User login
Common benign breast concerns for the primary care physician
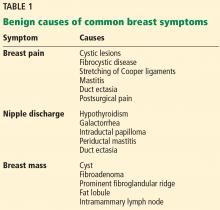
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
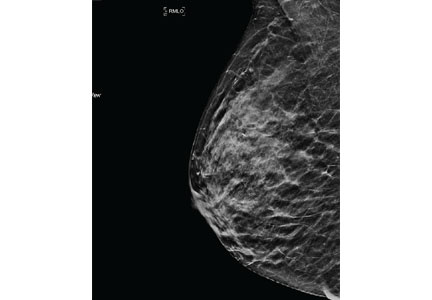
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
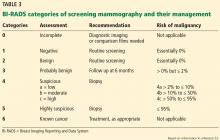
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
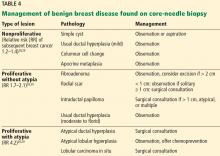
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
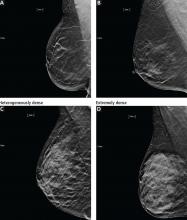
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
KEY POINTS
- The two most common breast symptoms are lumps and pain.
- Most breast problems are not caused by cancer.
- Evaluation of any breast problem begins with a focused history followed by a breast examination and, when necessary, imaging.
- If the results of the breast examination and imaging suggest a benign cause, no further follow-up is necessary.
- If there is discordance between imaging and breast examination results, or if there is a high clinical suspicion of cancer, then consider serial follow-up examinations at short intervals, referral to a breast surgeon for excision, or both.
Screening mammography starting at age 40: Still relevant
Screening mammography is not a perfect test, but it still plays an important role for women even in their 40s, when the incidence of breast cancer is low but the risk of a tumor being aggressive is especially high.
SCREENING DETECTS CANCER EARLY
The goal of screening mammography is to reduce breast cancer deaths by detecting cancers early, when treatment is more effective and less harmful.
Mammography detects tumors when they are smaller: the median size of breast cancers found with high-quality, two-view screening mammography is 1.0 to 1.5 cm, whereas cancers found by palpation are 2.0 to 2.5 cm.1 In general, tumors found when they are smaller require less treatment, and patients are more likely to survive.
Moreover, about 10% of invasive cancers smaller than 1 cm have spread to lymph nodes at the time of detection, compared with 35% of those 2 cm in size and 60% of those 4 cm or larger. Women who have a positive lymph node at the time of diagnosis usually undergo more intensive treatment with chemotherapy and more radical surgery than those who do not. The 5-year disease-free survival rate is more than 98% for breast cancer with a tumor smaller than 2 cm that has not spread to lymph nodes (stage I), compared with 86% for stage II disease (tumors 2.1–5 cm or one to three positive axillary lymph nodes).2
Treating breast cancer early is also less expensive. In a study of women enrolled in a health maintenance organization in Pennsylvania, 14% of those not screened presented with advanced breast cancer (stage III or IV) compared with 2% who had been screened. The cumulative cost of treating advanced breast cancer was two to three times that of treating early breast cancer (stage 0 or I), not accounting for time lost away from work and family, in addition to pain and suffering.3
SCREENING SAVES LIVES
Multiple prospective, randomized controlled trials have been conducted to assess whether inviting women between ages 40 and 74 to undergo screening mammography reduces the rate of death from breast cancer.4,5 Such trials tend to underestimate the effect of screening because not all women invited to be screened actually are screened, and some in the control group may undergo screening on their own.6
The Canadian National Breast Screening Study (NBSS) had additional problems that underestimated the benefits of screening. The quality of mammography came under question, and an issue with randomization became evident after the first round of screening, as the group invited to be screened had an excess of women presenting with palpable lumps and advanced breast cancer.6–8 Despite these issues, a meta-analysis of randomized controlled trials of screening mammography, including the NBSS data, found a 15% reduction in deaths.9 When the NBSS data were excluded, the reduction was 24%.10
In 2009, the United States Preventive Services Task Force (USPSTF)11 recommended against mammographic screening for women ages 40 to 49. Using results from trials including the NBSS, they estimated that the number of women needed to be invited to screening to prevent one breast cancer death was:
- 1,904 for ages 39 to 49
- 1,339 for ages 50 to 59
- 377 for ages 60 to 69.
But if the NBSS study were excluded, these results would be 950, 670, and 377, respectively.6
In a review on screening mammography, Feig12 points out that the USPSTF selected the number of women invited to be screened rather than the number that were actually screened to measure the absolute benefit of screening.
Hendrick and Helvie13 reported that the number of women who needed to be screened to prevent one cancer death was:
- 746 for ages 40 to 49
- 351 for ages 50 to 59
- 253 for ages 60 to 69.
The benefit of screening, if analyzed by number of life years gained rather than number of deaths prevented, is even more favorable to younger women with longer life expectancy. The number needed to be screened per life year gained is:
- 28 at ages 40 to 49
- 17 at ages 50 to 59
- 16 at ages 60 to 69.12
These data provide additional support for screening women starting at age 40.
Observational studies, which provide a better measure of effectiveness because only women who actually undergo routine mammography are compared with those who do not, also support this conclusion. An observational study in Sweden with 20 years of follow-up found that women of all ages who participated in screening had a 44% lower risk of death from breast cancer than with those who were not screened; for women in their 40s, the risk reduction was 48%.14 Similarly, an observational study conducted in British Columbia15 found a 40% decrease in deaths in women screened annually between ages 40 and 79, and a 39% decrease in deaths in women first screened between ages 40 and 49.
LOW RATE OF FALSE-POSITIVE RESULTS
Like many screening programs, screening mammography does not benefit all women equally.
False-positive results occur, for which women need additional imaging or a biopsy for findings that turn out not to be cancer. But the false-positive rate is not high: for every 1,000 women screened in the United States, 80 to 100 (10% or less) are recalled for additional evaluation, 15 (1.5%) undergo biopsy, and 2 to 5 have a cancer, so only about 1% of the women screened underwent an unnecessary biopsy.16
False-positive test results can provoke unnecessary anxiety, but evidence indicates that this tends to be a temporary effect, and even women who had a false-positive result tend to support mammography. In a report by Lerman et al,17 when mood was assessed 3 months after mammography, worry was reported by 26% of women who had had a false-positive report, compared with 9% of women who had had a normal mammogram. Another report addressing the consequences of false-positive mammograms found that although short-term anxiety increased, long-term anxiety did not.18 In a random telephone survey, 98% of adults who reported having had a false-positive cancer screening result stated that they were nevertheless glad that they had undergone screening.19
OVERDIAGNOSIS OCCURS BUT IS LIKELY UNCOMMON
Overdiagnosis of breast cancer is a possible drawback of screening mammography. Cancers may be detected that would not have become clinically apparent in a person’s lifetime20 or have affected ultimate prognosis,18 and so would not have needed to be treated.
Overdiagnosis from screening mammography usually refers to finding ductal carcinoma in situ (DCIS) on breast biopsy. Because no randomized controlled study has been done in which breast cancer was diagnosed and not treated, evidence of the danger from DCIS comes from retrospective reviews of 130 cases in which excised tissue initially interpreted as benign was actually cancerous. Over 10 to 30 years, 11% to 60% of these patients developed invasive breast cancer in the same quadrant from which tissue had been excised.21 This rate of cancer development could lead to underestimation of the invasive potential of DCIS because the patients studied all had low-grade DCIS; further, some of the baseline biopsies involved complete removal of the tumor, thereby preventing the development or progression of cancer.
All DCIS is not the same. An ongoing trial22 found a 5-year recurrence rate of 6.1% after surgery for low-grade or intermediate-grade DCIS, and 15% after surgery for high-grade DCIS. Swedish trials23 have shown that most women who die of “early” breast cancer have high-grade DCIS. These findings suggest that although screening mammography may result in overdiagnosis and overtreatment of low-grade DCIS, high-grade DCIS can be lethal and should be treated. Thus, overdiagnosis likely represents a small fraction of all breast cancers.
Most important, it is not yet possible to accurately predict the biologic behavior of an individual tumor. Current clinical practice is to treat patients with DCIS similar to the way we treat patients with early-stage breast cancer, as we cannot determine which types of DCIS may remain indolent and which ones may become invasive.
HOW FREQUENTLY SHOULD YOUNGER WOMEN BE SCREENED?
The frequency of screening mammography has been another area of controversy, but we believe that annual screening offers the greatest benefit, especially for younger women.
The optimum screening frequency depends on how fast breast cancer grows and spreads. Data suggest that tumors in younger women tend to be biologically aggressive and grow and spread more quickly, making the benefit of yearly mammography more dramatic for younger women. A model based on data from Swedish studies24–26 predicted that the mortality reduction from breast cancer in women ages 40 to 49 would be 36% with annual screening, 18% with screening every 2 years, and 4% with screening every 3 years. For women in their 50s, the model estimated a reduction of 46% for yearly mammography, and 39% and 34% for screening every 2 or 3 years, respectively.6
In a prospective cohort study of the Breast Cancer Surveillance Consortium,27 in women ages 40 to 49 with extremely dense breasts, screening every 2 years was associated with a higher risk of advanced-stage disease (IIb or higher) and large tumors (> 2 cm) than with annual screening. For women ages 50 to 74, screening every 2 years vs every year did not increase the odds of advanced-stage or larger tumors.
AN INFORMED DECISION
In agreement with the current recommendations from the American Cancer Society, the American College of Radiology, and the American Congress of Obstetricians and Gynecologists, we support starting breast cancer screening with mammography at age 40.
Not all cancers are visible on mammography (false negatives), as they may be masked by mammographically dense breast tissue. Women should be informed of the importance of seeking medical attention for breast symptoms, even if mammography is normal. We need to inform women of the benefits and risks of screening mammography, including the risk of false-positive results that could lead to additional imaging and anxiety, and the uncertainties related to the potential for overdiagnosis and overtreatment. This information, offered in an easily understandable format, can help the patient make an informed decision regarding screening mammography, based on her values and preferences.
- Güth U, Huang DJ, Huber M, et al. Tumor size and detection in breast cancer: self-examination and clinical breast examination are at their limit. Cancer Detect Prev 2008; 32:224–228.
- Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD; 2007:101–110. http://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf. Accessed April 9, 2015.
- Legorreta AP, Brooks RJ, Leibowitz AN, Solin LJ. Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med 1996; 156:2197–2201.
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L; Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006; 368:2053–2060.
- Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002; 137:347–360.
- Feig SA. Screening mammography benefit controversies: sorting the evidence. Radiol Clin North Am 2014; 52:455–480.
- Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 1992; 147:1477–1488.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Smart CR, Hendrick RE, Rutledge JH 3rd, Smith RA. Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer 1995; 75:1619–1626.
- Breast-cancer screening with mammography in women aged 40-49 years. Swedish Cancer Society and the Swedish National Board of Health and Welfare. Int J Cancer 1996; 68:693–699.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- Feig SA. Number needed to screen. Appropriate use of this new basis for screening mammography guidelines. AJR Am J Roentgenol 2012; 198:1214–1217.
- Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. AJR Am J Roentgenol 2012; 198:723–728.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003; 361:1405–1410.
- Coldman A, Phillips N, Warren L, Kan L. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer 2007; 120:1076–1080.
- Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006; 241:55–66.
- Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991; 114:657–661.
- Tosteson AN, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med 2014; 174:954–961.
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA 2004; 291:71–78.
- Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer 2013; 108:2205–2240.
- Feig SA. Ductal carcinoma in situ. Implications for screening mammography. Radiol Clin North Am 2000; 38:653–668,
- Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009; 27:5319–5324.
- Tabár L, Vitak B, Chen HH, et al. The Swedish two-county trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000; 38:625–651.
- Duffy SW, Chen HH, Tabar L, et al. Estimation of mean sojourn time in breast cancer screening using a Markov chair model of entry to and exit from the preclinical detectable phase. Stat Med 1995; 14:1521-1534.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part I: tumor attributes and the preclinical screening detectable phase. J Epidemiol Biostat 1997; 2:9–25.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part II: prediction of outcomes for different screening regimes. J Epidemiol Biostat 1997; 2:25–35.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.
Screening mammography is not a perfect test, but it still plays an important role for women even in their 40s, when the incidence of breast cancer is low but the risk of a tumor being aggressive is especially high.
SCREENING DETECTS CANCER EARLY
The goal of screening mammography is to reduce breast cancer deaths by detecting cancers early, when treatment is more effective and less harmful.
Mammography detects tumors when they are smaller: the median size of breast cancers found with high-quality, two-view screening mammography is 1.0 to 1.5 cm, whereas cancers found by palpation are 2.0 to 2.5 cm.1 In general, tumors found when they are smaller require less treatment, and patients are more likely to survive.
Moreover, about 10% of invasive cancers smaller than 1 cm have spread to lymph nodes at the time of detection, compared with 35% of those 2 cm in size and 60% of those 4 cm or larger. Women who have a positive lymph node at the time of diagnosis usually undergo more intensive treatment with chemotherapy and more radical surgery than those who do not. The 5-year disease-free survival rate is more than 98% for breast cancer with a tumor smaller than 2 cm that has not spread to lymph nodes (stage I), compared with 86% for stage II disease (tumors 2.1–5 cm or one to three positive axillary lymph nodes).2
Treating breast cancer early is also less expensive. In a study of women enrolled in a health maintenance organization in Pennsylvania, 14% of those not screened presented with advanced breast cancer (stage III or IV) compared with 2% who had been screened. The cumulative cost of treating advanced breast cancer was two to three times that of treating early breast cancer (stage 0 or I), not accounting for time lost away from work and family, in addition to pain and suffering.3
SCREENING SAVES LIVES
Multiple prospective, randomized controlled trials have been conducted to assess whether inviting women between ages 40 and 74 to undergo screening mammography reduces the rate of death from breast cancer.4,5 Such trials tend to underestimate the effect of screening because not all women invited to be screened actually are screened, and some in the control group may undergo screening on their own.6
The Canadian National Breast Screening Study (NBSS) had additional problems that underestimated the benefits of screening. The quality of mammography came under question, and an issue with randomization became evident after the first round of screening, as the group invited to be screened had an excess of women presenting with palpable lumps and advanced breast cancer.6–8 Despite these issues, a meta-analysis of randomized controlled trials of screening mammography, including the NBSS data, found a 15% reduction in deaths.9 When the NBSS data were excluded, the reduction was 24%.10
In 2009, the United States Preventive Services Task Force (USPSTF)11 recommended against mammographic screening for women ages 40 to 49. Using results from trials including the NBSS, they estimated that the number of women needed to be invited to screening to prevent one breast cancer death was:
- 1,904 for ages 39 to 49
- 1,339 for ages 50 to 59
- 377 for ages 60 to 69.
But if the NBSS study were excluded, these results would be 950, 670, and 377, respectively.6
In a review on screening mammography, Feig12 points out that the USPSTF selected the number of women invited to be screened rather than the number that were actually screened to measure the absolute benefit of screening.
Hendrick and Helvie13 reported that the number of women who needed to be screened to prevent one cancer death was:
- 746 for ages 40 to 49
- 351 for ages 50 to 59
- 253 for ages 60 to 69.
The benefit of screening, if analyzed by number of life years gained rather than number of deaths prevented, is even more favorable to younger women with longer life expectancy. The number needed to be screened per life year gained is:
- 28 at ages 40 to 49
- 17 at ages 50 to 59
- 16 at ages 60 to 69.12
These data provide additional support for screening women starting at age 40.
Observational studies, which provide a better measure of effectiveness because only women who actually undergo routine mammography are compared with those who do not, also support this conclusion. An observational study in Sweden with 20 years of follow-up found that women of all ages who participated in screening had a 44% lower risk of death from breast cancer than with those who were not screened; for women in their 40s, the risk reduction was 48%.14 Similarly, an observational study conducted in British Columbia15 found a 40% decrease in deaths in women screened annually between ages 40 and 79, and a 39% decrease in deaths in women first screened between ages 40 and 49.
LOW RATE OF FALSE-POSITIVE RESULTS
Like many screening programs, screening mammography does not benefit all women equally.
False-positive results occur, for which women need additional imaging or a biopsy for findings that turn out not to be cancer. But the false-positive rate is not high: for every 1,000 women screened in the United States, 80 to 100 (10% or less) are recalled for additional evaluation, 15 (1.5%) undergo biopsy, and 2 to 5 have a cancer, so only about 1% of the women screened underwent an unnecessary biopsy.16
False-positive test results can provoke unnecessary anxiety, but evidence indicates that this tends to be a temporary effect, and even women who had a false-positive result tend to support mammography. In a report by Lerman et al,17 when mood was assessed 3 months after mammography, worry was reported by 26% of women who had had a false-positive report, compared with 9% of women who had had a normal mammogram. Another report addressing the consequences of false-positive mammograms found that although short-term anxiety increased, long-term anxiety did not.18 In a random telephone survey, 98% of adults who reported having had a false-positive cancer screening result stated that they were nevertheless glad that they had undergone screening.19
OVERDIAGNOSIS OCCURS BUT IS LIKELY UNCOMMON
Overdiagnosis of breast cancer is a possible drawback of screening mammography. Cancers may be detected that would not have become clinically apparent in a person’s lifetime20 or have affected ultimate prognosis,18 and so would not have needed to be treated.
Overdiagnosis from screening mammography usually refers to finding ductal carcinoma in situ (DCIS) on breast biopsy. Because no randomized controlled study has been done in which breast cancer was diagnosed and not treated, evidence of the danger from DCIS comes from retrospective reviews of 130 cases in which excised tissue initially interpreted as benign was actually cancerous. Over 10 to 30 years, 11% to 60% of these patients developed invasive breast cancer in the same quadrant from which tissue had been excised.21 This rate of cancer development could lead to underestimation of the invasive potential of DCIS because the patients studied all had low-grade DCIS; further, some of the baseline biopsies involved complete removal of the tumor, thereby preventing the development or progression of cancer.
All DCIS is not the same. An ongoing trial22 found a 5-year recurrence rate of 6.1% after surgery for low-grade or intermediate-grade DCIS, and 15% after surgery for high-grade DCIS. Swedish trials23 have shown that most women who die of “early” breast cancer have high-grade DCIS. These findings suggest that although screening mammography may result in overdiagnosis and overtreatment of low-grade DCIS, high-grade DCIS can be lethal and should be treated. Thus, overdiagnosis likely represents a small fraction of all breast cancers.
Most important, it is not yet possible to accurately predict the biologic behavior of an individual tumor. Current clinical practice is to treat patients with DCIS similar to the way we treat patients with early-stage breast cancer, as we cannot determine which types of DCIS may remain indolent and which ones may become invasive.
HOW FREQUENTLY SHOULD YOUNGER WOMEN BE SCREENED?
The frequency of screening mammography has been another area of controversy, but we believe that annual screening offers the greatest benefit, especially for younger women.
The optimum screening frequency depends on how fast breast cancer grows and spreads. Data suggest that tumors in younger women tend to be biologically aggressive and grow and spread more quickly, making the benefit of yearly mammography more dramatic for younger women. A model based on data from Swedish studies24–26 predicted that the mortality reduction from breast cancer in women ages 40 to 49 would be 36% with annual screening, 18% with screening every 2 years, and 4% with screening every 3 years. For women in their 50s, the model estimated a reduction of 46% for yearly mammography, and 39% and 34% for screening every 2 or 3 years, respectively.6
In a prospective cohort study of the Breast Cancer Surveillance Consortium,27 in women ages 40 to 49 with extremely dense breasts, screening every 2 years was associated with a higher risk of advanced-stage disease (IIb or higher) and large tumors (> 2 cm) than with annual screening. For women ages 50 to 74, screening every 2 years vs every year did not increase the odds of advanced-stage or larger tumors.
AN INFORMED DECISION
In agreement with the current recommendations from the American Cancer Society, the American College of Radiology, and the American Congress of Obstetricians and Gynecologists, we support starting breast cancer screening with mammography at age 40.
Not all cancers are visible on mammography (false negatives), as they may be masked by mammographically dense breast tissue. Women should be informed of the importance of seeking medical attention for breast symptoms, even if mammography is normal. We need to inform women of the benefits and risks of screening mammography, including the risk of false-positive results that could lead to additional imaging and anxiety, and the uncertainties related to the potential for overdiagnosis and overtreatment. This information, offered in an easily understandable format, can help the patient make an informed decision regarding screening mammography, based on her values and preferences.
Screening mammography is not a perfect test, but it still plays an important role for women even in their 40s, when the incidence of breast cancer is low but the risk of a tumor being aggressive is especially high.
SCREENING DETECTS CANCER EARLY
The goal of screening mammography is to reduce breast cancer deaths by detecting cancers early, when treatment is more effective and less harmful.
Mammography detects tumors when they are smaller: the median size of breast cancers found with high-quality, two-view screening mammography is 1.0 to 1.5 cm, whereas cancers found by palpation are 2.0 to 2.5 cm.1 In general, tumors found when they are smaller require less treatment, and patients are more likely to survive.
Moreover, about 10% of invasive cancers smaller than 1 cm have spread to lymph nodes at the time of detection, compared with 35% of those 2 cm in size and 60% of those 4 cm or larger. Women who have a positive lymph node at the time of diagnosis usually undergo more intensive treatment with chemotherapy and more radical surgery than those who do not. The 5-year disease-free survival rate is more than 98% for breast cancer with a tumor smaller than 2 cm that has not spread to lymph nodes (stage I), compared with 86% for stage II disease (tumors 2.1–5 cm or one to three positive axillary lymph nodes).2
Treating breast cancer early is also less expensive. In a study of women enrolled in a health maintenance organization in Pennsylvania, 14% of those not screened presented with advanced breast cancer (stage III or IV) compared with 2% who had been screened. The cumulative cost of treating advanced breast cancer was two to three times that of treating early breast cancer (stage 0 or I), not accounting for time lost away from work and family, in addition to pain and suffering.3
SCREENING SAVES LIVES
Multiple prospective, randomized controlled trials have been conducted to assess whether inviting women between ages 40 and 74 to undergo screening mammography reduces the rate of death from breast cancer.4,5 Such trials tend to underestimate the effect of screening because not all women invited to be screened actually are screened, and some in the control group may undergo screening on their own.6
The Canadian National Breast Screening Study (NBSS) had additional problems that underestimated the benefits of screening. The quality of mammography came under question, and an issue with randomization became evident after the first round of screening, as the group invited to be screened had an excess of women presenting with palpable lumps and advanced breast cancer.6–8 Despite these issues, a meta-analysis of randomized controlled trials of screening mammography, including the NBSS data, found a 15% reduction in deaths.9 When the NBSS data were excluded, the reduction was 24%.10
In 2009, the United States Preventive Services Task Force (USPSTF)11 recommended against mammographic screening for women ages 40 to 49. Using results from trials including the NBSS, they estimated that the number of women needed to be invited to screening to prevent one breast cancer death was:
- 1,904 for ages 39 to 49
- 1,339 for ages 50 to 59
- 377 for ages 60 to 69.
But if the NBSS study were excluded, these results would be 950, 670, and 377, respectively.6
In a review on screening mammography, Feig12 points out that the USPSTF selected the number of women invited to be screened rather than the number that were actually screened to measure the absolute benefit of screening.
Hendrick and Helvie13 reported that the number of women who needed to be screened to prevent one cancer death was:
- 746 for ages 40 to 49
- 351 for ages 50 to 59
- 253 for ages 60 to 69.
The benefit of screening, if analyzed by number of life years gained rather than number of deaths prevented, is even more favorable to younger women with longer life expectancy. The number needed to be screened per life year gained is:
- 28 at ages 40 to 49
- 17 at ages 50 to 59
- 16 at ages 60 to 69.12
These data provide additional support for screening women starting at age 40.
Observational studies, which provide a better measure of effectiveness because only women who actually undergo routine mammography are compared with those who do not, also support this conclusion. An observational study in Sweden with 20 years of follow-up found that women of all ages who participated in screening had a 44% lower risk of death from breast cancer than with those who were not screened; for women in their 40s, the risk reduction was 48%.14 Similarly, an observational study conducted in British Columbia15 found a 40% decrease in deaths in women screened annually between ages 40 and 79, and a 39% decrease in deaths in women first screened between ages 40 and 49.
LOW RATE OF FALSE-POSITIVE RESULTS
Like many screening programs, screening mammography does not benefit all women equally.
False-positive results occur, for which women need additional imaging or a biopsy for findings that turn out not to be cancer. But the false-positive rate is not high: for every 1,000 women screened in the United States, 80 to 100 (10% or less) are recalled for additional evaluation, 15 (1.5%) undergo biopsy, and 2 to 5 have a cancer, so only about 1% of the women screened underwent an unnecessary biopsy.16
False-positive test results can provoke unnecessary anxiety, but evidence indicates that this tends to be a temporary effect, and even women who had a false-positive result tend to support mammography. In a report by Lerman et al,17 when mood was assessed 3 months after mammography, worry was reported by 26% of women who had had a false-positive report, compared with 9% of women who had had a normal mammogram. Another report addressing the consequences of false-positive mammograms found that although short-term anxiety increased, long-term anxiety did not.18 In a random telephone survey, 98% of adults who reported having had a false-positive cancer screening result stated that they were nevertheless glad that they had undergone screening.19
OVERDIAGNOSIS OCCURS BUT IS LIKELY UNCOMMON
Overdiagnosis of breast cancer is a possible drawback of screening mammography. Cancers may be detected that would not have become clinically apparent in a person’s lifetime20 or have affected ultimate prognosis,18 and so would not have needed to be treated.
Overdiagnosis from screening mammography usually refers to finding ductal carcinoma in situ (DCIS) on breast biopsy. Because no randomized controlled study has been done in which breast cancer was diagnosed and not treated, evidence of the danger from DCIS comes from retrospective reviews of 130 cases in which excised tissue initially interpreted as benign was actually cancerous. Over 10 to 30 years, 11% to 60% of these patients developed invasive breast cancer in the same quadrant from which tissue had been excised.21 This rate of cancer development could lead to underestimation of the invasive potential of DCIS because the patients studied all had low-grade DCIS; further, some of the baseline biopsies involved complete removal of the tumor, thereby preventing the development or progression of cancer.
All DCIS is not the same. An ongoing trial22 found a 5-year recurrence rate of 6.1% after surgery for low-grade or intermediate-grade DCIS, and 15% after surgery for high-grade DCIS. Swedish trials23 have shown that most women who die of “early” breast cancer have high-grade DCIS. These findings suggest that although screening mammography may result in overdiagnosis and overtreatment of low-grade DCIS, high-grade DCIS can be lethal and should be treated. Thus, overdiagnosis likely represents a small fraction of all breast cancers.
Most important, it is not yet possible to accurately predict the biologic behavior of an individual tumor. Current clinical practice is to treat patients with DCIS similar to the way we treat patients with early-stage breast cancer, as we cannot determine which types of DCIS may remain indolent and which ones may become invasive.
HOW FREQUENTLY SHOULD YOUNGER WOMEN BE SCREENED?
The frequency of screening mammography has been another area of controversy, but we believe that annual screening offers the greatest benefit, especially for younger women.
The optimum screening frequency depends on how fast breast cancer grows and spreads. Data suggest that tumors in younger women tend to be biologically aggressive and grow and spread more quickly, making the benefit of yearly mammography more dramatic for younger women. A model based on data from Swedish studies24–26 predicted that the mortality reduction from breast cancer in women ages 40 to 49 would be 36% with annual screening, 18% with screening every 2 years, and 4% with screening every 3 years. For women in their 50s, the model estimated a reduction of 46% for yearly mammography, and 39% and 34% for screening every 2 or 3 years, respectively.6
In a prospective cohort study of the Breast Cancer Surveillance Consortium,27 in women ages 40 to 49 with extremely dense breasts, screening every 2 years was associated with a higher risk of advanced-stage disease (IIb or higher) and large tumors (> 2 cm) than with annual screening. For women ages 50 to 74, screening every 2 years vs every year did not increase the odds of advanced-stage or larger tumors.
AN INFORMED DECISION
In agreement with the current recommendations from the American Cancer Society, the American College of Radiology, and the American Congress of Obstetricians and Gynecologists, we support starting breast cancer screening with mammography at age 40.
Not all cancers are visible on mammography (false negatives), as they may be masked by mammographically dense breast tissue. Women should be informed of the importance of seeking medical attention for breast symptoms, even if mammography is normal. We need to inform women of the benefits and risks of screening mammography, including the risk of false-positive results that could lead to additional imaging and anxiety, and the uncertainties related to the potential for overdiagnosis and overtreatment. This information, offered in an easily understandable format, can help the patient make an informed decision regarding screening mammography, based on her values and preferences.
- Güth U, Huang DJ, Huber M, et al. Tumor size and detection in breast cancer: self-examination and clinical breast examination are at their limit. Cancer Detect Prev 2008; 32:224–228.
- Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD; 2007:101–110. http://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf. Accessed April 9, 2015.
- Legorreta AP, Brooks RJ, Leibowitz AN, Solin LJ. Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med 1996; 156:2197–2201.
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L; Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006; 368:2053–2060.
- Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002; 137:347–360.
- Feig SA. Screening mammography benefit controversies: sorting the evidence. Radiol Clin North Am 2014; 52:455–480.
- Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 1992; 147:1477–1488.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Smart CR, Hendrick RE, Rutledge JH 3rd, Smith RA. Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer 1995; 75:1619–1626.
- Breast-cancer screening with mammography in women aged 40-49 years. Swedish Cancer Society and the Swedish National Board of Health and Welfare. Int J Cancer 1996; 68:693–699.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- Feig SA. Number needed to screen. Appropriate use of this new basis for screening mammography guidelines. AJR Am J Roentgenol 2012; 198:1214–1217.
- Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. AJR Am J Roentgenol 2012; 198:723–728.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003; 361:1405–1410.
- Coldman A, Phillips N, Warren L, Kan L. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer 2007; 120:1076–1080.
- Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006; 241:55–66.
- Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991; 114:657–661.
- Tosteson AN, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med 2014; 174:954–961.
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA 2004; 291:71–78.
- Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer 2013; 108:2205–2240.
- Feig SA. Ductal carcinoma in situ. Implications for screening mammography. Radiol Clin North Am 2000; 38:653–668,
- Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009; 27:5319–5324.
- Tabár L, Vitak B, Chen HH, et al. The Swedish two-county trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000; 38:625–651.
- Duffy SW, Chen HH, Tabar L, et al. Estimation of mean sojourn time in breast cancer screening using a Markov chair model of entry to and exit from the preclinical detectable phase. Stat Med 1995; 14:1521-1534.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part I: tumor attributes and the preclinical screening detectable phase. J Epidemiol Biostat 1997; 2:9–25.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part II: prediction of outcomes for different screening regimes. J Epidemiol Biostat 1997; 2:25–35.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.
- Güth U, Huang DJ, Huber M, et al. Tumor size and detection in breast cancer: self-examination and clinical breast examination are at their limit. Cancer Detect Prev 2008; 32:224–228.
- Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD; 2007:101–110. http://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf. Accessed April 9, 2015.
- Legorreta AP, Brooks RJ, Leibowitz AN, Solin LJ. Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med 1996; 156:2197–2201.
- Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L; Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006; 368:2053–2060.
- Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002; 137:347–360.
- Feig SA. Screening mammography benefit controversies: sorting the evidence. Radiol Clin North Am 2014; 52:455–480.
- Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 1992; 147:1477–1488.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Smart CR, Hendrick RE, Rutledge JH 3rd, Smith RA. Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer 1995; 75:1619–1626.
- Breast-cancer screening with mammography in women aged 40-49 years. Swedish Cancer Society and the Swedish National Board of Health and Welfare. Int J Cancer 1996; 68:693–699.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- Feig SA. Number needed to screen. Appropriate use of this new basis for screening mammography guidelines. AJR Am J Roentgenol 2012; 198:1214–1217.
- Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. AJR Am J Roentgenol 2012; 198:723–728.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003; 361:1405–1410.
- Coldman A, Phillips N, Warren L, Kan L. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer 2007; 120:1076–1080.
- Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006; 241:55–66.
- Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991; 114:657–661.
- Tosteson AN, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med 2014; 174:954–961.
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA 2004; 291:71–78.
- Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer 2013; 108:2205–2240.
- Feig SA. Ductal carcinoma in situ. Implications for screening mammography. Radiol Clin North Am 2000; 38:653–668,
- Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009; 27:5319–5324.
- Tabár L, Vitak B, Chen HH, et al. The Swedish two-county trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 2000; 38:625–651.
- Duffy SW, Chen HH, Tabar L, et al. Estimation of mean sojourn time in breast cancer screening using a Markov chair model of entry to and exit from the preclinical detectable phase. Stat Med 1995; 14:1521-1534.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part I: tumor attributes and the preclinical screening detectable phase. J Epidemiol Biostat 1997; 2:9–25.
- Chen HH, Duffy SW, Tabar L, et al. Markov chain models for progression of breast cancer. Part II: prediction of outcomes for different screening regimes. J Epidemiol Biostat 1997; 2:25–35.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med 2013; 173:807–816.