 day) or delayed (1 to 7 days after contrast administration). Immediate reactions are immunoglobulin E (IgE)-mediated anaphylactic reactions. Delayed reactions involve a T-cell mediated response that ranges from pruritus and urticaria (approximately 70%) to cardiac complications such as cardiovascular shock, arrhythmia, arrest, and Kounis syndrome. Other less prevalent complications include hypotension, bronchospasm, and CIN. Patients with the following factors may be at higher risk for contrast-induced reactions:
- asthma
- cardiac arrhythmias
- central myasthenia gravis
- >70 years of age
- pheochromocytoma
- sickle cell anemia
- hyperthyroidism
- dehydration
- hypotension.
Although some older literature reported correlations between seafood and shellfish allergies and iodine contrast reactions, more recent reports suggest there may not be a direct correlation, or any correlation at all.5,6
Iodinated CIE is a rare complication of contrast angiography. It was first reported in 1970 as transient cortical blindness after coronary angiography.7 Clinical manifestations include encephalopathy evidenced by AMS, affected orientation, and acute psychotic changes, including paranoia and hallucinations, seizures, cortical blindness, and focal neurologic deficits. Neuroimaging has been pivotal in confirming the diagnosis and in excluding thromboembolic and hemorrhagic complications of angiography.8
Encephalopathy has been documented after administration of

Continue to: Regardless of the mechanism...
Regardless of the mechanism, all the above-mentioned studies note a reversal of radiologic and neurologic findings without any deficits within 48 to 72 hours (median recovery time of 2.5 days).3 All reported cases of CIE, including ours, were found to be completely reversible without any neurologic or radiologic deficits after resolution (48 to 72 hours post-contrast administration).
Clinicians should have a high index of suspicion for CIE in patients with recent iodine-based contrast exposure. From a practical standpoint, such a mechanism could be easily missed because while use of a single-administration contrast agent may appear in procedure notes or medication administration records, it might not necessarily appear in documentation of currently administered medications. Also, such cases might not always present with unique radiologic findings, as illustrated by Ms. L’s case.
Bottom Line
Have a high index of suspicion for contrast-induced encephalopathy, especially in geriatric patients, even in the absence of radiologic findings. A full delirium/dementia workup is warranted to rule out other life-threatening causes of altered mental status. Timely recognition could enable implementation of medicationsparing approaches to the disorder, such as IV fluids and frequent reorientation.
Related Resources
- Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi:10.1155/2018/9278526.
- Hamra M, Bakhit Y, Khan M, et al. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13(4):331-335.
Drug Brand Names
Iohexol • Omnipaque
Iopamidol • Isovue-370
Iopromide • Ultravist
Ioxilan • Oxilan
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
1. Moreau F, Asdaghi N, Modi J, et al. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: the more you see the more you know. Cerebrovasc Dis Extra. 2013;3(1):130-136.
2. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76(11):1528-1533.
3. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling: a case report and review of the literature. Interv Neuroradiol. 2012;18(1):33-41.
4. Ito N, Nishio R, Ozuki T, et al. A state of delirium (confusion) following cerebral angiography with ioxilan: a case report. Nihon Igaku Hoshasen Gakkai Zasshi. 2002; 62(7):370-371.
5. Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-154.
6. Cohan R. AHRQ Patient Safety Network Reaction to Dye. US Department of Health and Human Services Agency for Healthcare Research and Quality. https://psnet.ahrq.gov/webmm/case/75/reaction-to-dye. Published September 2004. Accessed March 5, 2017.
7. Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness: an unusual complication of coronary angiography. Neurology. 1970;20(4):353-355.
8. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989;39(4):567-571.
9. Kocabay G, Karabay CY. Iopromide-induced encephalopathy following coronary angioplasty. Perfusion. 2011;26:67-70.
10. Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. Journal of Endovascular Therapy. 2001;8:111-113.
11. Sawaya RA, Hammoud R, Arnaout SJ, et al. Contrast induced encephalopathy following coronary angioplasty with iohexol. Southern Medical Journal. 2007;100(10):1054-1055.
MRI for Emergency Clinicians
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
The use of magnetic resonance imaging (MRI) by emergency physicians (EPs) is increasing steadily, as new MRI indications arise, technology evolves, and machines become faster and more widely available. It is therefore critically important that EPs understand the basics of this imaging modality, its uses, limitations, cautions, and contraindications.
A full explanation of the physics underpinning MRI is beyond this article’s scope. However, a comprehensive discussion of the topic is available in a 2013 review entitled, "Understanding MRI: basic MR physics for physicians."1 In short, three elements are necessary for an MRI machine to generate images: a strong magnetic field, radio waves, and a computer system. The body’s hydrogen nuclei with their single protons and north-south poles act as mini bar magnets with randomly aligned axes. However, when the body is subjected to the MRI machine magnetic field, these axes line up. When radio waves are applied to the magnetic field, the strength and direction of the magnetic field changes. Then, when the radio waves are turned off, the magnetic field strength and direction return to baseline and a signal is emitted. It is this signal that is interpreted by a computer system to generate images.2
Cautions and Limitations
Although limited availability is often cited as a reason for not obtaining MRI studies in the emergency department (ED), this limitation is institution specific and will likely improve over time. Recent statistics indicate that MRI availability in the United States is second only to that in Japan and climbing. MRI usage in the United States is the highest in the world.3
MRI cost (and the resultant patient bill) exceeds that of other commonly performed ED imaging roughly by a factor of 2:1 when compared to computed tomography (CT). This is unlikely to improve in the near term.
The time to complete an MRI study continues to fall for some indications, but significantly exceeds the time to obtain CT images. MRI scan times range from 20 to 60 minutes depending on test type.
Body habitus, particularly obesity, may limit the ability of certain patients to undergo MRI. Claustrophobia or the inability to lie still for the test’s entire duration may present a challenge for some patients. Be prepared to safely sedate patients with these issues. This is particularly relevant for pediatric patients. Consider a pre-MRI trial of sedation to assess which medication is best suited for individual patients.
Patients with certain medical devices may be unable to undergo MRI. Medical devices and implants from the U.S. and Europe manufactured within the past 30 years are non-ferromagnetic. This generally means they are MR-safe or MR-conditional. Realize, however, that certain non-ferromagnetic implants can heat during MR imaging.4 A free searchable database exists listing MRI-safe devices and implants along with limitations and cautions (http://www.mrisafety.com/TheList_search.asp).5
Pacemakers and defibrillators are worthy of special mention. Some are now considered MR-conditional in limited circumstances, and this situation will continue to evolve. Consult your radiologist and/or the physician who placed the medical device with any safety concerns.
Intraocular metallic foreign bodies are an MRI contraindication. If any concern exists for an intraocular metallic foreign body, perform an orbital CT before considering an MRI. Headphones and ear plugs are used during MRI examinations to prevent hearing damage due to machine noise or nerve and muscle stimulation.
A 2016 JAMA study of MRI in pregnancy involving more than 1.4 million deliveries concluded “exposure to MRI during the first trimester of pregnancy compared with non¬exposure was not associated with increased risk of harm to the fetus or in early childhood. Gadolinium MRI at any time during pregnancy was associated with an increased risk of a broad set of rheumatological, inflammatory, or infiltrative skin conditions and for stillbirth or neonatal death.”6
There is limited data on the use of MRI in pediatric patients, but a 2015 study noted, “to date, no studies have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies.”7
A variety of gadolinium-based contrast agents (GBCAs) are currently used. GBCA administrations in renally impaired patients has been linked to nephrogenic systemic fibrosis (NSF), a rare, progressive, potentially fatal, incompletely understood, systemic disorder with a spectrum of manifestations. Its occurrence has prompted alerts, and a recent set of recommendations for at-risk patients (ie, those with acute kidney injury or an eGFR < 30 mL/min/1.73 m2 and those who are dialysis dependent) specifies that (1) a low-risk GBCA should be used; (2) GBCA dose should be as low as possible; and (3) dialysis should be performed as indicated immediately after GBCA-enhanced MRI.8,9 Additionally, the EP may wish to obtain informed consent from at-risk patients prior to the administration of GBCAs.
Common MRI indications in the ED
Central nervous system MRI
Spinal cord compression may occur due to a neoplastic process, either primary or metastatic, infection (epidural abscess is a particular concern), or hematoma. CT myelography is another diagnostic option, but MRI offers ease of performance, superior resolution, multiplanar imaging, lack of ionizing radiation, and the ability to detect multiple lesions with a single scan. For non-traumatic myelopathy evaluation (most commonly due to cancer), perform a non-contrast MRI of the entire spinal canal since multiple lesions may be present. Repeat the MRI with contrast if the cause of the myelopathy is not clear after the non-contrast study.10 Gadolinium does help detect and define inflammatory, infectious, and neoplastic lesions, but spinal cord compression can be diagnosed without it if the patient cannot receive gadolinium (see Cautions and Limitations section).11 Only a non-enhanced MRI, limited to the traumatized area, is required in the evaluation of trauma-induced myelopathy.10
Dural venous sinus thrombosis (DVST) is best assessed with a combination of MRI and MR venography.10 DVST is clot formation within any of five major dural venous sinuses. DVST risk factors include: dehydration; infections, both systemic and local; pregnancy and the puerperium; neoplastic incursion; trauma; and coagulopathies.10,12 MR venography is an essential part of DVST evaluation since it assesses patency of the involved dural venous sinus.10
Carotid artery dissection is a leading cause of stroke in those younger than 45 years of age.13 Carotid and vertebral artery dissection, due to trauma, hypertension, vascular disease, or local infections, can be diagnosed with endovascular angiography.10,14 However, MRI in combination with MRA can be diagnostic as well.10,13,14 MRI delineates the intramural clots while MRA shows the degree and extent of endovascular compromise.10,13
Meningoencephalitis and vasculitis are usually diagnosed with a combination of clinical findings, laboratory data, CT, and lumbar puncture results. However, MRI is highly sensitive for the CNS lesions associated with infection or vasculitis. Consider MRI as an alternative to the usual work up in selected patients if aggressive early therapy for viral infection (eg, herpes) or vasculitis is being contemplated.10
Acute subarachnoid hemorrhage (SAH) is usually best demonstrated on CT. However, MRI may have a role, especially in posterior fossa SAH.10
Cerebral Ischemia (TIA and Stroke) - The 2018 guidelines for early management of patients with acute ischemic stroke both recommended and considered equal (in patients selected for mechanical thrombectomy) CT, diffusion weighted MRI or MRI perfusion.15 This guideline was promulgated by the American Heart Association/American Stroke Association and endorsed by the Society for Academic Emergency Medicine, among other professional organizations.
In a joint statement published by the American Society of Neuroradiology, the American College of Radiology, and the Society of Neurointerventional Surgery, MRI was reported to be equivalent to a non-contrast brain CT. MRI was also found to have superior accuracy in detecting microhemorrhages.16
Spine MRI
Spine and spinal cord emergencies must be promptly and correctly diagnosed to avoid or minimize functional loss. Knowledge of the most appropriate imaging modalities is essential to facilitate diagnosis and treatment for patients presenting with spine-related emergencies.
Low back pain prompts many ED visits and is a major cause of disability in the United States.
MRI is unwarranted for those patients with acute (< 6 weeks duration) low back pain in whom serious pathology, such as cauda equina, malignancy, epidural hematoma, or infection is not suspected. Manage most low back pain patients conservatively and without imaging.17
Trauma is the most common reason for spine MRI. CT, and now increasingly MRI, have supplanted plain radiography in the evaluation of spinal trauma. Currently, CT alone is considered sufficient in the evaluation of thoracic and lumbar skeletal injuries. This is not true for cervical spine injuries.18
Initially, use either the NEXUS or Canadian C-Spine Rule criteria to determine if a trauma patient needs any imaging. Then, consider whether CT or MRI or both will be required, while realizing that the literature on this thorny issue continues to evolve. CT is the current standard for detecting bony injuries. MRI is usually reserved for patient in whom a soft-tissue, particularly ligamentous, injury is suspected. MRI is also required for the evaluation of any patient suspected of having sustained spinal cord injury.18 The downside of our increased MRI usage in the evaluation of potentially spine-injured patients has been the detection of many clinically insignificant findings.
Acute cauda equina syndrome is a neurosurgical emergency requiring prompt recognition, imaging, and immediate neurosurgical consultation. Common findings include: recent onset or worsening severe low back pain; bowel and/or bladder dysfunction; neurological deficits; and saddle anesthesia. Many processes can lead to the syndrome, but the most common is disc herniation with resultant cauda equina compression. The American College of Radiology appropriateness criteria cite MRI as the correct imaging modality for the diagnosis of acute cauda equina syndrome.19 In patients who’ve undergone previous herniated disc surgery, MRI with and without contrast must be obtained to differentiate between contrast-enhancing granulation tissue at the site of the surgery and nonenhancing herniated disc tissue.18
Infection is an important item in the differential diagnosis of back pain, with or without radiculopathy, and particularly important to consider if the patient has infectious disease risk factors. These risk factors include: spinal instrumentation via injections or surgery; intravenous drug use; prosthetic heart valves; systemic infections; other infectious sources in the body; and immunocompromising conditions.18 All spinal elements, including the spinal cord, meninges, joints, discs, and vertebrae can be affected. Realize that infection can occur by direct inoculation or contiguous or hematogenous spread. An MRI with and without contrast is essential to confirm the diagnosis.19 Your neurosurgical consultant will likely recommend imaging the entire spinal axis, since infectious lesions may be present at multiple levels.18
Pregnant patients with abdominal pain - concern for appendicitis (see the Cautions and Limitations section above on MRI in pregnancy)
Appendicitis occurs commonly in pregnancy. Missing the diagnosis can lead to fetal loss and other untoward outcomes. The 2018 American College of Radiology guidelines list MRI and ultrasound as imaging studies of choice in gravid patients in whom appendicitis is a concern.20 Ultrasound is more commonly available and less expensive but is limited by high rates of appendiceal non-visualization, likely due to appendix displacement by the uterus, patient habitus, bowel gas, and discomfort during the exam.21
MRI has high sensitivity and very high specificity for the diagnosis of appendicitis. Abnormal diagnostic findings include an appendiceal diameter > 7 mm and surrounding inflammatory changes.22 The low negative predictive value of MRI obviates the need for risky surgeries in pregnant patients in whom appendicitis is ruled out. MRI also allows for the diagnosis of other etiologies of abdominal pain in these patients.21
Pediatric patients with abdominal pain -concern for appendicitis (see the Cautions and Limitations section above on MRI in pediatric patients)
For pediatric patients with possible appendicitis, ultrasound is the first imaging modality of choice, followed by CT. However, ultrasound is operator dependent, with wide variability in its ability to correctly diagnose appendicitis, often leading to equivocal results. CT involves ionizing radiation exposure.20 Non-contrast MRI is the emerging imaging modality for these patients. A systematic review of almost 2000 pediatric patients found MRI sensitivity and specificity to be 97% and 97% with a low negative appendectomy rate.23
Cost and image acquisition time are limitations for MRI use for children. Pediatric patients may require sedation with long acquisition times in order to ensure that high-quality images are obtained, potentially introducing more associated costs and safety concerns. Shorter image-acquisition times would make MRI a more widely applicable test.23
Orthopedics
Various orthopedic conditions can be investigated by MRI, but this is not commonly done in the ED. Acute knee trauma with a concern for ligamentous, cartilaginous, or meniscal injury is one example. The patient with a concern for occult fracture or injury to the shoulder, elbow, or scaphoid represent others.
However, the special case of the patient with hip trauma with negative radiographs who will not weight bear or has significant pain is worth considering. MRI to either diagnose or exclude occult hip, pelvic, or acetabular fracture is traditionally considered to be the criterion standard. However, a 2016 study called this widely-held belief into question. It found that CT and MRI were similarly sensitive and concluded that starting with CT was a reasonable approach.24 MRI can be considered if the diagnosis remains in doubt.
Musculoskeletal infections
A wide variety of bone, joint, and soft-tissue infections can be diagnosed by MRI, which is often the imaging modality of choice. Some of these infections may be limb- or even life-threatening. One, epidural abscess, is both life-threatening and function-threatening and has been discussed briefly already.
If you are concerned about the possibility of a serious soft-tissue or bone infection, strongly consider giving gadolinium contrast, which is particularly useful for detecting abscesses, sinus tracts, and spine infections, and for providing other important anatomic details.25
Conclusion
MRI utilization by EPs will continue to increase as the factors governing its use evolves. These factors include: decreasing scan times; wider availability; possible cost reductions; new and changing indications; more research; and the always-present pressure on EPs to care for a broader spectrum of evermore challenging patients. It therefore benefits us to understand more about this dynamic part of our practice. Look to the scientific literature on stroke, neurosurgical emergencies, orthopedics, pediatrics, infectious disease and other fields that impact emergency medicine practice and MRI use as they continue to change.
SIDEBAR
Summary of Cautions and Limitations of MRI Use
Lack of availability
Cost
Exam completion time
Claustrophobia
Patient’s inability to lie still
Implanted medical devices
Metallic foreign bodies
Obesity
Hearing damage
Pregnancy
Pediatric patients (the developing brain)
Nephrogenic systemic fibrosis due to gadolinium-based contrast agents
SIDEBAR
Common ED MRI indications
Central Nervous System
- Spinal cord compression
- Dural venous sinus thrombosis
- Arterial dissections - carotid or vertebral
- Meningoencephalitis and vasculitis evaluation (possible)
- Subarachnoid hemorrhage (possible)
- Cerebral ischemia - TIA/Stroke
Spinal cord/surrounding structure disease or trauma - epidural abscess, cauda equina syndrome, cord/nerve trauma
Pregnant patients with abdominal pain (concern for appendicitis)
Children with abdominal pain (concern for appendicitis)
Musculoskeletal infections Orthopedic trauma
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.
1. Currie S, Hoggard N, Craven IJ, Hadjivassiliou M, Wilkinson ID. Understanding MRI: Basic MR physics for physicians. Postgrad Med J. 2013;89:209-223.
2. Berger A. How does it work? Magnetic resonance imaging. BMJ. 2002;324:35.
3. Chung M, Dahabreh IJ, Hadar N, et al. Emerging MRI technologies for imaging musculoskeletal disorders under loading stress. Comparative Effectiveness Technical Briefs, No. 7. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.ncbi.nlm.nih.gov/books/NBK82287/
4. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451.
5. MRI Safety. http://www.mrisafety.com/TheList_search.asp.
6. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952-961.
7. Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73-104.
8. Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents—review of literature and guidelines. Insights Imaging. 2015;6(5):553-558.
9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5:1-17.
10. Quint DJ. Indications for emergent MRI of the central nervous system. JAMA. 2000;283(7):853-855.
11. Broder J. Imaging the cervical, thoracic, and lumbar spine. In: Broder J, ed. Diagnostic Imaging for the Emergency Physician. Philadelphia, PA: Elsevier; 2011:73-157.
12. Villringer A, Einhäupl KM. Dural sinus and cerebral venous thrombosis. New Horiz. 1997;5(4):332-341.
13. Ben Hassen W, Machet A, Edjlali-Goujon M, et al. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95(12):1151-1161.
14. Jacobs A, Lanfermann H, Neveling M, Szelies B, Schröder R, Heiss W-D. MRI-and MRI-guided therapy of carotid and vertebral artery dissections. J Neurol Sci. 1997;147(1):27-34.
15. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
16. Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117-127.
17. Lavi ES, Pal A, Bleicher D, Kang K, Sidani C. MR imaging of the spine: Urgent and emergent indications. Semin Ultrasound CT MR. (2018), doi: https://doi.org/10.1053/j.sult.2018.10.006
18. Kawakyu-O’Connor D, Bordia R, Nicola R. Magnetic resonance imaging of spinal emergencies. Magn Reson Imaging Clin N Am. 2016;24(2):325-344.
19. Patel ND, Broderick DF, Burns J, et al. ACR appropriateness criteria low back pain. J Am Coll of Radiol. 2016;13(9):1069-1078.
20. Garcia EM, Camacho MA, Karolyi DR, et al. ACR appropriateness criteria -right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15(11):S373-S387.
21. Duke E, Kalb B, Arif-Tiwari H, et al. A systematic review and meta-analysis of diagnostic performance of MRI for evaluation of acute appendicitis. AJR Am J Roentgenol. 2016;206(3):508-517.
22. Yu HS, Gupta A, Soto JA, et al. Emergency abdominal MRI: Current uses and trends. Br J Radiol. 2016;89(1061). doi:10.1259/bjr.20150804
23. Kim JR, Suh CH, Yoon HM, et al. Performance of MRI for suspected appendicitis in pediatric patients and negative appendectomy rate: A systematic review and meta-analysis. J Magn Reson Imaging. 2017;47(3):767-778.
24. Rehman H, Clement RG, Perks F, White TO. Imaging of occult hip fractures: CT or MRI? Injury. 2016;47(6):1297-1301.
25. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin N Am. 2017;31:299-324.