User login
Gastroparesis in a patient with diabetic ketoacidosis
A 40-year-old man with type 1 diabetes mellitus and recurrent renal calculi presented to the emergency department with nausea, vomiting, and abdominal pain for the past day. He had been checking his blood glucose level regularly, and it had usually been within the normal range until 2 or 3 days previously, when he stopped taking his insulin because he ran out and could not afford to buy more.
He said he initially vomited clear mucus but then had 2 episodes of black vomit. His abdominal pain was diffuse but more intense in his flanks. He said he had never had nausea or vomiting before this episode.
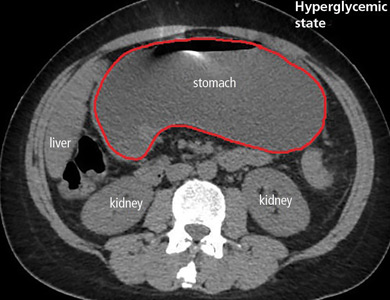
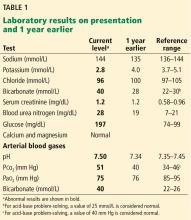
In the emergency department, his heart rate was 136 beats per minute and respiratory rate 24 breaths per minute. He appeared to be in mild distress, and physical examination revealed a distended abdomen, decreased bowel sounds on auscultation, tympanic sound elicited by percussion, and diffuse abdominal tenderness to palpation without rebound tenderness or rigidity. His blood glucose level was 993 mg/dL, and his anion gap was 36 mmol/L.
The patient was treated with hydration, insulin, and a nasogastric tube to relieve the pressure. The following day, his symptoms had significantly improved, his abdomen was less distended, his bowel sounds had returned, and his plasma glucose levels were in the normal range. The nasogastric tube was removed after he started to have bowel movements; he was given liquids by mouth and eventually solid food. Since his condition had significantly improved and he had started to have bowel movements, no follow-up imaging was done. The next day, he was symptom-free, his laboratory values were normal, and he was discharged home.
GASTROPARESIS
Gastroparesis is defined by delayed gastric emptying in the absence of a mechanical obstruction, with symptoms of nausea, vomiting, bloating, and abdominal pain. Most commonly it is idiopathic or caused by long-standing uncontrolled diabetes.
Diabetic gastroparesis is thought to result from impaired neural control of gastric function. Damage to the pacemaker interstitial cells of Cajal and underlying smooth muscle may be contributing factors.1 It is usually chronic, with a mean duration of symptoms of 26.5 months.2 However, acute gastroparesis can occur after an acute elevation in the plasma glucose concentration, which can affect gastric sensory and motor function3 via relaxation of the proximal stomach, decrease in antral pressure waves, and increase in pyloric pressure waves.4
Patients with diabetic ketoacidosis often present with symptoms similar to those of gastroparesis, including nausea, vomiting, and abdominal pain.5 But acute gastroparesis can coexist with diabetic ketoacidosis, as in our patient, and the gastroparesis can go undiagnosed, since imaging studies are not routinely done for diabetic ketoacidosis unless there is another reason—as in our patient.
More study is needed to answer questions on long-term outcomes for patients presenting with acute gastroparesis: Do they develop chronic gastroparesis? And is there is a correlation with progression of neuropathy?
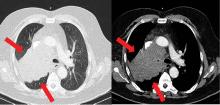
The diagnosis usually requires a high level of suspicion in patients with nausea, vomiting, fullness, abdominal pain, and bloating; exclusion of gastric outlet obstruction by a mass or antral stenosis; and evidence of delayed gastric emptying. Gastric outlet obstruction can be ruled out by endoscopy, abdominal CT, or magnetic resonance enterography. Delayed gastric emptying can be quantified with scintigraphy and endoscopy. In our patient, gastroparesis was diagnosed on the basis of the clinical symptoms and CT findings.
Treatment is usually directed at symptoms, with better glycemic control and dietary modification for moderate cases, and prokinetics and a gastrostomy tube for severe cases.
TAKE-HOME POINTS
- Gastroparesis is usually chronic but can present acutely with acute severe hyperglycemia.
- Gastrointestinal tract motor function is affected by plasma glucose levels and can change over brief intervals.
- Diabetic ketoacidosis symptoms can mask acute gastroparesis, as imaging studies are not routinely done.
- Acute gastroparesis can be diagnosed clinically along with abdominal CT or endoscopy to rule out gastric outlet obstruction.
- Acute gastroparesis caused by diabetic ketoacidosis can resolve promptly with tight control of plasma glucose levels, anion gap closing, and nasogastric tube placement.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127(5):1592–1622. pmid:15521026
- Dudekula A, O’Connell M, Bielefeldt K. Hospitalizations and testing in gastroparesis. J Gastroenterol Hepatol 2011; 26(8):1275–1282. doi:10.1111/j.1440-1746.2011.06735.x
- Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990; 33(11):675–680. pmid:2076799
- Mearin F, Malagelada JR. Gastroparesis and dyspepsia in patients with diabetes mellitus. Eur J Gastroenterol Hepatol 1995; 7(8):717–723. pmid:7496857
- Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc 1992; 40(11):1100–1104. pmid:1401693
A 40-year-old man with type 1 diabetes mellitus and recurrent renal calculi presented to the emergency department with nausea, vomiting, and abdominal pain for the past day. He had been checking his blood glucose level regularly, and it had usually been within the normal range until 2 or 3 days previously, when he stopped taking his insulin because he ran out and could not afford to buy more.
He said he initially vomited clear mucus but then had 2 episodes of black vomit. His abdominal pain was diffuse but more intense in his flanks. He said he had never had nausea or vomiting before this episode.
In the emergency department, his heart rate was 136 beats per minute and respiratory rate 24 breaths per minute. He appeared to be in mild distress, and physical examination revealed a distended abdomen, decreased bowel sounds on auscultation, tympanic sound elicited by percussion, and diffuse abdominal tenderness to palpation without rebound tenderness or rigidity. His blood glucose level was 993 mg/dL, and his anion gap was 36 mmol/L.
The patient was treated with hydration, insulin, and a nasogastric tube to relieve the pressure. The following day, his symptoms had significantly improved, his abdomen was less distended, his bowel sounds had returned, and his plasma glucose levels were in the normal range. The nasogastric tube was removed after he started to have bowel movements; he was given liquids by mouth and eventually solid food. Since his condition had significantly improved and he had started to have bowel movements, no follow-up imaging was done. The next day, he was symptom-free, his laboratory values were normal, and he was discharged home.
GASTROPARESIS
Gastroparesis is defined by delayed gastric emptying in the absence of a mechanical obstruction, with symptoms of nausea, vomiting, bloating, and abdominal pain. Most commonly it is idiopathic or caused by long-standing uncontrolled diabetes.
Diabetic gastroparesis is thought to result from impaired neural control of gastric function. Damage to the pacemaker interstitial cells of Cajal and underlying smooth muscle may be contributing factors.1 It is usually chronic, with a mean duration of symptoms of 26.5 months.2 However, acute gastroparesis can occur after an acute elevation in the plasma glucose concentration, which can affect gastric sensory and motor function3 via relaxation of the proximal stomach, decrease in antral pressure waves, and increase in pyloric pressure waves.4
Patients with diabetic ketoacidosis often present with symptoms similar to those of gastroparesis, including nausea, vomiting, and abdominal pain.5 But acute gastroparesis can coexist with diabetic ketoacidosis, as in our patient, and the gastroparesis can go undiagnosed, since imaging studies are not routinely done for diabetic ketoacidosis unless there is another reason—as in our patient.
More study is needed to answer questions on long-term outcomes for patients presenting with acute gastroparesis: Do they develop chronic gastroparesis? And is there is a correlation with progression of neuropathy?
The diagnosis usually requires a high level of suspicion in patients with nausea, vomiting, fullness, abdominal pain, and bloating; exclusion of gastric outlet obstruction by a mass or antral stenosis; and evidence of delayed gastric emptying. Gastric outlet obstruction can be ruled out by endoscopy, abdominal CT, or magnetic resonance enterography. Delayed gastric emptying can be quantified with scintigraphy and endoscopy. In our patient, gastroparesis was diagnosed on the basis of the clinical symptoms and CT findings.
Treatment is usually directed at symptoms, with better glycemic control and dietary modification for moderate cases, and prokinetics and a gastrostomy tube for severe cases.
TAKE-HOME POINTS
- Gastroparesis is usually chronic but can present acutely with acute severe hyperglycemia.
- Gastrointestinal tract motor function is affected by plasma glucose levels and can change over brief intervals.
- Diabetic ketoacidosis symptoms can mask acute gastroparesis, as imaging studies are not routinely done.
- Acute gastroparesis can be diagnosed clinically along with abdominal CT or endoscopy to rule out gastric outlet obstruction.
- Acute gastroparesis caused by diabetic ketoacidosis can resolve promptly with tight control of plasma glucose levels, anion gap closing, and nasogastric tube placement.
A 40-year-old man with type 1 diabetes mellitus and recurrent renal calculi presented to the emergency department with nausea, vomiting, and abdominal pain for the past day. He had been checking his blood glucose level regularly, and it had usually been within the normal range until 2 or 3 days previously, when he stopped taking his insulin because he ran out and could not afford to buy more.
He said he initially vomited clear mucus but then had 2 episodes of black vomit. His abdominal pain was diffuse but more intense in his flanks. He said he had never had nausea or vomiting before this episode.
In the emergency department, his heart rate was 136 beats per minute and respiratory rate 24 breaths per minute. He appeared to be in mild distress, and physical examination revealed a distended abdomen, decreased bowel sounds on auscultation, tympanic sound elicited by percussion, and diffuse abdominal tenderness to palpation without rebound tenderness or rigidity. His blood glucose level was 993 mg/dL, and his anion gap was 36 mmol/L.
The patient was treated with hydration, insulin, and a nasogastric tube to relieve the pressure. The following day, his symptoms had significantly improved, his abdomen was less distended, his bowel sounds had returned, and his plasma glucose levels were in the normal range. The nasogastric tube was removed after he started to have bowel movements; he was given liquids by mouth and eventually solid food. Since his condition had significantly improved and he had started to have bowel movements, no follow-up imaging was done. The next day, he was symptom-free, his laboratory values were normal, and he was discharged home.
GASTROPARESIS
Gastroparesis is defined by delayed gastric emptying in the absence of a mechanical obstruction, with symptoms of nausea, vomiting, bloating, and abdominal pain. Most commonly it is idiopathic or caused by long-standing uncontrolled diabetes.
Diabetic gastroparesis is thought to result from impaired neural control of gastric function. Damage to the pacemaker interstitial cells of Cajal and underlying smooth muscle may be contributing factors.1 It is usually chronic, with a mean duration of symptoms of 26.5 months.2 However, acute gastroparesis can occur after an acute elevation in the plasma glucose concentration, which can affect gastric sensory and motor function3 via relaxation of the proximal stomach, decrease in antral pressure waves, and increase in pyloric pressure waves.4
Patients with diabetic ketoacidosis often present with symptoms similar to those of gastroparesis, including nausea, vomiting, and abdominal pain.5 But acute gastroparesis can coexist with diabetic ketoacidosis, as in our patient, and the gastroparesis can go undiagnosed, since imaging studies are not routinely done for diabetic ketoacidosis unless there is another reason—as in our patient.
More study is needed to answer questions on long-term outcomes for patients presenting with acute gastroparesis: Do they develop chronic gastroparesis? And is there is a correlation with progression of neuropathy?
The diagnosis usually requires a high level of suspicion in patients with nausea, vomiting, fullness, abdominal pain, and bloating; exclusion of gastric outlet obstruction by a mass or antral stenosis; and evidence of delayed gastric emptying. Gastric outlet obstruction can be ruled out by endoscopy, abdominal CT, or magnetic resonance enterography. Delayed gastric emptying can be quantified with scintigraphy and endoscopy. In our patient, gastroparesis was diagnosed on the basis of the clinical symptoms and CT findings.
Treatment is usually directed at symptoms, with better glycemic control and dietary modification for moderate cases, and prokinetics and a gastrostomy tube for severe cases.
TAKE-HOME POINTS
- Gastroparesis is usually chronic but can present acutely with acute severe hyperglycemia.
- Gastrointestinal tract motor function is affected by plasma glucose levels and can change over brief intervals.
- Diabetic ketoacidosis symptoms can mask acute gastroparesis, as imaging studies are not routinely done.
- Acute gastroparesis can be diagnosed clinically along with abdominal CT or endoscopy to rule out gastric outlet obstruction.
- Acute gastroparesis caused by diabetic ketoacidosis can resolve promptly with tight control of plasma glucose levels, anion gap closing, and nasogastric tube placement.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127(5):1592–1622. pmid:15521026
- Dudekula A, O’Connell M, Bielefeldt K. Hospitalizations and testing in gastroparesis. J Gastroenterol Hepatol 2011; 26(8):1275–1282. doi:10.1111/j.1440-1746.2011.06735.x
- Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990; 33(11):675–680. pmid:2076799
- Mearin F, Malagelada JR. Gastroparesis and dyspepsia in patients with diabetes mellitus. Eur J Gastroenterol Hepatol 1995; 7(8):717–723. pmid:7496857
- Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc 1992; 40(11):1100–1104. pmid:1401693
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127(5):1592–1622. pmid:15521026
- Dudekula A, O’Connell M, Bielefeldt K. Hospitalizations and testing in gastroparesis. J Gastroenterol Hepatol 2011; 26(8):1275–1282. doi:10.1111/j.1440-1746.2011.06735.x
- Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990; 33(11):675–680. pmid:2076799
- Mearin F, Malagelada JR. Gastroparesis and dyspepsia in patients with diabetes mellitus. Eur J Gastroenterol Hepatol 1995; 7(8):717–723. pmid:7496857
- Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc 1992; 40(11):1100–1104. pmid:1401693
Is neuroimaging necessary to evaluate syncope?
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
Patient selection for acute stroke thrombectomy stirs controversy
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
HONOLULU – A little more than a year ago, results from the DAWN and DEFUSE 3 trials substantially broadened the time window for endovascular thrombectomy of acute ischemic stroke by selecting patients using brain imaging. Stroke clinicians are now trying to reconcile widespread, routine use of this life-changing treatment against an uncertain need to replicate the higher-end perfusion CT and analytical software imaging that these landmark trials used for patient selection. This has produced a schism in what experts advise for using endovascular thrombectomy on acute ischemic stroke patients.
“Go open the artery, people!” Michael D. Hill, MD, exhorted during a talk at the International Stroke Conference, sponsored by the American Heart Association. “Don’t get over-selective; more people will benefit than you think,” said Dr. Hill, a professor of clinical neurosciences at the University of Calgary (Alta.).
“We are over-selecting, and depriving patients,” commented Raul C. Nogueira, MD, professor of neurology at Emory University in Atlanta and a lead investigator of the DAWN trial, speaking from the audience during a discussion at the session where Dr. Hill spoke.
“The prevalence of treatable [acute ischemic stroke] patients is far higher than the prevalence of patients who are not good candidates, so you just want to exclude the ‘wipe-outs’; that’s what we do,” Dr. Hill explained. “Fortunately, endovascular therapy is very safe, and you’re not going to harm many patients. With other treatments [used routinely in medicine] some patients don’t benefit, but when you have a large effect size we use the treatment on almost everyone. The effect size from thrombectomy is so large it’s an argument to treat almost everyone, although the patients in the trials were selected by imaging.”
Dr. Hill repeatedly stressed that for most patients a non-contrast CT image is usually adequate to identify patients with salvageable brain tissue and a low risk for hemorrhage from intervention, and he endorsed also doing CT angiography to further inform the diagnosis. But he dismissed CT perfusion imaging as unnecessary. “Noncontrast CT and CT angiography are more than adequate to make treatment decisions,” he said. “The prevalence of poor collaterals is quite low, about 10%,” which means that about 90% of acute ischemic stroke patients will have more slowly progressing infarction,” making them amenable to treatment in an expanded time window and boosting the volume of salvageable tissue.
But these appeals for more liberal use of thrombectomy without the perfusion CT imaging used in DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21)and DEFUSE-3 (N Engl J Med. 2018 Feb 22;378[8]:708-18) received push back. Maarten G. Lansberg, MD, a co-investigator on the DEFUSE 3 trial, highlighted the speed and simplicity of CT perfusion imaging, and its utility in helping to better target thrombectomy to the right patients. It’s “speedy, simple, and safe,” it “excludes patients who will not benefit” from thrombectomy, and it helps when the patient’s history and noncontrast CT images are inconclusive, said Dr. Lansberg, a neurologist at Stanford (Calif.) University.
“Clinical presentation will only tell you so much.” With imaging that includes CT perfusion, “you can find out, in 5, 10 minutes, whether there is an occlusion, its location, the extent of dead tissue – that’s all really helpful,” said Marc Fisher, MD, professor of neurology at Harvard Medical School in Boston. “There is a tension now between doing treatment really fast and the concept of slow and fast evolvers. For slow evolvers, the concern about speed is irrelevant because it can take days” for their brains to have substantial damage. “For the fast evolvers, time matters, but they could also possibly be harmed; that’s why we need more data.”
A pitch for more data also came from Pooja Khatri, MD, who also spoke at the session. “There is a real tension now between personalizing the imaging and figuring out exactly the right patients against the time trade off for doing that. Some argue to keep it simple and move fast, and by doing that you’ll wash out any difference from doing more fancy stuff. Plus some places, even in developed countries, can’t afford the image-processing software” used in the DAWN and DEFUSE 3 trials. The correct approach remains unclear and has created “an area ripe for a trial,” declared Dr. Khatri, professor of neurology at director of acute stroke at the University of Cincinnati.
Dr. Hill has received honoraria from Merck and received research funding from Boehringer Ingelheim, Covidien, Medtronic, and Stryker. He has an ownership interest in Calgary Scientific and holds a patent on acute stroke triage methods. Dr. Nogueira has financial relationships with many companies. Dr. Lansberg and Dr. Fisher had no disclosures. Dr. Khatri has been a consultant to Lumosa and has received research funding from Cerenovus/Johnson & Johnson, Genentech, and Nervive.
REPORTING FROM ISC 2019
Developing clinical mastery at HM19
Boosting your bedside diagnostic skills
A new three-session minitrack devoted to the clinical mastery of diagnostic and treatment skills at the hospitalized patient’s bedside should be a highlight of the Society of Hospital Medicine’s 2019 annual conference.
The “Clinical Mastery” track is designed to help hospitalists enhance their skills in making expert diagnoses at the bedside, said Dustin T. Smith, MD, SFHM, course director for HM19, and associate professor of medicine at Emory University, Atlanta. “We feel that all of the didactic sessions offered at HM19 are highly useful for hospitalists, but there is growing interest in having sessions devoted to learning clinical pearls that can aid in practicing medicine and acquiring the skill set of a master clinician.”
The three clinical mastery sessions at HM19 will address neurologic symptoms, ECG interpretation, and the role of point-of-care ultrasound (POCUS), currently a hot topic in hospital medicine. Recent advances in ultrasound technology have resulted in probes that can cost as little as $2,000, fit inside a lab coat pocket, and be read from a smartphone – making ultrasound far easier to bring to the bedside of hospitalized patients, said Ria Dancel, MD, FHM, associate professor of internal medicine and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Dancel will copresent the POCUS clinical mastery track at HM19. “Our focus will be on how POCUS and the physical exam relate to each other. These are not competing technologies but complementary, reflecting the evolution in bedside medicine. Because these new devices will soon be in the pockets of your colleagues, residents, physician assistants, and others, you should at least have the knowledge and vocabulary to communicate with them,” she said.
POCUS is a new technology that is not yet in wide use at the hospital bedside, but clearly a wave is building, said Dr. Dancel’s copresenter, Michael Janjigian, MD, associate professor in the department of medicine at NYU Langone Health in New York City.
“We’re at the inflection point where the cost of the machine and the availability of training means that hospitals need to decide if it’s time to embrace it,” he said. Hospitalists may also consider petitioning their hospital’s leadership to offer the machines and training.
“Hospitalists’ competencies and strengths lie primarily in making diagnoses,” Dr. Janjigian said. “We like to think of ourselves as master diagnosticians. Our session at HM19 will explore the strengths and weaknesses of both the physical exam and POCUS, presenting clinical scenarios common to hospital medicine. This course is designed for those who have never picked up an ultrasound probe and want to better understand why they should, and for those who want a better sense of how they might integrate it into their practice.”
While radiology and cardiology have been using ultrasound for decades, internists are finding uses at the bedside to speed diagnosis or focus their next diagnostic steps, Dr. Dancel noted. For certain diagnoses, the physical exam is still the tool of choice. But when looking for fluid around the heart or ascites buildup in the abdomen or when looking at the heart itself, she said, there is no better tool at the bedside than ultrasound.
In January 2019, the SHM issued a position statement on POCUS1, which is intended to inform hospitalists about the technology and its uses, encourage them to be more integrally involved in decision making processes surrounding POCUS program management for their hospitals, and promote development of standards for hospitalists in POCUS training and assessment. The SHM has also developed a pathway to teach the use of ultrasound, the Point-of-Care Ultrasound Certificate of Completion.
In order to qualify, clinicians complete online training modules, attend two live learning courses, compile a portfolio of ultrasound video clips on the job that are reviewed by a panel of experts, and then pass a final exam. The exam will be offered at HM19 for clinicians who have completed preliminary work for this new certificate – as well as precourses devoted to ultrasound and other procedures – and another workshop on POCUS.
Earning the POCUS certificate of completion requires a lot of effort, Dr. Dancel acknowledged. “It is a big commitment, and we don’t want hospitalists thinking that just because they have completed the certificate that they have fully mastered ultrasound. We encourage hospitalists to find a proctor in their own hospitals and to work with them to continue to refine their skills.”
Dr. Dancel and Dr. Janjigian reported no relevant disclosures.
References
1. Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2. doi: 10.12788/jhm.3079.
Boosting your bedside diagnostic skills
Boosting your bedside diagnostic skills
A new three-session minitrack devoted to the clinical mastery of diagnostic and treatment skills at the hospitalized patient’s bedside should be a highlight of the Society of Hospital Medicine’s 2019 annual conference.
The “Clinical Mastery” track is designed to help hospitalists enhance their skills in making expert diagnoses at the bedside, said Dustin T. Smith, MD, SFHM, course director for HM19, and associate professor of medicine at Emory University, Atlanta. “We feel that all of the didactic sessions offered at HM19 are highly useful for hospitalists, but there is growing interest in having sessions devoted to learning clinical pearls that can aid in practicing medicine and acquiring the skill set of a master clinician.”
The three clinical mastery sessions at HM19 will address neurologic symptoms, ECG interpretation, and the role of point-of-care ultrasound (POCUS), currently a hot topic in hospital medicine. Recent advances in ultrasound technology have resulted in probes that can cost as little as $2,000, fit inside a lab coat pocket, and be read from a smartphone – making ultrasound far easier to bring to the bedside of hospitalized patients, said Ria Dancel, MD, FHM, associate professor of internal medicine and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Dancel will copresent the POCUS clinical mastery track at HM19. “Our focus will be on how POCUS and the physical exam relate to each other. These are not competing technologies but complementary, reflecting the evolution in bedside medicine. Because these new devices will soon be in the pockets of your colleagues, residents, physician assistants, and others, you should at least have the knowledge and vocabulary to communicate with them,” she said.
POCUS is a new technology that is not yet in wide use at the hospital bedside, but clearly a wave is building, said Dr. Dancel’s copresenter, Michael Janjigian, MD, associate professor in the department of medicine at NYU Langone Health in New York City.
“We’re at the inflection point where the cost of the machine and the availability of training means that hospitals need to decide if it’s time to embrace it,” he said. Hospitalists may also consider petitioning their hospital’s leadership to offer the machines and training.
“Hospitalists’ competencies and strengths lie primarily in making diagnoses,” Dr. Janjigian said. “We like to think of ourselves as master diagnosticians. Our session at HM19 will explore the strengths and weaknesses of both the physical exam and POCUS, presenting clinical scenarios common to hospital medicine. This course is designed for those who have never picked up an ultrasound probe and want to better understand why they should, and for those who want a better sense of how they might integrate it into their practice.”
While radiology and cardiology have been using ultrasound for decades, internists are finding uses at the bedside to speed diagnosis or focus their next diagnostic steps, Dr. Dancel noted. For certain diagnoses, the physical exam is still the tool of choice. But when looking for fluid around the heart or ascites buildup in the abdomen or when looking at the heart itself, she said, there is no better tool at the bedside than ultrasound.
In January 2019, the SHM issued a position statement on POCUS1, which is intended to inform hospitalists about the technology and its uses, encourage them to be more integrally involved in decision making processes surrounding POCUS program management for their hospitals, and promote development of standards for hospitalists in POCUS training and assessment. The SHM has also developed a pathway to teach the use of ultrasound, the Point-of-Care Ultrasound Certificate of Completion.
In order to qualify, clinicians complete online training modules, attend two live learning courses, compile a portfolio of ultrasound video clips on the job that are reviewed by a panel of experts, and then pass a final exam. The exam will be offered at HM19 for clinicians who have completed preliminary work for this new certificate – as well as precourses devoted to ultrasound and other procedures – and another workshop on POCUS.
Earning the POCUS certificate of completion requires a lot of effort, Dr. Dancel acknowledged. “It is a big commitment, and we don’t want hospitalists thinking that just because they have completed the certificate that they have fully mastered ultrasound. We encourage hospitalists to find a proctor in their own hospitals and to work with them to continue to refine their skills.”
Dr. Dancel and Dr. Janjigian reported no relevant disclosures.
References
1. Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2. doi: 10.12788/jhm.3079.
A new three-session minitrack devoted to the clinical mastery of diagnostic and treatment skills at the hospitalized patient’s bedside should be a highlight of the Society of Hospital Medicine’s 2019 annual conference.
The “Clinical Mastery” track is designed to help hospitalists enhance their skills in making expert diagnoses at the bedside, said Dustin T. Smith, MD, SFHM, course director for HM19, and associate professor of medicine at Emory University, Atlanta. “We feel that all of the didactic sessions offered at HM19 are highly useful for hospitalists, but there is growing interest in having sessions devoted to learning clinical pearls that can aid in practicing medicine and acquiring the skill set of a master clinician.”
The three clinical mastery sessions at HM19 will address neurologic symptoms, ECG interpretation, and the role of point-of-care ultrasound (POCUS), currently a hot topic in hospital medicine. Recent advances in ultrasound technology have resulted in probes that can cost as little as $2,000, fit inside a lab coat pocket, and be read from a smartphone – making ultrasound far easier to bring to the bedside of hospitalized patients, said Ria Dancel, MD, FHM, associate professor of internal medicine and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Dancel will copresent the POCUS clinical mastery track at HM19. “Our focus will be on how POCUS and the physical exam relate to each other. These are not competing technologies but complementary, reflecting the evolution in bedside medicine. Because these new devices will soon be in the pockets of your colleagues, residents, physician assistants, and others, you should at least have the knowledge and vocabulary to communicate with them,” she said.
POCUS is a new technology that is not yet in wide use at the hospital bedside, but clearly a wave is building, said Dr. Dancel’s copresenter, Michael Janjigian, MD, associate professor in the department of medicine at NYU Langone Health in New York City.
“We’re at the inflection point where the cost of the machine and the availability of training means that hospitals need to decide if it’s time to embrace it,” he said. Hospitalists may also consider petitioning their hospital’s leadership to offer the machines and training.
“Hospitalists’ competencies and strengths lie primarily in making diagnoses,” Dr. Janjigian said. “We like to think of ourselves as master diagnosticians. Our session at HM19 will explore the strengths and weaknesses of both the physical exam and POCUS, presenting clinical scenarios common to hospital medicine. This course is designed for those who have never picked up an ultrasound probe and want to better understand why they should, and for those who want a better sense of how they might integrate it into their practice.”
While radiology and cardiology have been using ultrasound for decades, internists are finding uses at the bedside to speed diagnosis or focus their next diagnostic steps, Dr. Dancel noted. For certain diagnoses, the physical exam is still the tool of choice. But when looking for fluid around the heart or ascites buildup in the abdomen or when looking at the heart itself, she said, there is no better tool at the bedside than ultrasound.
In January 2019, the SHM issued a position statement on POCUS1, which is intended to inform hospitalists about the technology and its uses, encourage them to be more integrally involved in decision making processes surrounding POCUS program management for their hospitals, and promote development of standards for hospitalists in POCUS training and assessment. The SHM has also developed a pathway to teach the use of ultrasound, the Point-of-Care Ultrasound Certificate of Completion.
In order to qualify, clinicians complete online training modules, attend two live learning courses, compile a portfolio of ultrasound video clips on the job that are reviewed by a panel of experts, and then pass a final exam. The exam will be offered at HM19 for clinicians who have completed preliminary work for this new certificate – as well as precourses devoted to ultrasound and other procedures – and another workshop on POCUS.
Earning the POCUS certificate of completion requires a lot of effort, Dr. Dancel acknowledged. “It is a big commitment, and we don’t want hospitalists thinking that just because they have completed the certificate that they have fully mastered ultrasound. We encourage hospitalists to find a proctor in their own hospitals and to work with them to continue to refine their skills.”
Dr. Dancel and Dr. Janjigian reported no relevant disclosures.
References
1. Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2. doi: 10.12788/jhm.3079.
Assessing liver fibrosis without biopsy in patients with HCV or NAFLD
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
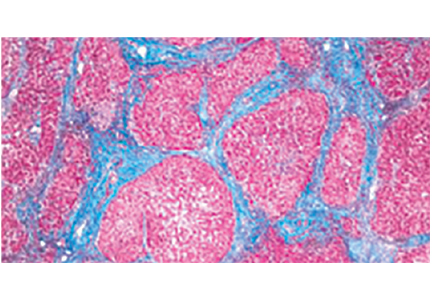
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
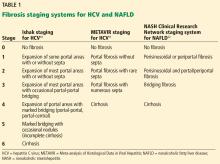
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
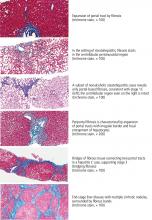
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
KEY POINTS
- Liver biopsy remains the gold standard for determining fibrosis stage but is expensive and entails risk of complications.
- For patients infected with HCV, fibrosis stage should be determined with transient elastography, a transthoracic ultrasonographic technique that measures shear-wave velocity.
- For patients with cirrhosis, transient elastography combined with a platelet count can detect developing portal hypertension and determine whether to screen for esophageal varices.
- For NAFLD, combined elastography and NAFLD fibrosis score—which incorporates patient characteristics and laboratory test results—should be used to determine the need for liver biopsy.
Flu or strep? Rapid tests can mislead
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
A 62-year-old woman presented to our emergency department with fever, chills, hoarseness, pain on swallowing, and a painful neck. Her symptoms had begun 1 day earlier. Because acetaminophen brought no improvement, she went to an urgent care facility, where a nasal swab polymerase chain reaction test was positive for influenza A, and a throat swab rapid test was positive for group A streptococci. She was then referred to our emergency department.
She reported no pre-existing conditions predisposing her to infection. Her temperature was 99.9°F (37.7°C), pulse 112 beats per minute, and respiratory rate 24 breaths per minute. The physical examination was unremarkable except for bilateral anterior cervical adenopathy and bilateral anterior neck tenderness. Her pharynx was not injected, and no exudate, palatal edema, or petechiae were noted.
Results of initial laboratory testing were as follows:
- White blood cell count 20.5 × 109/L (reference range 3.9–11)
- Neutrophils 76% (42%–75%)
- Bands 15% (0%–5%)
- Lymphocytes 3% (21%–51%)
- Erythrocyte sedimentation rate 75 mm/h (< 20 mm/h)
- C-reactive protein 247.14 mg/L (≤ 3 mg/L)
- Serum aminotransferase levels were normal.
- Polymerase chain reaction testing of a nasal swab was negative for viral infection.
Throat swabs and blood samples were sent for culture.
She was started on ceftriaxone 1 g intravenously every 24 hours, with close observation in the medical intensive care unit, where she was admitted because of epiglottitis. On hospital day 3, the throat culture was reported as negative, but the blood culture was reported as positive for Haemophilus influenzae. Thus, the clinical diagnosis was acute epiglottitis due to H influenzae, not group A streptococci.
The patient completed 10 days of ceftriaxone therapy; her recovery was uneventful, and she was discharged on hospital day 10.
INFLUENZA: CHALLENGES TO PROMPT, ACCURATE DIAGNOSIS
During influenza season, emergency departments are inundated with adults with influenza A and other viral respiratory infections. This makes prompt, accurate diagnosis a challenge,1 given the broad differential diagnosis.2,3 Adults with influenza and its complications as well as unrelated conditions can present a special challenge.4
Our patient presented with acute-onset influenza A and was then found to have acute epiglottitis, an unexpected complication of influenza A.5 A positive rapid test for group A streptococci done at an urgent care facility led emergency department physicians to assume that the acute epiglottitis was due to group A streptococci. Unless correlated with clinical findings, results of rapid diagnostic tests may mislead the unwary practitioner. Accurate diagnosis should be based mainly on the history and physical findings. Results of rapid diagnostic tests can be helpful if interpreted in the clinical context.6–8
The rapid test for streptococci is appropriate for the diagnosis of pharyngitis due to group A streptococci in people under age 30 with acute-onset sore throat, fever, and bilateral acute cervical adenopathy, without fatigue or myalgias. However, the rapid test does not differentiate colonization from infection. Group A streptococci are common colonizers with viral pharyngitis. In 30% of cases of Epstein-Barr virus pharyngitis, there is colonization with group A streptococci. A positive rapid test in such cases can result in the wrong diagnosis, ie, pharyngitis due to group A streptococci rather than Epstein-Barr virus.
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
- Cunha BA. The clinical diagnosis of severe viral influenza A. Infection 2008; 36(1):92–93. doi:10.1007/s15010-007-7255-9
- Cunha BA, Klein NC, Strollo S, Syed U, Mickail N, Laguerre M. Legionnaires’ disease mimicking swine influenza (H1N1) pneumonia during the “herald wave” of the pandemic. Heart Lung 2010; 39(3):242–248. doi:10.1016/j.hrtlng.2009.10.009
- Cunha BA, Raza M. During influenza season: all influenza-like illnesses are not due to influenza: dengue mimicking influenza. J Emerg Med 2015; 48(5):e117–e120. doi:10.1016/j.jemermed.2014.12.051
- Cunha CB. Infectious disease differential diagnosis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:493–526.
- Cunha BA. Pharyngitis. In: Cunha CB, Cunha BA, eds. Antibiotic Essentials. Jaypee Brothers Medical Pub: New Delhi, India; 2017:42–47.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis 2013; 32(6):787–793. doi:10.1007/s10096-012-1809-1
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Ann Emerg Med 2001; 38(6):648–652. doi:10.1067/mem.2001.119850
- Cunha BA. A positive rapid strep test in a young adult with acute pharyngitis: be careful what you wish for! IDCases 2017; 10:58–59. doi:10.1016/j.idcr.2017.08.012
A paraneoplastic potassium and acid-base disturbance
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
Cancer screening: A modest proposal for prevention
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
Physiological versus pathological cardiac remodeling in athletes
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Functional MRI detects consciousness after brain damage
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.
Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
FROM SCIENCE ADVANCES
Key clinical point:
Major finding: A brain-wide coordination pattern of high complexity became increasingly common when moving from patients with unresponsive wakefulness syndrome (UWS) to patients in a minimally conscious state (MCS) to healthy control individuals.
Study details: A study involving blood oxygen level–dependent (BOLD) fMRI scans at rest or under anesthesia in 159 participants at four independent research facilities.
Disclosures: The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
Source: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.