User login
Chronic Microaspiration and Frailty: A Geriatric Smoking Gun?
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
Frailty is a highly prevalent syndrome in nursing homes, occurring in at least 50% of patients.1 The frailty phenotype has been described by Fried and colleagues as impairment in ≥ 3 of 5 domains: unintentional weight loss, self-reported exhaustion, muscle weakness, slow gait speed, and low physical activity. By this definition, frailty is highly associated with poor quality of life and mortality.2,3
In recent years, there has been evolving evidence of a relationship between frailty and chronic systemic inflammation.4-6 Some degree of chronic inflammation is likely inherent to the aging process and increases the risk of frailty (so-called inflammaging) but is seen to a greater degree in many pathologic conditions in nursing homes, including cancer, organ failure, and chronic infection.4,6-8
Dysphagia also is highly prevalent in nursing homes, affecting up to 60% of patients and is a strong predictor of hospital utilization and of mortality.9,10 Overt aspiration pneumonitis and pneumonia are perhaps the best studied sequelae, but chronic occult microaspiration also is prevalent in this population.11 Just as normal systemic inflammatory changes in aging may increase vulnerability to frailty with additional illness burden, normal aging changes in swallowing function may increase vulnerability to dysphagia and to microaspiration with additional illness burden.12,13 In older adults, important risk factors for microaspiration include not only overt dysphagia, dementia, and other neurologic illnesses, but also general debility, weakness, and immobility.14
Matsuse and colleagues have described diffuse aspiration bronchiolitis (DAB) in patients with chronic microaspiration.14 DAB often goes undiagnosed.14-16 As in frailty, weight loss and chronic anemia may be seen, and many of these patients are bedridden.14,17 Episodes of macroaspiration and overt lobar pneumonia also may occur.14 Lung biopsy or autopsy reveals chronic bronchiolar inflammation and sometimes pulmonary fibrosis, but to date there have been no reports suggesting chronic systemic inflammation or elevated proinflammatory cytokines.14,15,17 We present 3 patients with progressive weight loss, functional decline, and frailty in whom chronic microaspiration likely played a significant role.
Case 1 Presentation
A 68-year-old man with a 6-year history of rapidly progressive Parkinson disease was admitted to the Haley’s Cove Community Living Center (CLC) on the James A. Haley Veterans’ Hospital campus in Tampa, Florida for long-term care. The patient’s medical history also was significant for bipolar illness and for small cell carcinoma of the lung in sustained remission.
Medications included levodopa/carbidopa 50 mg/200 mg 4 times daily, entacapone 200 mg 4 times daily, lithium carbonate 600 mg every night at bedtime, lamotrigine 150 mg daily, quetiapine 200 mg every night at bedtime, pravastatin 40 mg every night at bedtime, omeprazole 20 mg daily, tamsulosin 0.4 mg every night at bedtime, and aspirin 81 mg daily. He initially did well, but after 6 months the nursing staff began to notice the patient coughing during and after meals. Speech pathology evaluation revealed moderate oropharyngeal dysphagia, and his diet was downgraded to nectar-thickened liquids.
Over the subsequent 10 months, he became progressively weaker in physical therapy and more inactive, with about a 20-lb weight loss and mild hypoalbuminemia of 3.0 gm/dL. He had developed 3 episodes of aspiration pneumonia during this period; a repeat swallow evaluation after the last episode revealed worsened dysphagia, and his physician suggested nil per os (NPO) status and an alternative feeding route. His guardian declined placement of a percutaneous endoscopic gastrostomy (PEG) tube, he was transferred to the inpatient hospice unit, and died 2 weeks later. An autopsy was declined.
Case 2 Presentation
A 66-year-old man with a medical history of multiple traumatic brain injuries (TBIs) was admitted to the CLC for long-term care. Sequelae of the TBIs included moderate dementia, spastic paraparesis with multiple pressure injuries, a well-controlled seizure disorder, and severe oropharyngeal dysphagia with NPO status and a percutaneous endoscopic gastrostomy (PEG) tube. His medical history included TBIs and hepatitis C virus infection; medications included levetiracetam 1,000 mg twice daily, lamotrigine 25 mg twice daily, and cholecalciferol 2,000 U daily. He had multiple stage III pressure injuries and an ischial stage IV injury at the time of admission.
His 11-month stay in the CLC was characterized by progressively worsening weakness and inactivity, with a 25-lb weight loss in spite of adequate tube feeding. Serum albumin remained in the 2.0 to 2.5 gm/dL range, hemoglobin in the 7 to 9 gm/dL range without any obvious source of anemia. Most of the pressure injuries worsened during his stay in spite of aggressive wound care, and he developed a second stage IV sacral wound. A single C-reactive protein (CRP) level 2 months prior to his death was markedly elevated at 19.5 mg/dL. In spite of maintaining NPO status, he developed 3 episodes of aspiration pneumonia, all of which responded well to treatment. Ultimately, he was found pulseless and apneic and resuscitation was unsuccessful. An autopsy revealed purulent material in the small airways.
Case 3 Presentation
A 65-year-old man with a long history of paranoid schizophrenia and severe gastroesophageal reflux disease had resided in the CLC for about 10 years. Medications included risperidone microspheres 37.5 mg every 2 weeks, valproic acid 500 mg 3 times daily and 1,000 mg every night at bedtime, lansoprazole 30 mg twice daily, ranitidine 150 mg every night at bedtime, sucralfate 1,000 mg 3 times daily, simvastatin 20 mg every night at bedtime, and tamsulosin 0.4 mg every night at bedtime. He had done well for many years but developed some drooling and a modest resting tremor (but no other signs of pseudoparkinsonism) about 8 years after admission.
There had been no changes to his risperidone dosage. He also lost about 20 lb over a period of 1 year and became increasingly weak and dependent in gait, serum albumin dropped as low as 1.6 gm/dL, hemoglobin dropped to the 7 to 8 gm/dL range (without any other obvious source of anemia), and he developed a gradually worsening right-sided pleural effusion. CRP was chronically elevated at this point, in the 6 to 15 mg/dL range and as high as 17.2 mg/dL. Ultimately, he developed 3 episodes of aspiration pneumonia over a period of 2 months. Swallowing evaluation at that time revealed severe oropharyngeal dysphagia and a PEG tube was placed. Due to concerns for possible antipsychotic-induced dysphagia, risperidone was discontinued, and quetiapine 400 mg a day was substituted. He did well over the subsequent year with no further pneumonia and advancement back to a regular diet. He regained all of the lost weight and began independent ambulation. Albumin improved to the 3 gm/dL range, hemoglobin to the 12 to 13 gm/dL range, and CRP had decreased to 0.7 mg/dL. The pleural effusion (believed to have been a parapneumonic effusion) had resolved.
Discussion
All 3 patients met the Fried criteria for frailty, although there were several confounding issues.2 All 3 patients lost between 20 and 25 lb; all had clearly become weaker according to nursing and rehabilitation staff (although none were formally assessed for grip strength); and all had clear declines in their activity level. Patient 3 had a clear decrement in gait speed, but patient 1 had severe gait impairment due to Parkinson disease (although his gait in therapy had clearly worsened). Patient 2 was paraparetic and unable to ambulate. There also was evidence of limited biomarkers of systemic inflammation; all 3 patients’ albumin had decreased, and patients 2 and 3 had significant decrease in hemoglobin; but these commonplace clinical biomarkers are obviously multifactorially determined. We have limited data on our patients’ CRP levels; serial levels would have been more specific for systemic inflammation but were infrequently performed on the patients.
Multimorbidity and medical complexity are more the rule than the exception in frail geriatric patients,and it is difficult to separate the role of microaspiration from other confounding conditions that might have contributed to these patients’ evolving systemic inflammation and frailty.18 It might be argued that the decline for patient 1 was related to the underlying Parkinson disease (a progressive neurologic illness in which systemic inflammation has been reported), or that the decline of patient 2 was related to the worsening pressure injuries rather than to covert microaspiration.19 However, the TBIs for patient 2 and the schizophrenia for patient 3 would not be expected to be associated with frailty or with systemic inflammation. Furthermore, the frailty symptoms of patient 3 and inflammatory biomarkers improved after the risperidone, which was likely responsible for his microaspiration, was discontinued. All 3 patients were at risk for oropharyngeal dysphagia (antipsychotic medication is clearly associated with dysphagia20); patient 2 demonstrated pathologic evidence of DAB at autopsy.
There is evolving evidence that chronic systemic inflammation and immune activation are key mechanisms in the pathogenesis of frailty.4-6 It is known that elevated serum levels of proinflammatory cytokines, including tumor necrosis factor-α, interleukin-6, and CRP are directly associated with frailty and are inversely associated with levels of albumin, hemoglobin, insulin-like growth factor-1, and several micronutrients in frail individuals.4-7,21,22 Chronic inflammation contributes to the pathophysiology of frailty through detrimental effects on a broad range of systems, including the musculoskeletal, endocrine, and hematopoietic systems and through nutritional dysregulation.2,4,23 These changes may lead to further deleterious effects, creating a downward spiral of worsening frailty. For example, it seems likely that our patients’ progressive weakness further compromised airway protection, creating a vicious cycle of worsening microaspiration and chronic inflammation.
Conclusions
To date, the role of chronic microaspiration and DAB in chronic systemic inflammation or in frailty has not been explored. Given the prevalence of microaspiration in nursing home residents and the devastating consequences of frailty, though, this seems to be a crucial area of investigation. It is equally crucial for long-term care staff, both providers and nursing staff, to have a heightened awareness of covert microaspiration and a low threshold for referral to speech pathology for further investigation. Staff also should be aware of the utility of the Fried criteria to improve identification of frailty in general. It is probable that covert microaspiration will prove to be an important part of the differential diagnosis of frailty.
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003
1. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940-945. doi:10.1016/j.jamda.2015.06.025
2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. doi:10.1093/gerona/56.3.m146
3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi:10.1016/j.jamda.2013.03.022
4. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. doi:10.2147/CIA.S45300.
5. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1-8. doi:10.1016/j.arr.2016.08.006
6. Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777-1783. doi:10.1111/jgs.14876
7. Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-882. doi:10.1016/j.jamda.2013.05.009
8. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;72(9):1218-1225. doi:10.1093/gerona/glw240
9. Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs. 2000;26(8):35-48. doi:10.3928/0098-9134-20000801-09
10. Altman KW, Yu GP, Schaefer SD. Consequences of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784-789. doi:10.1001/archoto.2010.129
11. Sakai K, Hirano H, Watanabe Y, et al. An examination of factors related to aspiration and silent aspiration in older adults requiring long-term care in rural Japan. J Oral Rehabil. 2016;43(2):103-110. doi:10.1111/joor.12349
12. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180-184. doi:10.1007/BF00366381
13. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270-274. doi:10.1007/s00455-006-9051-6
14. Matsuse T, Oka T, Kida K, Fukuchi Y. Importance of diffuse aspiration bronchiolitis caused by chronic occult aspiration in the elderly. Chest. 1996;110(5):1289-1293. doi:10.1378/chest.110.5.1289
15. Cardasis JJ, MacMahon H, Husain AN. The spectrum of lung disease due to chronic occult aspiration. Ann Am Thorac Soc. 2014;11(6):865-873. doi:10.1513/AnnalsATS.201310-360OC
16. Pereira-Silva JL, Silva CIS, Araujo Neto CA, Andrade TL, Muller NL. Chronic pulmonary microaspiration: high-resolution computed tomographic findings in 13 patients. J Thorac Imaging. 2014;29(5):298-303. doi:10.1097/RTI.0000000000000091
17. Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815-823. doi:10.1378/chest.14-1049
18. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Aging. 2017;46(6):882-888. doi:10.1093/ageing/afx150
19. Calabrese V, Santoro A, Monti D, et al. Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80-91. doi:10.1016/j.freeradbiomed.2017.10.379
20. Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467-471. doi:10.1007/s00455-017-9802-6
21. Velissaris D, Pantzaris N, Koniari I, et al. C-reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461-465. doi:10.14740/jocmr2959w
22. Hubbard RE, O’Mahoney MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103-3109. doi:10.1111/j.1582-4934.2009.00733.x
23. Argiles JM, Busquets S, Stemmler B, Lotez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-106. doi:10.1016/j.coph.2015.04.003
Implementation of a Protocol to Manage Patients at Risk for Hospitalization Due to an Ambulatory Care Sensitive Condition
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
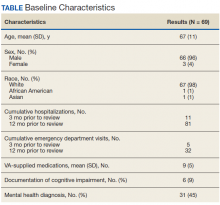
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
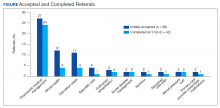
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Creating an Intensive Care Unit From a Postanesthesia Care Unit for the COVID-19 Surge at the Veterans Affairs Ann Arbor Healthcare System
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The Role of Process Improvements in Reducing Heart Failure Readmissions
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.
During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.
We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.
During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.
We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.
During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.
We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
A Multidisciplinary Ambulation Protocol to Reduce Postoperative Venous Thromboembolism After Colorectal Surgery
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
A Curriculum for Training Medical Faculty to Teach Mental Health Care—and Their Responses to the Learning
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.
First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.
2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21
Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.
First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.
2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21
Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.
First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.
2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21
Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
Remdesivir Reduces Time to Recovery in Adults Hospitalized With COVID-19: A Meaningful Step in Therapeutic Discovery
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
US News releases latest top hospitals list, adds COVID heroes
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
Non–COVID-19 VA Hospital Admissions Drop During the Pandemic
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.