User login
Leadership & Professional Development: Having a Backup Plan
“Confidence comes from being prepared.”
—John Wooden
Hospital medicine is a field that requires a constant state of readiness and flexibility. With respect to patient care, constant preparedness is required because conditions change. This necessitates always having a backup plan, or Plan B. For example, your patient with a gastrointestinal (GI) bleed should have two large-bore intravenous (IV) catheters and packed red blood cells (RBCs) typed and crossed. If the patient becomes unstable, the response is not just doing more of the same (IV fluids and proton pump inhibitors); the focus shifts to your Plan B: call GI, transfuse blood, transfer the patient to the intensive care unit.
In contrast to clinical scenarios, there is often a lack of readiness to deal with rapid changes in workflow. Without a plan, efficiency decreases, stress levels rise, and both patients and providers alike suffer the consequences. Patients spending extended periods of time in the Emergency Department (ED) receive less timely services and often don’t benefit from the expertise that they would receive in inpatient units.1 This is particularly true in an era in which many hospitals are experiencing higher overall volume and surges are more common.
Ideally, readiness should manifest as the ability to adapt to changes at the individual, hospitalist team, and leadership levels. Having a Plan B in the practice of hospital medicine is a focused exercise for anticipating future problems and addressing them prospectively. When thinking about a Plan B, the following are some steps to consider:
1. Identify Triggers. In the earlier example of the GI bleed, our triggers for Plan B would be a change in vitals or a brisk drop in hemoglobin. Regarding hospital workflow, the triggers might include low service or bed capacity or a decreased number of expected discharges for the day. Perhaps a high ED census or increased surgical volume will trigger your plan to handle the surge.
2. Define Your Response. At both an individual and service level, there are steps you might consider in your Plan B. On teaching services, this might mean prioritizing rounding on patients that you’re expecting to discharge so they’re able to leave the hospital sooner. For patients on observation status who are boarding in the ED for extended periods, there might be opportunities to safely discharge them with follow-up or even complete their work-up in the ED. There may be circumstances in which providers should exceed the usual service capacity and conditions in which it is truly unsafe to exceed that limit. If there are resources available to increase staffing, consider how to best utilize them.
3. Engage Broadly and Proactively. It is very difficult to execute a Plan B (or frankly a Plan A) without buy-in from your stakeholders. This starts with the rank and file, those on your team who will actually execute the plan. The leadership of your department or division, the ED, and nursing will also likely need to provide input. If financial resources for flexing up staff are part of your plan, the hospital administration might need to weigh in. It is best to engage stakeholders early on rather than during a crisis.
4. Constant Assessment and Improvement. Going back to our example of our patient with a GI bleed, you’re constantly reevaluating your patient to determine if your Plan B is working. Similarly, you should collect data and reassess the effectiveness of your plan. There are likely opportunities to improve it.
There are no textbook chapters or medical school lectures to prepare hospitalists for these real-world crises. Yet failing to have a Plan B is to surrender a tremendous amount of personal control in the face of chaos, to jeopardize patient care, and to ultimately forgo the opportunity to achieve a level of mastery in a field predicated on readiness.
1. Institute of Medicine, Committee on the Future of Emergency Care in the United States Health System. Hospital-Based Emergency Care at the Breaking Point. Washington, District of Columbia: The National Academies Press; 2006.
“Confidence comes from being prepared.”
—John Wooden
Hospital medicine is a field that requires a constant state of readiness and flexibility. With respect to patient care, constant preparedness is required because conditions change. This necessitates always having a backup plan, or Plan B. For example, your patient with a gastrointestinal (GI) bleed should have two large-bore intravenous (IV) catheters and packed red blood cells (RBCs) typed and crossed. If the patient becomes unstable, the response is not just doing more of the same (IV fluids and proton pump inhibitors); the focus shifts to your Plan B: call GI, transfuse blood, transfer the patient to the intensive care unit.
In contrast to clinical scenarios, there is often a lack of readiness to deal with rapid changes in workflow. Without a plan, efficiency decreases, stress levels rise, and both patients and providers alike suffer the consequences. Patients spending extended periods of time in the Emergency Department (ED) receive less timely services and often don’t benefit from the expertise that they would receive in inpatient units.1 This is particularly true in an era in which many hospitals are experiencing higher overall volume and surges are more common.
Ideally, readiness should manifest as the ability to adapt to changes at the individual, hospitalist team, and leadership levels. Having a Plan B in the practice of hospital medicine is a focused exercise for anticipating future problems and addressing them prospectively. When thinking about a Plan B, the following are some steps to consider:
1. Identify Triggers. In the earlier example of the GI bleed, our triggers for Plan B would be a change in vitals or a brisk drop in hemoglobin. Regarding hospital workflow, the triggers might include low service or bed capacity or a decreased number of expected discharges for the day. Perhaps a high ED census or increased surgical volume will trigger your plan to handle the surge.
2. Define Your Response. At both an individual and service level, there are steps you might consider in your Plan B. On teaching services, this might mean prioritizing rounding on patients that you’re expecting to discharge so they’re able to leave the hospital sooner. For patients on observation status who are boarding in the ED for extended periods, there might be opportunities to safely discharge them with follow-up or even complete their work-up in the ED. There may be circumstances in which providers should exceed the usual service capacity and conditions in which it is truly unsafe to exceed that limit. If there are resources available to increase staffing, consider how to best utilize them.
3. Engage Broadly and Proactively. It is very difficult to execute a Plan B (or frankly a Plan A) without buy-in from your stakeholders. This starts with the rank and file, those on your team who will actually execute the plan. The leadership of your department or division, the ED, and nursing will also likely need to provide input. If financial resources for flexing up staff are part of your plan, the hospital administration might need to weigh in. It is best to engage stakeholders early on rather than during a crisis.
4. Constant Assessment and Improvement. Going back to our example of our patient with a GI bleed, you’re constantly reevaluating your patient to determine if your Plan B is working. Similarly, you should collect data and reassess the effectiveness of your plan. There are likely opportunities to improve it.
There are no textbook chapters or medical school lectures to prepare hospitalists for these real-world crises. Yet failing to have a Plan B is to surrender a tremendous amount of personal control in the face of chaos, to jeopardize patient care, and to ultimately forgo the opportunity to achieve a level of mastery in a field predicated on readiness.
“Confidence comes from being prepared.”
—John Wooden
Hospital medicine is a field that requires a constant state of readiness and flexibility. With respect to patient care, constant preparedness is required because conditions change. This necessitates always having a backup plan, or Plan B. For example, your patient with a gastrointestinal (GI) bleed should have two large-bore intravenous (IV) catheters and packed red blood cells (RBCs) typed and crossed. If the patient becomes unstable, the response is not just doing more of the same (IV fluids and proton pump inhibitors); the focus shifts to your Plan B: call GI, transfuse blood, transfer the patient to the intensive care unit.
In contrast to clinical scenarios, there is often a lack of readiness to deal with rapid changes in workflow. Without a plan, efficiency decreases, stress levels rise, and both patients and providers alike suffer the consequences. Patients spending extended periods of time in the Emergency Department (ED) receive less timely services and often don’t benefit from the expertise that they would receive in inpatient units.1 This is particularly true in an era in which many hospitals are experiencing higher overall volume and surges are more common.
Ideally, readiness should manifest as the ability to adapt to changes at the individual, hospitalist team, and leadership levels. Having a Plan B in the practice of hospital medicine is a focused exercise for anticipating future problems and addressing them prospectively. When thinking about a Plan B, the following are some steps to consider:
1. Identify Triggers. In the earlier example of the GI bleed, our triggers for Plan B would be a change in vitals or a brisk drop in hemoglobin. Regarding hospital workflow, the triggers might include low service or bed capacity or a decreased number of expected discharges for the day. Perhaps a high ED census or increased surgical volume will trigger your plan to handle the surge.
2. Define Your Response. At both an individual and service level, there are steps you might consider in your Plan B. On teaching services, this might mean prioritizing rounding on patients that you’re expecting to discharge so they’re able to leave the hospital sooner. For patients on observation status who are boarding in the ED for extended periods, there might be opportunities to safely discharge them with follow-up or even complete their work-up in the ED. There may be circumstances in which providers should exceed the usual service capacity and conditions in which it is truly unsafe to exceed that limit. If there are resources available to increase staffing, consider how to best utilize them.
3. Engage Broadly and Proactively. It is very difficult to execute a Plan B (or frankly a Plan A) without buy-in from your stakeholders. This starts with the rank and file, those on your team who will actually execute the plan. The leadership of your department or division, the ED, and nursing will also likely need to provide input. If financial resources for flexing up staff are part of your plan, the hospital administration might need to weigh in. It is best to engage stakeholders early on rather than during a crisis.
4. Constant Assessment and Improvement. Going back to our example of our patient with a GI bleed, you’re constantly reevaluating your patient to determine if your Plan B is working. Similarly, you should collect data and reassess the effectiveness of your plan. There are likely opportunities to improve it.
There are no textbook chapters or medical school lectures to prepare hospitalists for these real-world crises. Yet failing to have a Plan B is to surrender a tremendous amount of personal control in the face of chaos, to jeopardize patient care, and to ultimately forgo the opportunity to achieve a level of mastery in a field predicated on readiness.
1. Institute of Medicine, Committee on the Future of Emergency Care in the United States Health System. Hospital-Based Emergency Care at the Breaking Point. Washington, District of Columbia: The National Academies Press; 2006.
1. Institute of Medicine, Committee on the Future of Emergency Care in the United States Health System. Hospital-Based Emergency Care at the Breaking Point. Washington, District of Columbia: The National Academies Press; 2006.
© 2020 Society of Hospital Medicine
Promoting Gender Equity at the Journal of Hospital Medicine
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.
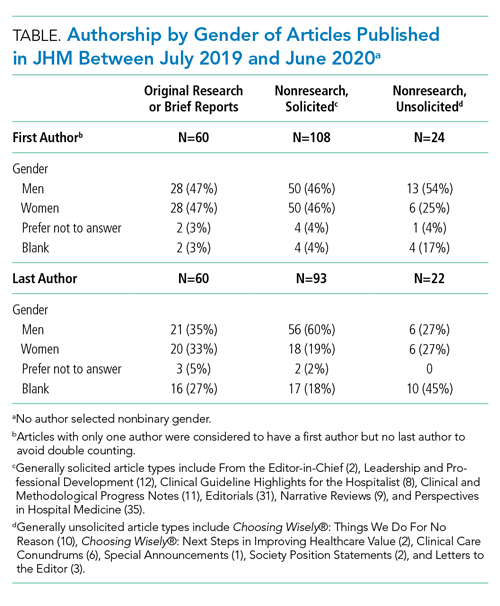
Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.

Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.

Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
© 2020 Society of Hospital Medicine
Ultrabrief Screens for Detecting Delirium in Postoperative Cognitively Intact Older Adults
Delirium is the most common postsurgical complication for older adults, with incidence of 15%-54%, depending on surgery type.1 Increasing numbers of older adults are undergoing surgery2; and those who develop delirium experience negative consequences including longer lengths of stay, higher likelihood of institutional discharge, and increased morbidity and mortality.3 The American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults and the European Society of Anaesthesiology4 recommend routine screening for delirium in those at risk.
Ultrabrief screens are designed to rule out delirium quickly and identify a subset of patients who require further testing.5 Our group, and others, have previously published ultrabrief screens for the general medicine, nonsurgical population and for patients with dementia.5,6 The UB-2 is an ultrabrief screen consisting of “Months of the year backward” (MOYB) and “What day of the week is it?”, which has a sensitivity of 93% and specificity of 64% in hospitalized older adults and takes less than 40 seconds to administer.5 However, no such screens for delirium have been developed for the group with relatively high cognitive and physical functioning undergoing scheduled major surgery in which delirium may present differently. Thus, the purpose of this study was to develop an ultrabrief screen for postoperative delirium using data from a large study of delirium in cognitively intact, older adults undergoing scheduled major noncardiac surgery.
METHODS
We performed a secondary data analysis on 560 patients enrolled between June 18, 2010, and August 8, 2013, in the Successful Aging After Elective Surgery (SAGES) study,7 an ongoing prospective cohort study of older adults undergoing major elective surgeries (eg, total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy). Exclusion criteria included evidence of dementia, delirium, prior hospitalization within 3 months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. The Institutional Review Boards of Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, and Hebrew SeniorLife, all in Boston, Massachusetts, approved the study.
SAGES Delirium Assessment and Additional Variables
The presence or absence of delirium was based on daily in-hospital assessments by trained research staff using the Confusion Assessment Method (CAM)8 long form. The Delirium Symptom Interview (DSI)9 and information related to acute changes in mental status were also included as provided by nursing staff and/or family. Delirium severity was determined using the CAM-S.10 Participants in The SAGES Study had an initial baseline, presurgical assessment in their homes. Cognitive and physical functioning, depression, comorbidities, laboratory, and self-reported demographic data were collected.
Statistical Analyses
We included CAM delirium data from postoperative days (POD) 1 and 2 for each participant, if available; postoperative day 0 was not included because of potential residual anesthetic effects. We chose these days because most delirium began on POD1 or 2, and patients started being discharged on POD3. We considered all one-, two-, and three-item combinations of the 12 cognitive items of the 3D-CAM11 because of their demonstrated high information content for CAM diagnostic features per Item Response Theory.12 There were 12 possible one-item screens, 66 two-item screens, and 220 three-item screens. Sensitivity, specificity, and 95% confidence intervals for each were compared with CAM delirium determination. An ideal ultrabrief screen for delirium has high sensitivity with moderate specificity; general guidelines considered based on investigator consensus included screens with a sensitivity higher than 0.90 and specificity greater than 0.70. Because these screens are used to quickly rule out delirium, we also present the percent positive screen among the entire population (whether delirium is present or not). Screens with a positive screen rate of more than 50% are unlikely to be helpful in ruling out delirium quickly in a large enough fraction of the population. We also required that in multiple item screens, no two items should assess the same CAM feature. For instance, we would eliminate a two-item screen with MOYB and four-digit span since both items measure CAM Feature 2 (Inattention). Finally, we evaluated screen performance separately on POD1 and POD2. Switching screens by POD can be confusing, so we chose a single best screen that retained excellent performance over both days. Data analyses used SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The dataset included 560 adults who had an average age of 76.6 years (SD = 5.2), were 58% women, and were highly educated (15.0 years; SD = 2.9; Table). Postoperative delirium occurred during one or more days in 134 individuals (24%). A total of 1,100 delirium assessments were used, with 113 that were CAM positive (10.3%). For POD1, we used 551 assessments, 61 of which were positive (11.1%); for POD2, 549 assessments were used, with 51 positive (9.3%). Appendix Tables present the positive screen rates, sensitivities, specificities, and 95% confidence intervals of all 12 one-item screens and the 12 best performing two- and three-item screens in order of decreasing sensitivity.

The best ultrabrief screen from POD1 included the following three items: “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?”, with a sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.73 (95% CI, 0.69-0.77). The same combination of items has a sensitivity of 0.88 (95% CI, 0.77-0.96) and a specificity of 0.70 (95% CI, 0.66-0.74) on POD2. When POD1 and POD2 are combined, the sensitivity is 0.92 (95% CI, 0.85-0.96) and specificity is 0.72 (95% CI, 0.69-0.74). We consider this to be our best screen overall.
DISCUSSION
We identified a three-item screen for delirium after elective surgery consisting of “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?” In our own prior work, we identified a two-item screen consisting of MOYB and “What is the day of the week?” as the best ultrabrief screen for delirium in general medicine populations (termed the “UB-2”)5 and a subsequent screen for patients with delirium superimposed on dementia (DSD) including “What type of place is this?”, Days of the Week Backward, and “Does the patient appear sleepy?”6 All three contain a test of attention (a cardinal feature of delirium) and a test of orientation, although the specific test for that varies. Both the surgical and DSD screens include “Does the patient appear sleepy?”, which addresses a reduced level of consciousness. This might be particularly important in the postoperative setting because of residual effects of anesthesia and/or postoperative analgesic medications contributing to delirium. Work done by others confirms our current findings, which is that MOYB is the best single item for most groups. Belleli et al13 and Han et al14 included MOYB as the single attentional item in the 4AT and B-CAM, respectively. The Nu-DESC has been used as a screen in surgical patients; however, it involves only nursing observations and no direct questioning of the patient.15
The Figure describes how our “best screen” could be integrated into clinical care. One or more “positive” or incorrect responses on these three items constitutes a positive screen that should be further evaluated with the CAM or 3D-CAM. If all three items are correct or negative, this effectively rules out delirium; however, continued periodic screening on a daily (or per shift) basis is indicated. On repeat testing, if any of the previously negative or correct items becomes positive or incorrect, this would be evidence for Acute Change, CAM Feature 1. Finally, it should be noted that, if all three items in our best screen are positive, full CAM criteria for delirium diagnosis are met within the screen itself, and no further testing is required. We envision this process being facilitated by use of an app-based program that generates optimal screening items based on patient and setting characteristics.

There are several limitations that must be noted. First, our three-item screen may not generalize to nonsurgical candidates or those undergoing emergent surgery and should be tested in these groups. Second, the SAGES sample is relatively homogenous with respect to racial and ethnic diversity and was highly educated with little functional impairment and no dementia. Therefore, results may not be generalizable to populations with lower educational attainment and/or preexisting mental and physical disabilities. A third limitation is that screen items were included in the reference standard delirium assessment, leading to a potential bias toward increased sensitivity. Finally, all screens were derived from secondary data analysis and further research will be needed to prospectively validate the results. Despite these limitations, this study has several strengths including the use of a well-characterized surgical population and a rigorous approach to delirium measurement. It is one of the first studies to identify a screening tool targeted to identifying delirium in postoperative older adults.
Future research should prospectively validate our screening tool and test its implementation in a real-world clinical environment. As part of this process, clinicians should document barriers and facilitators to widespread implementation. The goal of such screens is to facilitate early identification of postoperative delirium, which will allow timely intervention to address underlying causes and prevent adverse consequences, thereby improving the outcomes of vulnerable older surgical patients.
1. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73-81. https://doi.org/10.1001/jama.2012.6857
2. Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2):160-168. https://doi.org/10.1001/jamasurg.2017.4007
3. Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications after elective surgery in older adults. JAMA Surg. 2015;150(12):1134-1140. https://doi.org/10.1001/jamasurg.2015.2606
4. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192-214. https://doi.org/10.1097/EJA.0000000000000594
5. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. https://doi.org/10.1002/jhm.2418
6. Steensma E, Zhou W, Ngo L, et al. Ultra-brief screeners for detecting delirium superimposed on dementia. J Am Med Dir Assoc. 2019;20(11):1391-1396.e1. https://doi.org/10.1016/j.jamda.2019.05.011
7. Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818.e1-818.e810. https://doi.org/10.1016/j.jamda.2012.08.004
8. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941
9. Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14-21. https://doi.org/10.1177/002383099200500103
10. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526-533. https://doi.org/10.7326/M13-1927
11. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. https://doi.org/10.7326/M14-0865
12. Yang FM, Jones RN, Inouye SK, et al. Selecting optimal screening items for delirium: an application of item response theory. BMC Med Res Methodol. 2013;13(1):8. https://doi.org/10.1186/1471-2288-13-8
13. Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496-502. https://doi.org/10.1093/ageing/afu021
14. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen and the Brief Confusion Assessment Method. Ann Emerg Med. 2013;62(5):457-465. https://doi.org/10.1016/j.annemergmed.2013.05.003
15. Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29(4):368-375. https://doi.org/10.1016/j.jpainsymman.2004.07.009
Delirium is the most common postsurgical complication for older adults, with incidence of 15%-54%, depending on surgery type.1 Increasing numbers of older adults are undergoing surgery2; and those who develop delirium experience negative consequences including longer lengths of stay, higher likelihood of institutional discharge, and increased morbidity and mortality.3 The American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults and the European Society of Anaesthesiology4 recommend routine screening for delirium in those at risk.
Ultrabrief screens are designed to rule out delirium quickly and identify a subset of patients who require further testing.5 Our group, and others, have previously published ultrabrief screens for the general medicine, nonsurgical population and for patients with dementia.5,6 The UB-2 is an ultrabrief screen consisting of “Months of the year backward” (MOYB) and “What day of the week is it?”, which has a sensitivity of 93% and specificity of 64% in hospitalized older adults and takes less than 40 seconds to administer.5 However, no such screens for delirium have been developed for the group with relatively high cognitive and physical functioning undergoing scheduled major surgery in which delirium may present differently. Thus, the purpose of this study was to develop an ultrabrief screen for postoperative delirium using data from a large study of delirium in cognitively intact, older adults undergoing scheduled major noncardiac surgery.
METHODS
We performed a secondary data analysis on 560 patients enrolled between June 18, 2010, and August 8, 2013, in the Successful Aging After Elective Surgery (SAGES) study,7 an ongoing prospective cohort study of older adults undergoing major elective surgeries (eg, total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy). Exclusion criteria included evidence of dementia, delirium, prior hospitalization within 3 months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. The Institutional Review Boards of Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, and Hebrew SeniorLife, all in Boston, Massachusetts, approved the study.
SAGES Delirium Assessment and Additional Variables
The presence or absence of delirium was based on daily in-hospital assessments by trained research staff using the Confusion Assessment Method (CAM)8 long form. The Delirium Symptom Interview (DSI)9 and information related to acute changes in mental status were also included as provided by nursing staff and/or family. Delirium severity was determined using the CAM-S.10 Participants in The SAGES Study had an initial baseline, presurgical assessment in their homes. Cognitive and physical functioning, depression, comorbidities, laboratory, and self-reported demographic data were collected.
Statistical Analyses
We included CAM delirium data from postoperative days (POD) 1 and 2 for each participant, if available; postoperative day 0 was not included because of potential residual anesthetic effects. We chose these days because most delirium began on POD1 or 2, and patients started being discharged on POD3. We considered all one-, two-, and three-item combinations of the 12 cognitive items of the 3D-CAM11 because of their demonstrated high information content for CAM diagnostic features per Item Response Theory.12 There were 12 possible one-item screens, 66 two-item screens, and 220 three-item screens. Sensitivity, specificity, and 95% confidence intervals for each were compared with CAM delirium determination. An ideal ultrabrief screen for delirium has high sensitivity with moderate specificity; general guidelines considered based on investigator consensus included screens with a sensitivity higher than 0.90 and specificity greater than 0.70. Because these screens are used to quickly rule out delirium, we also present the percent positive screen among the entire population (whether delirium is present or not). Screens with a positive screen rate of more than 50% are unlikely to be helpful in ruling out delirium quickly in a large enough fraction of the population. We also required that in multiple item screens, no two items should assess the same CAM feature. For instance, we would eliminate a two-item screen with MOYB and four-digit span since both items measure CAM Feature 2 (Inattention). Finally, we evaluated screen performance separately on POD1 and POD2. Switching screens by POD can be confusing, so we chose a single best screen that retained excellent performance over both days. Data analyses used SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The dataset included 560 adults who had an average age of 76.6 years (SD = 5.2), were 58% women, and were highly educated (15.0 years; SD = 2.9; Table). Postoperative delirium occurred during one or more days in 134 individuals (24%). A total of 1,100 delirium assessments were used, with 113 that were CAM positive (10.3%). For POD1, we used 551 assessments, 61 of which were positive (11.1%); for POD2, 549 assessments were used, with 51 positive (9.3%). Appendix Tables present the positive screen rates, sensitivities, specificities, and 95% confidence intervals of all 12 one-item screens and the 12 best performing two- and three-item screens in order of decreasing sensitivity.

The best ultrabrief screen from POD1 included the following three items: “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?”, with a sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.73 (95% CI, 0.69-0.77). The same combination of items has a sensitivity of 0.88 (95% CI, 0.77-0.96) and a specificity of 0.70 (95% CI, 0.66-0.74) on POD2. When POD1 and POD2 are combined, the sensitivity is 0.92 (95% CI, 0.85-0.96) and specificity is 0.72 (95% CI, 0.69-0.74). We consider this to be our best screen overall.
DISCUSSION
We identified a three-item screen for delirium after elective surgery consisting of “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?” In our own prior work, we identified a two-item screen consisting of MOYB and “What is the day of the week?” as the best ultrabrief screen for delirium in general medicine populations (termed the “UB-2”)5 and a subsequent screen for patients with delirium superimposed on dementia (DSD) including “What type of place is this?”, Days of the Week Backward, and “Does the patient appear sleepy?”6 All three contain a test of attention (a cardinal feature of delirium) and a test of orientation, although the specific test for that varies. Both the surgical and DSD screens include “Does the patient appear sleepy?”, which addresses a reduced level of consciousness. This might be particularly important in the postoperative setting because of residual effects of anesthesia and/or postoperative analgesic medications contributing to delirium. Work done by others confirms our current findings, which is that MOYB is the best single item for most groups. Belleli et al13 and Han et al14 included MOYB as the single attentional item in the 4AT and B-CAM, respectively. The Nu-DESC has been used as a screen in surgical patients; however, it involves only nursing observations and no direct questioning of the patient.15
The Figure describes how our “best screen” could be integrated into clinical care. One or more “positive” or incorrect responses on these three items constitutes a positive screen that should be further evaluated with the CAM or 3D-CAM. If all three items are correct or negative, this effectively rules out delirium; however, continued periodic screening on a daily (or per shift) basis is indicated. On repeat testing, if any of the previously negative or correct items becomes positive or incorrect, this would be evidence for Acute Change, CAM Feature 1. Finally, it should be noted that, if all three items in our best screen are positive, full CAM criteria for delirium diagnosis are met within the screen itself, and no further testing is required. We envision this process being facilitated by use of an app-based program that generates optimal screening items based on patient and setting characteristics.

There are several limitations that must be noted. First, our three-item screen may not generalize to nonsurgical candidates or those undergoing emergent surgery and should be tested in these groups. Second, the SAGES sample is relatively homogenous with respect to racial and ethnic diversity and was highly educated with little functional impairment and no dementia. Therefore, results may not be generalizable to populations with lower educational attainment and/or preexisting mental and physical disabilities. A third limitation is that screen items were included in the reference standard delirium assessment, leading to a potential bias toward increased sensitivity. Finally, all screens were derived from secondary data analysis and further research will be needed to prospectively validate the results. Despite these limitations, this study has several strengths including the use of a well-characterized surgical population and a rigorous approach to delirium measurement. It is one of the first studies to identify a screening tool targeted to identifying delirium in postoperative older adults.
Future research should prospectively validate our screening tool and test its implementation in a real-world clinical environment. As part of this process, clinicians should document barriers and facilitators to widespread implementation. The goal of such screens is to facilitate early identification of postoperative delirium, which will allow timely intervention to address underlying causes and prevent adverse consequences, thereby improving the outcomes of vulnerable older surgical patients.
Delirium is the most common postsurgical complication for older adults, with incidence of 15%-54%, depending on surgery type.1 Increasing numbers of older adults are undergoing surgery2; and those who develop delirium experience negative consequences including longer lengths of stay, higher likelihood of institutional discharge, and increased morbidity and mortality.3 The American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults and the European Society of Anaesthesiology4 recommend routine screening for delirium in those at risk.
Ultrabrief screens are designed to rule out delirium quickly and identify a subset of patients who require further testing.5 Our group, and others, have previously published ultrabrief screens for the general medicine, nonsurgical population and for patients with dementia.5,6 The UB-2 is an ultrabrief screen consisting of “Months of the year backward” (MOYB) and “What day of the week is it?”, which has a sensitivity of 93% and specificity of 64% in hospitalized older adults and takes less than 40 seconds to administer.5 However, no such screens for delirium have been developed for the group with relatively high cognitive and physical functioning undergoing scheduled major surgery in which delirium may present differently. Thus, the purpose of this study was to develop an ultrabrief screen for postoperative delirium using data from a large study of delirium in cognitively intact, older adults undergoing scheduled major noncardiac surgery.
METHODS
We performed a secondary data analysis on 560 patients enrolled between June 18, 2010, and August 8, 2013, in the Successful Aging After Elective Surgery (SAGES) study,7 an ongoing prospective cohort study of older adults undergoing major elective surgeries (eg, total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy). Exclusion criteria included evidence of dementia, delirium, prior hospitalization within 3 months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. The Institutional Review Boards of Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, and Hebrew SeniorLife, all in Boston, Massachusetts, approved the study.
SAGES Delirium Assessment and Additional Variables
The presence or absence of delirium was based on daily in-hospital assessments by trained research staff using the Confusion Assessment Method (CAM)8 long form. The Delirium Symptom Interview (DSI)9 and information related to acute changes in mental status were also included as provided by nursing staff and/or family. Delirium severity was determined using the CAM-S.10 Participants in The SAGES Study had an initial baseline, presurgical assessment in their homes. Cognitive and physical functioning, depression, comorbidities, laboratory, and self-reported demographic data were collected.
Statistical Analyses
We included CAM delirium data from postoperative days (POD) 1 and 2 for each participant, if available; postoperative day 0 was not included because of potential residual anesthetic effects. We chose these days because most delirium began on POD1 or 2, and patients started being discharged on POD3. We considered all one-, two-, and three-item combinations of the 12 cognitive items of the 3D-CAM11 because of their demonstrated high information content for CAM diagnostic features per Item Response Theory.12 There were 12 possible one-item screens, 66 two-item screens, and 220 three-item screens. Sensitivity, specificity, and 95% confidence intervals for each were compared with CAM delirium determination. An ideal ultrabrief screen for delirium has high sensitivity with moderate specificity; general guidelines considered based on investigator consensus included screens with a sensitivity higher than 0.90 and specificity greater than 0.70. Because these screens are used to quickly rule out delirium, we also present the percent positive screen among the entire population (whether delirium is present or not). Screens with a positive screen rate of more than 50% are unlikely to be helpful in ruling out delirium quickly in a large enough fraction of the population. We also required that in multiple item screens, no two items should assess the same CAM feature. For instance, we would eliminate a two-item screen with MOYB and four-digit span since both items measure CAM Feature 2 (Inattention). Finally, we evaluated screen performance separately on POD1 and POD2. Switching screens by POD can be confusing, so we chose a single best screen that retained excellent performance over both days. Data analyses used SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The dataset included 560 adults who had an average age of 76.6 years (SD = 5.2), were 58% women, and were highly educated (15.0 years; SD = 2.9; Table). Postoperative delirium occurred during one or more days in 134 individuals (24%). A total of 1,100 delirium assessments were used, with 113 that were CAM positive (10.3%). For POD1, we used 551 assessments, 61 of which were positive (11.1%); for POD2, 549 assessments were used, with 51 positive (9.3%). Appendix Tables present the positive screen rates, sensitivities, specificities, and 95% confidence intervals of all 12 one-item screens and the 12 best performing two- and three-item screens in order of decreasing sensitivity.

The best ultrabrief screen from POD1 included the following three items: “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?”, with a sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.73 (95% CI, 0.69-0.77). The same combination of items has a sensitivity of 0.88 (95% CI, 0.77-0.96) and a specificity of 0.70 (95% CI, 0.66-0.74) on POD2. When POD1 and POD2 are combined, the sensitivity is 0.92 (95% CI, 0.85-0.96) and specificity is 0.72 (95% CI, 0.69-0.74). We consider this to be our best screen overall.
DISCUSSION
We identified a three-item screen for delirium after elective surgery consisting of “Does the patient report feeling confused?”, MOYB, and “Does the patient appear sleepy?” In our own prior work, we identified a two-item screen consisting of MOYB and “What is the day of the week?” as the best ultrabrief screen for delirium in general medicine populations (termed the “UB-2”)5 and a subsequent screen for patients with delirium superimposed on dementia (DSD) including “What type of place is this?”, Days of the Week Backward, and “Does the patient appear sleepy?”6 All three contain a test of attention (a cardinal feature of delirium) and a test of orientation, although the specific test for that varies. Both the surgical and DSD screens include “Does the patient appear sleepy?”, which addresses a reduced level of consciousness. This might be particularly important in the postoperative setting because of residual effects of anesthesia and/or postoperative analgesic medications contributing to delirium. Work done by others confirms our current findings, which is that MOYB is the best single item for most groups. Belleli et al13 and Han et al14 included MOYB as the single attentional item in the 4AT and B-CAM, respectively. The Nu-DESC has been used as a screen in surgical patients; however, it involves only nursing observations and no direct questioning of the patient.15
The Figure describes how our “best screen” could be integrated into clinical care. One or more “positive” or incorrect responses on these three items constitutes a positive screen that should be further evaluated with the CAM or 3D-CAM. If all three items are correct or negative, this effectively rules out delirium; however, continued periodic screening on a daily (or per shift) basis is indicated. On repeat testing, if any of the previously negative or correct items becomes positive or incorrect, this would be evidence for Acute Change, CAM Feature 1. Finally, it should be noted that, if all three items in our best screen are positive, full CAM criteria for delirium diagnosis are met within the screen itself, and no further testing is required. We envision this process being facilitated by use of an app-based program that generates optimal screening items based on patient and setting characteristics.

There are several limitations that must be noted. First, our three-item screen may not generalize to nonsurgical candidates or those undergoing emergent surgery and should be tested in these groups. Second, the SAGES sample is relatively homogenous with respect to racial and ethnic diversity and was highly educated with little functional impairment and no dementia. Therefore, results may not be generalizable to populations with lower educational attainment and/or preexisting mental and physical disabilities. A third limitation is that screen items were included in the reference standard delirium assessment, leading to a potential bias toward increased sensitivity. Finally, all screens were derived from secondary data analysis and further research will be needed to prospectively validate the results. Despite these limitations, this study has several strengths including the use of a well-characterized surgical population and a rigorous approach to delirium measurement. It is one of the first studies to identify a screening tool targeted to identifying delirium in postoperative older adults.
Future research should prospectively validate our screening tool and test its implementation in a real-world clinical environment. As part of this process, clinicians should document barriers and facilitators to widespread implementation. The goal of such screens is to facilitate early identification of postoperative delirium, which will allow timely intervention to address underlying causes and prevent adverse consequences, thereby improving the outcomes of vulnerable older surgical patients.
1. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73-81. https://doi.org/10.1001/jama.2012.6857
2. Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2):160-168. https://doi.org/10.1001/jamasurg.2017.4007
3. Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications after elective surgery in older adults. JAMA Surg. 2015;150(12):1134-1140. https://doi.org/10.1001/jamasurg.2015.2606
4. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192-214. https://doi.org/10.1097/EJA.0000000000000594
5. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. https://doi.org/10.1002/jhm.2418
6. Steensma E, Zhou W, Ngo L, et al. Ultra-brief screeners for detecting delirium superimposed on dementia. J Am Med Dir Assoc. 2019;20(11):1391-1396.e1. https://doi.org/10.1016/j.jamda.2019.05.011
7. Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818.e1-818.e810. https://doi.org/10.1016/j.jamda.2012.08.004
8. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941
9. Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14-21. https://doi.org/10.1177/002383099200500103
10. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526-533. https://doi.org/10.7326/M13-1927
11. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. https://doi.org/10.7326/M14-0865
12. Yang FM, Jones RN, Inouye SK, et al. Selecting optimal screening items for delirium: an application of item response theory. BMC Med Res Methodol. 2013;13(1):8. https://doi.org/10.1186/1471-2288-13-8
13. Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496-502. https://doi.org/10.1093/ageing/afu021
14. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen and the Brief Confusion Assessment Method. Ann Emerg Med. 2013;62(5):457-465. https://doi.org/10.1016/j.annemergmed.2013.05.003
15. Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29(4):368-375. https://doi.org/10.1016/j.jpainsymman.2004.07.009
1. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73-81. https://doi.org/10.1001/jama.2012.6857
2. Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2):160-168. https://doi.org/10.1001/jamasurg.2017.4007
3. Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications after elective surgery in older adults. JAMA Surg. 2015;150(12):1134-1140. https://doi.org/10.1001/jamasurg.2015.2606
4. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192-214. https://doi.org/10.1097/EJA.0000000000000594
5. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. https://doi.org/10.1002/jhm.2418
6. Steensma E, Zhou W, Ngo L, et al. Ultra-brief screeners for detecting delirium superimposed on dementia. J Am Med Dir Assoc. 2019;20(11):1391-1396.e1. https://doi.org/10.1016/j.jamda.2019.05.011
7. Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818.e1-818.e810. https://doi.org/10.1016/j.jamda.2012.08.004
8. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941
9. Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14-21. https://doi.org/10.1177/002383099200500103
10. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526-533. https://doi.org/10.7326/M13-1927
11. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. https://doi.org/10.7326/M14-0865
12. Yang FM, Jones RN, Inouye SK, et al. Selecting optimal screening items for delirium: an application of item response theory. BMC Med Res Methodol. 2013;13(1):8. https://doi.org/10.1186/1471-2288-13-8
13. Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496-502. https://doi.org/10.1093/ageing/afu021
14. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen and the Brief Confusion Assessment Method. Ann Emerg Med. 2013;62(5):457-465. https://doi.org/10.1016/j.annemergmed.2013.05.003
15. Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29(4):368-375. https://doi.org/10.1016/j.jpainsymman.2004.07.009
© Society of Hospital Medicine
The Effects of Care Team Roles on Situation Awareness in the Pediatric Intensive Care Unit: A Prospective Cross-Sectional Study
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).
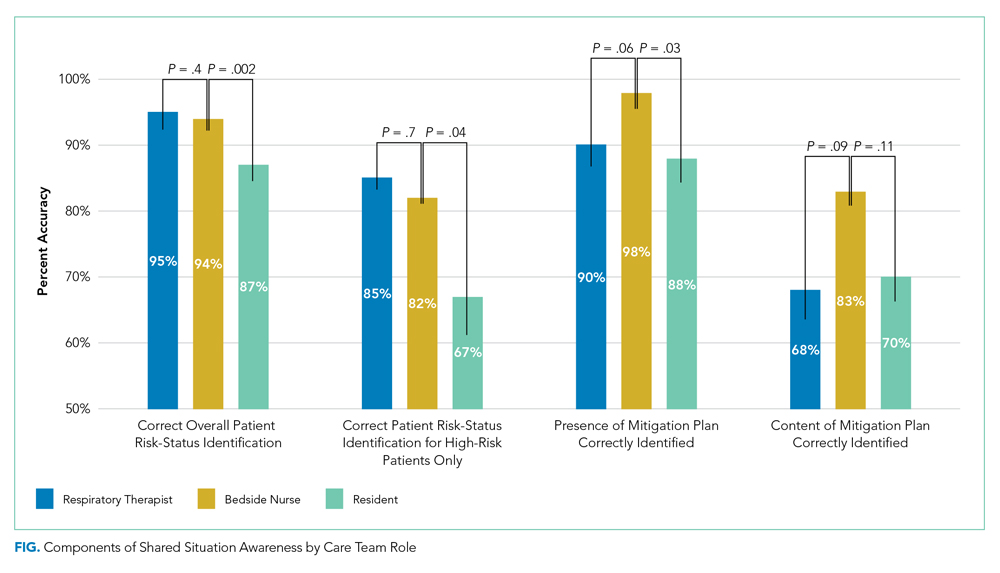
When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: 2019 American Thoracic Society/Infectious Diseases Society of America Update on Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)
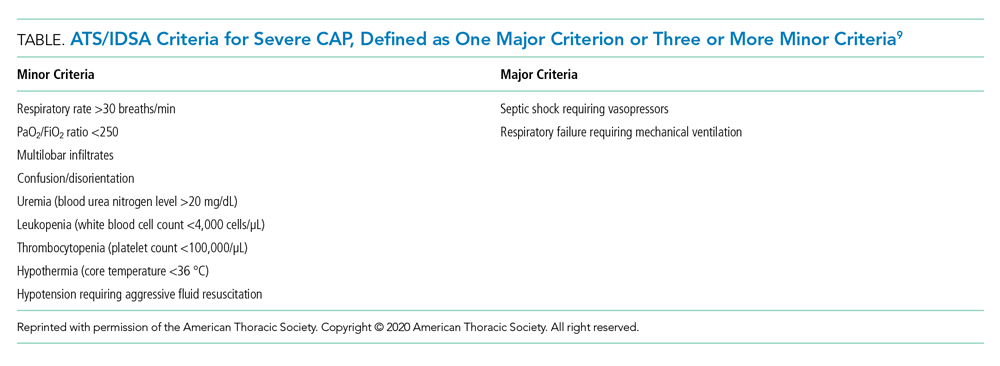
The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)

The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)

The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
© 2020 Society of Hospital Medicine
Clinical Progress Note: Goal-Directed N-acetylcysteine Treatment of Acetaminophen Toxicity
According to the most recent Annual Report of the American Association of Poison Control Centers’ National Poison Data System, there were 51,081 patient exposures to acetaminophen or acetaminophen-containing products treated in a healthcare facility in 2018.1 The toxicity of acetaminophen is mediated by its metabolism into the electrophile N-acetyl-p-benzoquinone imine (NAPQI).2 If the amount of acetaminophen ingested is higher than recommended dosages, glutathione stores may become depleted as the liver tries to detoxify NAPQI and may no longer able to keep up with the demand. As the patient progresses 24 to 36 hours after ingestion, the onset of hepatic injury becomes apparent with elevations in aspartate aminotransferase (AST). AST elevations, should they occur, are almost always present by 36 hours.2 Maximum hepatic dysfunction, as well as elevations in prothrombin time, bilirubin, and serum creatinine, occurs 72 to 96 hours after a toxic ingestion. As a result of marked age-associated differences in the process of conjugating acetaminophen metabolites, infants and young children may be less susceptible to acetaminophen-associated hepatotoxicity, compared with their adult counterparts.3,4
This clinical progress note addresses acetaminophen toxicity treatment, focusing on N-acetylcysteine (NAC) dosing protocols and the 2017 American College of Medical Toxicology (ACMT) position statement, Duration of Intravenous Acetylcysteine Therapy Following Acetaminophen Overdose.5 We conducted a literature search via the PubMed database. The authors began by using the following Medical Subject Headings terms “acetaminophen overdose [title],” which yielded 299 articles, “acetaminophen hepatotoxicity [title],” which yielded 283 articles, and “acetaminophen N-acetylcysteine [title],” which yielded 335 titles. Variations of these terms were used to ensure exhaustive search results. The search results were reviewed for applicability to acetaminophen poisoning and especially goal-directed N-acetylcysteine treatment strategies.
INITIAL TREATMENT
Clarifying a patient’s history of ingestion is a crucial first step to the diagnosis and risk evaluation of acetaminophen-poisoned patients. The initial laboratory workup should include, at minimum, measurement (at 4 or more hours after ingestion) of serum acetaminophen level, AST and alanine aminotransferase (ALT), bilirubin, alkaline phosphatase, creatinine, prothrombin time and international normalized ratio (PT/INR), lactate, and ethanol levels. Gastric decontamination is not appropriate in most cases because of the rapid absorption of acetaminophen.2 Activated charcoal is most effective when given within the first 2 hours following ingestion. For acetaminophen-poisoned patients with severe hepatic toxicity, treating clinicians should consider consultation with a medical toxicologist, an individual with expertise, or the local Poison Control Center.5
The Rumack-Matthew nomogram provides guidance on the initiation of antidotal treatment for patients with acetaminophen overdose. This nomogram was developed to exhibit high sensitivity in patients at risk for hepatic damage from acetaminophen overdose.6 The mainstay for treatment of acetaminophen poisonings is NAC. Early administration of NAC can replenish depleted hepatic glutathione stores, which restores the liver’s ability to detoxify the toxic metabolite NAPQI. When administered within 6 to 8 hours following ingestion, NAC is nearly universally effective at preventing the subsequent hepatic damage from NAPQI.7 Consideration of individualized factors should lead the treating clinician to use the Rumack-Matthew nomogram within the appropriate contexts. Factors such as an unreliable timeline of ingestion by patients, concomitantly ingested medications, and patient-specific factors that might alter the absorption of acetaminophen or impact serum acetaminophen concentrations should be considered. While this nomogram is helpful for decision-making pertaining to the initiation of NAC, further management and continuation of treatment must be patient specific and determined by the treating clinician.
In the United States, intravenous and oral formulations of NAC are available for treatment of acetaminophen toxicity. These formulations are equally efficacious in treating acetaminophen toxicity, with the exception of established hepatic failure, for which only the intravenous route has been studied.8 Oral administration results in vomiting in approximately 20% of patients, while intravenous administration may uncommonly result in severe anaphylactoid reactions. Three scenarios exist in which the intravenous route is preferred over the oral route: acetaminophen toxicity in pregnant women, acetaminophen-induced hepatic failure, and intractable vomiting preventing treatment with oral therapy.2 The intravenous formulation is Food and Drug Administration–approved for a 21-hour treatment duration, while the oral formulation is traditionally administered for a 72-hour duration.9,10
LIMITATIONS OF CURRENT TREATMENT ALGORITHMS
Treatment of acetaminophen poisoning must be tailored on an individual basis and should not follow predetermined durations of treatment. Not only might the FDA-approved 21-hour course of intravenous NAC be inadequate, but the standard 72-hour oral regimen duration may be excessive for many patients. Both the 21-hour and 72-hour protocols were developed based on the half-life and expected elimination of acetaminophen from the body. The traditional 72-hour oral protocol used in the United States was approved in 1985 and was based on the observation that, in patients who developed acetaminophen-induced fatal hepatotoxicity, the acetaminophen half-life was 12 hours, requiring five half-lives to eliminate the drug, for a total of 60 hours.11 The FDA then added an additional 12 hours to provide an additional margin of safety. Researchers in the United Kingdom developed an intravenous treatment protocol based on a shorter 4-hour acetaminophen half-life, calculated from observations of a larger pool of acetaminophen-poisoned patients and not just those with fatal hepatotoxicity.12 This 21-hour protocol—slightly longer than the five 4-hour half-lives needed—was subsequently approved in the United States as the intravenous formulation’s duration in 2004.
Case reports of hepatic failure have described treatment failures of intravenous NAC after the completion of the standard 21 hour treatment regimen.13 In a case report by Smith et al, a patient with significant single ingestion is described who ultimately developed hepatotoxicity despite receiving the FDA-approved 21-hour infusion.13 This patient ingested approximately 96 immediate-release, 500-mg acetaminophen tablets over approximately 1 hour. The initial acetaminophen concentration was 264 mcg/mL at 2.25 hours after ingestion. At 21 hours after ingestion, the patient’s acetaminophen levels remained elevated at 116 mcg/mL, but therapy with NAC was discontinued per protocol because AST and ALT levels were normal. A second peak in serum acetaminophen levels occurred after discontinuation of NAC, with levels reaching 228 mcg/mL approximately 48 hours after ingestion. Liver function worsened over the next few days with elevations in AST of greater than 4,000 U/L, PT of 51.4 seconds, and ammonia of 165 mcg/dL. Intravenous NAC was restarted and hepatic function improved gradually. The patient was ultimately discharged from the hospital. This case and others highlight the possible pitfalls of applying a protocolized, fixed treatment duration of NAC to all patients.
GOAL-DIRECTED TREATMENT RECOMMENDATIONS
Based on individual ingestion timing, quantities, and pharmacokinetics, patients should have pertinent laboratory markers monitored at least every 24 hours from presentation. The clinician may then consider the need to continue or discontinue NAC treatment every 24 hours from the initiation of NAC. Although notable limitations exist for both intravenous and oral treatment regimens, the literature uniformly recommends treating until serum acetaminophen levels are undetectable, regardless of NAC administration route. Based on the metabolism of acetaminophen, as long as serum concentrations of acetaminophen persist, further metabolism to the toxic NAPQI metabolite is possible. The ACMT addressed these concerns with the 2017 position paper, which strongly recommends all of the following criteria to be present prior to discontinuation of intravenous NAC: undetectable acetaminophen concentration, improving hepatic aminotransferases, and improving prognostic markers, including creatinine, lactate, pH, PT/INR, and phosphate.5 In cases in which laboratory and clinical parameters are abnormal, continued treatment beyond the established standard NAC treatment durations is warranted. However, once NAC treatment is extended beyond established protocol lengths, the subsequent dosing regimens that should be employed in these patients are not well studied. The ACMT Position Statement states the administration of an additional NAC bolus or extending the duration of the 6.25 mg/kg per hour maintenance infusion may be appropriate in this subset of patients requiring continued treatment.
Based on the positive outcomes in most patients receiving the 21-hour intravenous NAC protocol, the necessity of an additional 2 days of NAC treatment with the 72-hour oral regimen is uncertain. The ACMT Position Statement states “evidence supports using shorter oral NAC courses, provided that liver enzymes and synthetic function are normal or improving, and plasma acetaminophen concentration is undetectable.”5 Based on this and available literature, patients with a lower risk for developing hepatotoxicity may have oral NAC therapy discontinued after 24 to 48 hours of treatment if specific lab and patient parameters are within defined criteria.
The ACMT does not differentiate between adult and pediatric recommendations. However, the data used to develop the ACMT goal-directed treatment recommendations included both adult and pediatric patients.14,15 The same goal-directed treatment principles may be applied to pediatric patients, though studies have not specifically addressed pediatric goal-directed treatment of acetaminophen poisoning.
CONCLUSION
Acetaminophen-poisoned patients require evaluation not just prior to initiation of treatment, but also when deciding to discontinue NAC therapy. New literature supports reconsideration of standardized 21- and 72-hour NAC treatment protocols, as well as the notion that treatment duration should be guided by laboratory parameters rather than by route of NAC administration. Goal-directed or patient-tailored treatment is also supported by the ACMT.5 Treatment with NAC should continue in patients displaying hepatic toxicity or persistent elevations in acetaminophen levels. In these cases, the optimal treatment regimen in these cases remains unknown and further research into this area is merited.
1. Gummin D, Mowry J, Spyker D, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220‐1413. https://doi.org/10.1080/15563650.2019.1677022
2. Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies, Tenth Edition. McGraw Hill Education; 2015.
3. Miller RP, Roberts RJ, Fischer LJ. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther. 1976;19:284-294. https://doi.org/10.1002/cpt1976193284
4. Rumack BH. Acetaminophen overdose in young children: treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child. 1984;138(5):428-433. https://doi.org/10.1001/archpedi.1984.02140430006003
5. American College of Medical Toxicology. ACMT position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017;13(1):126-127. https://doi.org/10.1007/s13181-016-0542-z
6. Matthew H. Acute acetaminophen poisoning. Clin Toxicol. 1973;6(1):9-11. https://doi.org/10.3109/15563657308991037
7. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319(24):1557-1562. https://doi.org/10.1056/nejm198812153192401
8. Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303(6809):1026-1029. https://doi.org/10.1136/bmj.303.6809.1026
9. Acetadote ® (acetylcysteine injection). Package Insert. Cumberland Pharmaceuticals; 2004.
10. Acetylcysteine Solution. Package insert. Roxanne Laboratories; 2007.
11. Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila). 2017;55(8):879-892. https://doi.org/10.1080/15563650.2017.1317349
12. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S-298S. https://doi.org/10.1111/j.1365-2125.1980.tb01812.x
13. Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N-acetylcysteine therapy. Ann Pharmacother. 2008;42(9):1333-1339. https://doi.org/10.1345/aph.1k680
14. Woo OF, Mueller PD, Olsen KR, Anderson B, Kim SY. Shorter duration of oral N-Acetylcysteine therapy for acute acetaminophen overdose. Ann Emerg Med. 2000; 35(4):363-368. https://doi.org/10.1016/S0196-0644(00)70055-2
15. Betten D, Cantrell F, Thomas S, Williams S, Clark R. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50(3):272-279. https://doi.org/10.1016/j.annemergmed.2006.11.010
According to the most recent Annual Report of the American Association of Poison Control Centers’ National Poison Data System, there were 51,081 patient exposures to acetaminophen or acetaminophen-containing products treated in a healthcare facility in 2018.1 The toxicity of acetaminophen is mediated by its metabolism into the electrophile N-acetyl-p-benzoquinone imine (NAPQI).2 If the amount of acetaminophen ingested is higher than recommended dosages, glutathione stores may become depleted as the liver tries to detoxify NAPQI and may no longer able to keep up with the demand. As the patient progresses 24 to 36 hours after ingestion, the onset of hepatic injury becomes apparent with elevations in aspartate aminotransferase (AST). AST elevations, should they occur, are almost always present by 36 hours.2 Maximum hepatic dysfunction, as well as elevations in prothrombin time, bilirubin, and serum creatinine, occurs 72 to 96 hours after a toxic ingestion. As a result of marked age-associated differences in the process of conjugating acetaminophen metabolites, infants and young children may be less susceptible to acetaminophen-associated hepatotoxicity, compared with their adult counterparts.3,4
This clinical progress note addresses acetaminophen toxicity treatment, focusing on N-acetylcysteine (NAC) dosing protocols and the 2017 American College of Medical Toxicology (ACMT) position statement, Duration of Intravenous Acetylcysteine Therapy Following Acetaminophen Overdose.5 We conducted a literature search via the PubMed database. The authors began by using the following Medical Subject Headings terms “acetaminophen overdose [title],” which yielded 299 articles, “acetaminophen hepatotoxicity [title],” which yielded 283 articles, and “acetaminophen N-acetylcysteine [title],” which yielded 335 titles. Variations of these terms were used to ensure exhaustive search results. The search results were reviewed for applicability to acetaminophen poisoning and especially goal-directed N-acetylcysteine treatment strategies.
INITIAL TREATMENT
Clarifying a patient’s history of ingestion is a crucial first step to the diagnosis and risk evaluation of acetaminophen-poisoned patients. The initial laboratory workup should include, at minimum, measurement (at 4 or more hours after ingestion) of serum acetaminophen level, AST and alanine aminotransferase (ALT), bilirubin, alkaline phosphatase, creatinine, prothrombin time and international normalized ratio (PT/INR), lactate, and ethanol levels. Gastric decontamination is not appropriate in most cases because of the rapid absorption of acetaminophen.2 Activated charcoal is most effective when given within the first 2 hours following ingestion. For acetaminophen-poisoned patients with severe hepatic toxicity, treating clinicians should consider consultation with a medical toxicologist, an individual with expertise, or the local Poison Control Center.5
The Rumack-Matthew nomogram provides guidance on the initiation of antidotal treatment for patients with acetaminophen overdose. This nomogram was developed to exhibit high sensitivity in patients at risk for hepatic damage from acetaminophen overdose.6 The mainstay for treatment of acetaminophen poisonings is NAC. Early administration of NAC can replenish depleted hepatic glutathione stores, which restores the liver’s ability to detoxify the toxic metabolite NAPQI. When administered within 6 to 8 hours following ingestion, NAC is nearly universally effective at preventing the subsequent hepatic damage from NAPQI.7 Consideration of individualized factors should lead the treating clinician to use the Rumack-Matthew nomogram within the appropriate contexts. Factors such as an unreliable timeline of ingestion by patients, concomitantly ingested medications, and patient-specific factors that might alter the absorption of acetaminophen or impact serum acetaminophen concentrations should be considered. While this nomogram is helpful for decision-making pertaining to the initiation of NAC, further management and continuation of treatment must be patient specific and determined by the treating clinician.
In the United States, intravenous and oral formulations of NAC are available for treatment of acetaminophen toxicity. These formulations are equally efficacious in treating acetaminophen toxicity, with the exception of established hepatic failure, for which only the intravenous route has been studied.8 Oral administration results in vomiting in approximately 20% of patients, while intravenous administration may uncommonly result in severe anaphylactoid reactions. Three scenarios exist in which the intravenous route is preferred over the oral route: acetaminophen toxicity in pregnant women, acetaminophen-induced hepatic failure, and intractable vomiting preventing treatment with oral therapy.2 The intravenous formulation is Food and Drug Administration–approved for a 21-hour treatment duration, while the oral formulation is traditionally administered for a 72-hour duration.9,10
LIMITATIONS OF CURRENT TREATMENT ALGORITHMS
Treatment of acetaminophen poisoning must be tailored on an individual basis and should not follow predetermined durations of treatment. Not only might the FDA-approved 21-hour course of intravenous NAC be inadequate, but the standard 72-hour oral regimen duration may be excessive for many patients. Both the 21-hour and 72-hour protocols were developed based on the half-life and expected elimination of acetaminophen from the body. The traditional 72-hour oral protocol used in the United States was approved in 1985 and was based on the observation that, in patients who developed acetaminophen-induced fatal hepatotoxicity, the acetaminophen half-life was 12 hours, requiring five half-lives to eliminate the drug, for a total of 60 hours.11 The FDA then added an additional 12 hours to provide an additional margin of safety. Researchers in the United Kingdom developed an intravenous treatment protocol based on a shorter 4-hour acetaminophen half-life, calculated from observations of a larger pool of acetaminophen-poisoned patients and not just those with fatal hepatotoxicity.12 This 21-hour protocol—slightly longer than the five 4-hour half-lives needed—was subsequently approved in the United States as the intravenous formulation’s duration in 2004.
Case reports of hepatic failure have described treatment failures of intravenous NAC after the completion of the standard 21 hour treatment regimen.13 In a case report by Smith et al, a patient with significant single ingestion is described who ultimately developed hepatotoxicity despite receiving the FDA-approved 21-hour infusion.13 This patient ingested approximately 96 immediate-release, 500-mg acetaminophen tablets over approximately 1 hour. The initial acetaminophen concentration was 264 mcg/mL at 2.25 hours after ingestion. At 21 hours after ingestion, the patient’s acetaminophen levels remained elevated at 116 mcg/mL, but therapy with NAC was discontinued per protocol because AST and ALT levels were normal. A second peak in serum acetaminophen levels occurred after discontinuation of NAC, with levels reaching 228 mcg/mL approximately 48 hours after ingestion. Liver function worsened over the next few days with elevations in AST of greater than 4,000 U/L, PT of 51.4 seconds, and ammonia of 165 mcg/dL. Intravenous NAC was restarted and hepatic function improved gradually. The patient was ultimately discharged from the hospital. This case and others highlight the possible pitfalls of applying a protocolized, fixed treatment duration of NAC to all patients.
GOAL-DIRECTED TREATMENT RECOMMENDATIONS
Based on individual ingestion timing, quantities, and pharmacokinetics, patients should have pertinent laboratory markers monitored at least every 24 hours from presentation. The clinician may then consider the need to continue or discontinue NAC treatment every 24 hours from the initiation of NAC. Although notable limitations exist for both intravenous and oral treatment regimens, the literature uniformly recommends treating until serum acetaminophen levels are undetectable, regardless of NAC administration route. Based on the metabolism of acetaminophen, as long as serum concentrations of acetaminophen persist, further metabolism to the toxic NAPQI metabolite is possible. The ACMT addressed these concerns with the 2017 position paper, which strongly recommends all of the following criteria to be present prior to discontinuation of intravenous NAC: undetectable acetaminophen concentration, improving hepatic aminotransferases, and improving prognostic markers, including creatinine, lactate, pH, PT/INR, and phosphate.5 In cases in which laboratory and clinical parameters are abnormal, continued treatment beyond the established standard NAC treatment durations is warranted. However, once NAC treatment is extended beyond established protocol lengths, the subsequent dosing regimens that should be employed in these patients are not well studied. The ACMT Position Statement states the administration of an additional NAC bolus or extending the duration of the 6.25 mg/kg per hour maintenance infusion may be appropriate in this subset of patients requiring continued treatment.
Based on the positive outcomes in most patients receiving the 21-hour intravenous NAC protocol, the necessity of an additional 2 days of NAC treatment with the 72-hour oral regimen is uncertain. The ACMT Position Statement states “evidence supports using shorter oral NAC courses, provided that liver enzymes and synthetic function are normal or improving, and plasma acetaminophen concentration is undetectable.”5 Based on this and available literature, patients with a lower risk for developing hepatotoxicity may have oral NAC therapy discontinued after 24 to 48 hours of treatment if specific lab and patient parameters are within defined criteria.
The ACMT does not differentiate between adult and pediatric recommendations. However, the data used to develop the ACMT goal-directed treatment recommendations included both adult and pediatric patients.14,15 The same goal-directed treatment principles may be applied to pediatric patients, though studies have not specifically addressed pediatric goal-directed treatment of acetaminophen poisoning.
CONCLUSION
Acetaminophen-poisoned patients require evaluation not just prior to initiation of treatment, but also when deciding to discontinue NAC therapy. New literature supports reconsideration of standardized 21- and 72-hour NAC treatment protocols, as well as the notion that treatment duration should be guided by laboratory parameters rather than by route of NAC administration. Goal-directed or patient-tailored treatment is also supported by the ACMT.5 Treatment with NAC should continue in patients displaying hepatic toxicity or persistent elevations in acetaminophen levels. In these cases, the optimal treatment regimen in these cases remains unknown and further research into this area is merited.
According to the most recent Annual Report of the American Association of Poison Control Centers’ National Poison Data System, there were 51,081 patient exposures to acetaminophen or acetaminophen-containing products treated in a healthcare facility in 2018.1 The toxicity of acetaminophen is mediated by its metabolism into the electrophile N-acetyl-p-benzoquinone imine (NAPQI).2 If the amount of acetaminophen ingested is higher than recommended dosages, glutathione stores may become depleted as the liver tries to detoxify NAPQI and may no longer able to keep up with the demand. As the patient progresses 24 to 36 hours after ingestion, the onset of hepatic injury becomes apparent with elevations in aspartate aminotransferase (AST). AST elevations, should they occur, are almost always present by 36 hours.2 Maximum hepatic dysfunction, as well as elevations in prothrombin time, bilirubin, and serum creatinine, occurs 72 to 96 hours after a toxic ingestion. As a result of marked age-associated differences in the process of conjugating acetaminophen metabolites, infants and young children may be less susceptible to acetaminophen-associated hepatotoxicity, compared with their adult counterparts.3,4
This clinical progress note addresses acetaminophen toxicity treatment, focusing on N-acetylcysteine (NAC) dosing protocols and the 2017 American College of Medical Toxicology (ACMT) position statement, Duration of Intravenous Acetylcysteine Therapy Following Acetaminophen Overdose.5 We conducted a literature search via the PubMed database. The authors began by using the following Medical Subject Headings terms “acetaminophen overdose [title],” which yielded 299 articles, “acetaminophen hepatotoxicity [title],” which yielded 283 articles, and “acetaminophen N-acetylcysteine [title],” which yielded 335 titles. Variations of these terms were used to ensure exhaustive search results. The search results were reviewed for applicability to acetaminophen poisoning and especially goal-directed N-acetylcysteine treatment strategies.
INITIAL TREATMENT
Clarifying a patient’s history of ingestion is a crucial first step to the diagnosis and risk evaluation of acetaminophen-poisoned patients. The initial laboratory workup should include, at minimum, measurement (at 4 or more hours after ingestion) of serum acetaminophen level, AST and alanine aminotransferase (ALT), bilirubin, alkaline phosphatase, creatinine, prothrombin time and international normalized ratio (PT/INR), lactate, and ethanol levels. Gastric decontamination is not appropriate in most cases because of the rapid absorption of acetaminophen.2 Activated charcoal is most effective when given within the first 2 hours following ingestion. For acetaminophen-poisoned patients with severe hepatic toxicity, treating clinicians should consider consultation with a medical toxicologist, an individual with expertise, or the local Poison Control Center.5
The Rumack-Matthew nomogram provides guidance on the initiation of antidotal treatment for patients with acetaminophen overdose. This nomogram was developed to exhibit high sensitivity in patients at risk for hepatic damage from acetaminophen overdose.6 The mainstay for treatment of acetaminophen poisonings is NAC. Early administration of NAC can replenish depleted hepatic glutathione stores, which restores the liver’s ability to detoxify the toxic metabolite NAPQI. When administered within 6 to 8 hours following ingestion, NAC is nearly universally effective at preventing the subsequent hepatic damage from NAPQI.7 Consideration of individualized factors should lead the treating clinician to use the Rumack-Matthew nomogram within the appropriate contexts. Factors such as an unreliable timeline of ingestion by patients, concomitantly ingested medications, and patient-specific factors that might alter the absorption of acetaminophen or impact serum acetaminophen concentrations should be considered. While this nomogram is helpful for decision-making pertaining to the initiation of NAC, further management and continuation of treatment must be patient specific and determined by the treating clinician.
In the United States, intravenous and oral formulations of NAC are available for treatment of acetaminophen toxicity. These formulations are equally efficacious in treating acetaminophen toxicity, with the exception of established hepatic failure, for which only the intravenous route has been studied.8 Oral administration results in vomiting in approximately 20% of patients, while intravenous administration may uncommonly result in severe anaphylactoid reactions. Three scenarios exist in which the intravenous route is preferred over the oral route: acetaminophen toxicity in pregnant women, acetaminophen-induced hepatic failure, and intractable vomiting preventing treatment with oral therapy.2 The intravenous formulation is Food and Drug Administration–approved for a 21-hour treatment duration, while the oral formulation is traditionally administered for a 72-hour duration.9,10
LIMITATIONS OF CURRENT TREATMENT ALGORITHMS
Treatment of acetaminophen poisoning must be tailored on an individual basis and should not follow predetermined durations of treatment. Not only might the FDA-approved 21-hour course of intravenous NAC be inadequate, but the standard 72-hour oral regimen duration may be excessive for many patients. Both the 21-hour and 72-hour protocols were developed based on the half-life and expected elimination of acetaminophen from the body. The traditional 72-hour oral protocol used in the United States was approved in 1985 and was based on the observation that, in patients who developed acetaminophen-induced fatal hepatotoxicity, the acetaminophen half-life was 12 hours, requiring five half-lives to eliminate the drug, for a total of 60 hours.11 The FDA then added an additional 12 hours to provide an additional margin of safety. Researchers in the United Kingdom developed an intravenous treatment protocol based on a shorter 4-hour acetaminophen half-life, calculated from observations of a larger pool of acetaminophen-poisoned patients and not just those with fatal hepatotoxicity.12 This 21-hour protocol—slightly longer than the five 4-hour half-lives needed—was subsequently approved in the United States as the intravenous formulation’s duration in 2004.
Case reports of hepatic failure have described treatment failures of intravenous NAC after the completion of the standard 21 hour treatment regimen.13 In a case report by Smith et al, a patient with significant single ingestion is described who ultimately developed hepatotoxicity despite receiving the FDA-approved 21-hour infusion.13 This patient ingested approximately 96 immediate-release, 500-mg acetaminophen tablets over approximately 1 hour. The initial acetaminophen concentration was 264 mcg/mL at 2.25 hours after ingestion. At 21 hours after ingestion, the patient’s acetaminophen levels remained elevated at 116 mcg/mL, but therapy with NAC was discontinued per protocol because AST and ALT levels were normal. A second peak in serum acetaminophen levels occurred after discontinuation of NAC, with levels reaching 228 mcg/mL approximately 48 hours after ingestion. Liver function worsened over the next few days with elevations in AST of greater than 4,000 U/L, PT of 51.4 seconds, and ammonia of 165 mcg/dL. Intravenous NAC was restarted and hepatic function improved gradually. The patient was ultimately discharged from the hospital. This case and others highlight the possible pitfalls of applying a protocolized, fixed treatment duration of NAC to all patients.
GOAL-DIRECTED TREATMENT RECOMMENDATIONS
Based on individual ingestion timing, quantities, and pharmacokinetics, patients should have pertinent laboratory markers monitored at least every 24 hours from presentation. The clinician may then consider the need to continue or discontinue NAC treatment every 24 hours from the initiation of NAC. Although notable limitations exist for both intravenous and oral treatment regimens, the literature uniformly recommends treating until serum acetaminophen levels are undetectable, regardless of NAC administration route. Based on the metabolism of acetaminophen, as long as serum concentrations of acetaminophen persist, further metabolism to the toxic NAPQI metabolite is possible. The ACMT addressed these concerns with the 2017 position paper, which strongly recommends all of the following criteria to be present prior to discontinuation of intravenous NAC: undetectable acetaminophen concentration, improving hepatic aminotransferases, and improving prognostic markers, including creatinine, lactate, pH, PT/INR, and phosphate.5 In cases in which laboratory and clinical parameters are abnormal, continued treatment beyond the established standard NAC treatment durations is warranted. However, once NAC treatment is extended beyond established protocol lengths, the subsequent dosing regimens that should be employed in these patients are not well studied. The ACMT Position Statement states the administration of an additional NAC bolus or extending the duration of the 6.25 mg/kg per hour maintenance infusion may be appropriate in this subset of patients requiring continued treatment.
Based on the positive outcomes in most patients receiving the 21-hour intravenous NAC protocol, the necessity of an additional 2 days of NAC treatment with the 72-hour oral regimen is uncertain. The ACMT Position Statement states “evidence supports using shorter oral NAC courses, provided that liver enzymes and synthetic function are normal or improving, and plasma acetaminophen concentration is undetectable.”5 Based on this and available literature, patients with a lower risk for developing hepatotoxicity may have oral NAC therapy discontinued after 24 to 48 hours of treatment if specific lab and patient parameters are within defined criteria.
The ACMT does not differentiate between adult and pediatric recommendations. However, the data used to develop the ACMT goal-directed treatment recommendations included both adult and pediatric patients.14,15 The same goal-directed treatment principles may be applied to pediatric patients, though studies have not specifically addressed pediatric goal-directed treatment of acetaminophen poisoning.
CONCLUSION
Acetaminophen-poisoned patients require evaluation not just prior to initiation of treatment, but also when deciding to discontinue NAC therapy. New literature supports reconsideration of standardized 21- and 72-hour NAC treatment protocols, as well as the notion that treatment duration should be guided by laboratory parameters rather than by route of NAC administration. Goal-directed or patient-tailored treatment is also supported by the ACMT.5 Treatment with NAC should continue in patients displaying hepatic toxicity or persistent elevations in acetaminophen levels. In these cases, the optimal treatment regimen in these cases remains unknown and further research into this area is merited.
1. Gummin D, Mowry J, Spyker D, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220‐1413. https://doi.org/10.1080/15563650.2019.1677022
2. Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies, Tenth Edition. McGraw Hill Education; 2015.
3. Miller RP, Roberts RJ, Fischer LJ. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther. 1976;19:284-294. https://doi.org/10.1002/cpt1976193284
4. Rumack BH. Acetaminophen overdose in young children: treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child. 1984;138(5):428-433. https://doi.org/10.1001/archpedi.1984.02140430006003
5. American College of Medical Toxicology. ACMT position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017;13(1):126-127. https://doi.org/10.1007/s13181-016-0542-z
6. Matthew H. Acute acetaminophen poisoning. Clin Toxicol. 1973;6(1):9-11. https://doi.org/10.3109/15563657308991037
7. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319(24):1557-1562. https://doi.org/10.1056/nejm198812153192401
8. Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303(6809):1026-1029. https://doi.org/10.1136/bmj.303.6809.1026
9. Acetadote ® (acetylcysteine injection). Package Insert. Cumberland Pharmaceuticals; 2004.
10. Acetylcysteine Solution. Package insert. Roxanne Laboratories; 2007.
11. Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila). 2017;55(8):879-892. https://doi.org/10.1080/15563650.2017.1317349
12. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S-298S. https://doi.org/10.1111/j.1365-2125.1980.tb01812.x
13. Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N-acetylcysteine therapy. Ann Pharmacother. 2008;42(9):1333-1339. https://doi.org/10.1345/aph.1k680
14. Woo OF, Mueller PD, Olsen KR, Anderson B, Kim SY. Shorter duration of oral N-Acetylcysteine therapy for acute acetaminophen overdose. Ann Emerg Med. 2000; 35(4):363-368. https://doi.org/10.1016/S0196-0644(00)70055-2
15. Betten D, Cantrell F, Thomas S, Williams S, Clark R. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50(3):272-279. https://doi.org/10.1016/j.annemergmed.2006.11.010
1. Gummin D, Mowry J, Spyker D, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220‐1413. https://doi.org/10.1080/15563650.2019.1677022
2. Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies, Tenth Edition. McGraw Hill Education; 2015.
3. Miller RP, Roberts RJ, Fischer LJ. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther. 1976;19:284-294. https://doi.org/10.1002/cpt1976193284
4. Rumack BH. Acetaminophen overdose in young children: treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child. 1984;138(5):428-433. https://doi.org/10.1001/archpedi.1984.02140430006003
5. American College of Medical Toxicology. ACMT position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017;13(1):126-127. https://doi.org/10.1007/s13181-016-0542-z
6. Matthew H. Acute acetaminophen poisoning. Clin Toxicol. 1973;6(1):9-11. https://doi.org/10.3109/15563657308991037
7. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319(24):1557-1562. https://doi.org/10.1056/nejm198812153192401
8. Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303(6809):1026-1029. https://doi.org/10.1136/bmj.303.6809.1026
9. Acetadote ® (acetylcysteine injection). Package Insert. Cumberland Pharmaceuticals; 2004.
10. Acetylcysteine Solution. Package insert. Roxanne Laboratories; 2007.
11. Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila). 2017;55(8):879-892. https://doi.org/10.1080/15563650.2017.1317349
12. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S-298S. https://doi.org/10.1111/j.1365-2125.1980.tb01812.x
13. Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N-acetylcysteine therapy. Ann Pharmacother. 2008;42(9):1333-1339. https://doi.org/10.1345/aph.1k680
14. Woo OF, Mueller PD, Olsen KR, Anderson B, Kim SY. Shorter duration of oral N-Acetylcysteine therapy for acute acetaminophen overdose. Ann Emerg Med. 2000; 35(4):363-368. https://doi.org/10.1016/S0196-0644(00)70055-2
15. Betten D, Cantrell F, Thomas S, Williams S, Clark R. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50(3):272-279. https://doi.org/10.1016/j.annemergmed.2006.11.010
© 2020 Society of Hospital Medicine
The Changing Landscape of Uncomplicated Gram-Negative Bacteremia: A Narrative Review to Guide Inpatient Management
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.
Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33
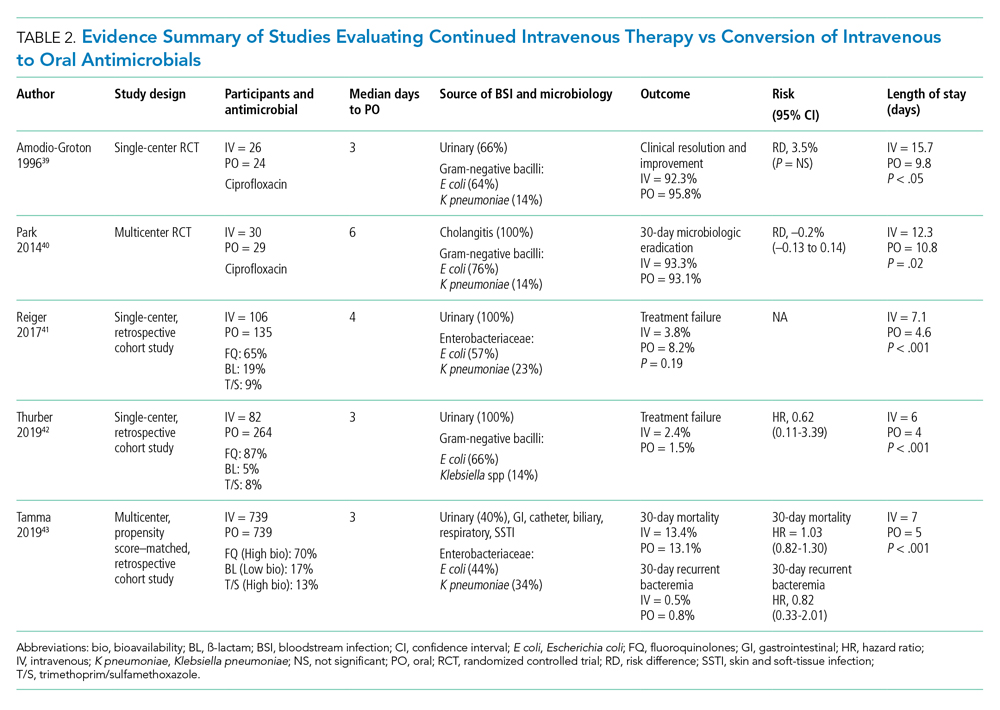
Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45
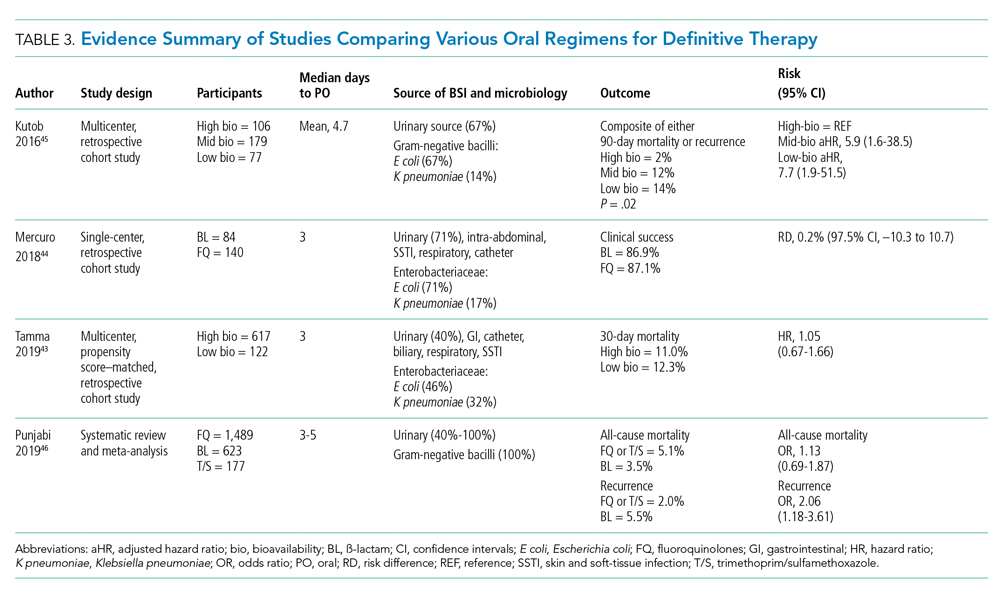
It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.
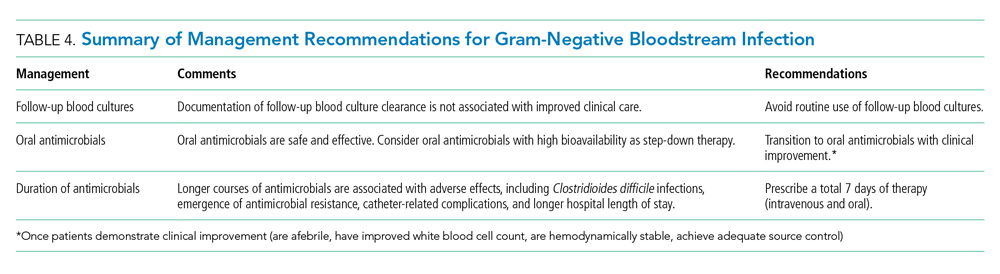
Disclosures
The authors have no conflicts of interest to disclose.
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.

Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33

Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45

It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.

Disclosures
The authors have no conflicts of interest to disclose.
Uncomplicated bacteremia, while not precisely defined in the literature, generally implies bacteremia in the absence of a persistent or difficult-to-eradicate infectious source. Bacteremia secondary to focal infections such as skin and soft-tissue infection, pneumonia, pyelonephritis, or urinary tract infection (UTI) accounts for up to 25% of bloodstream infections (BSIs) and usually resolves with prompt and appropriate antimicrobial therapy.1,2 Current practice guidelines do not adequately address key aspects of the optimal management of gram-negative (GN)–BSI commonly encountered in hospital care.3-7 Notably, antimicrobial duration, criteria to transition from intravenous (IV) to oral step-down therapy, choice of oral antimicrobials, and reassessment of follow-up blood cultures have not been addressed. In the absence of consensus guidelines, clinicians rely on “conventional wisdom” and clinical experience, which may not be supported by scientific rigor. A growing body of research now challenges some long-standing practices once thought to be standard of care.
In this narrative review, we aim to examine and synthesize emerging information to provide an evidence-based framework in the management of hospitalized patients with GN-BSI. We highlight the unintended consequences and potential harms of excessive antimicrobial exposure and focus on areas in the fundamental approach to duration of therapy, the role of oral antimicrobials, and usefulness of follow-up blood cultures. A comprehensive search of the published literature was performed in PubMed with an emphasis on articles published during 2015-2019 with use of search terms including gram-negative bacteremia, duration, antibiotics, adverse effects, intravascular catheter, and follow-up blood cultures.
ANTIMICROBIAL RISKS: ‘PRIMUM NON NOCERE’
Antimicrobial overuse is common and may be driven by concerns for undertreatment. Clinicians may believe that prolonged antimicrobial therapy maximizes cure rates, with treatment duration often defined arbitrarily by a fixed number of “Constantine-units” (dating back to the ancient Roman emperor’s decree of 7 days in a week).8-10 Recent publications refute this notion and point out that the harms of overprescribing outweigh the perceived benefits of longer treatment duration.
Antimicrobials are lifesaving but not benign; adverse effects are common and costly to our patients and healthcare system. Among 1,488 hospitalized adults who received at least 24 hours of systemic antimicrobials, 20% had an antimicrobial-associated adverse event, mostly gastrointestinal, renal, or hematologic in nature.11 Prolonged duration of antimicrobials is further associated with adverse effects such as antimicrobial-associated diarrhea, increased rates of Clostridioides difficile infection (CDI), emergence of antimicrobial resistance, and longer hospital length of stay (LOS).11-15 Vaughn and colleagues conducted the largest observational study to date, evaluating antimicrobial prescriptions for the treatment of nearly 6,500 adults with community-acquired pneumonia in a 43-hospital consortium in Michigan.14 More than two-thirds of patients received antimicrobial courses (median 8 days) that exceeded guideline-recommended duration. Patients who received longer antimicrobial courses did not have reduced mortality, readmission, or emergency department visits. More importantly, each excess day of treatment was associated with a relative 5% increase in the odds of antimicrobial-associated adverse effects reported by patients. This is further supported by national and state hospital data that antimicrobial-associated adverse events are an independent predictor of longer LOS.12
CDI is commonly linked to destructive changes to the indigenous microbiota of the intestinal flora caused by antimicrobial administration. Stevens and colleagues identified 7,792 hospitalized patients who received at least 2 consecutive days of antimicrobial therapy13; comparing 241 cases of CDI with the control group, they observed a dose-dependent risk of CDI associated with increasing cumulative dose, number of antimicrobials, and days of antimicrobial exposure. Compared with patients who received fewer than 4 days of antimicrobials, the adjusted hazard ratios (aHR) for those who received 4-7 days or 8-18 days of therapy were 1.4 (95% CI, 0.8-2.4) and 3.0 (95% CI, 1.9-5.0), respectively. This correlates to a threefold increase in CDI risk for patients who received more than 7 days of antimicrobials. More specifically, the empiric use of antipseudomonal ß-lactams (APBL) for more than 48 hours was also found to be an independent risk factor for CDI among 808 patients with Enterobacteriaceae BSI.16 The risk of CDI within 90 days of BSI was higher among those who received >48 hours of APBL than it was among those who received ≤48 hours (HR, 3.6; 95% CI, 1.5-9.9).
While C difficile may be the most well-known pathogen implicated in antimicrobial usage, the incidence of multidrug-resistant (MDR) organisms, either as infectious or colonizing pathogens, is also tied to antimicrobial exposure. Among patients receiving systemic antimicrobials, 6% developed an MDR infection within 90 days.11 Over a 5-year period, Teshome and colleagues evaluated 7,118 critically ill patients and demonstrated that prolonged exposures to APBLs increased the risk of new antimicrobial resistance within 60 days.15 This resistance pattern was not an institutional or environmental finding but a patient-level finding. For each additional day of cefepime or piperacillin/tazobactam received, the risk of new antimicrobial resistance was increased by 8%. The authors concluded that defining a piperacillin/tazobactam course as 10 vs 7 days would result in a 24% higher relative risk of resistance per patient related to those 3 additional days of antimicrobial exposure.
Catheter complications including thrombophlebitis, infiltration, and infection are serious and frequent problems associated with IV medication administration.17 Even with short-term use, peripherally inserted central catheters (PICCs) carry a substantial risk of venous thrombosis (superficial and deep veins). The incidence of deep vein thrombosis (DVT) for PICCs is estimated between 5% and 15% for hospitalized patients and 2% and 5% for ambulatory patients.18 A recent randomized controlled trial (RCT) of oral vs IV antimicrobials for bone and joint infections reported that, compared with patients randomized to oral antimicrobials, those randomized to IV antimicrobials were more likely to have catheter complications (9.4% vs 1.0%; P < .001) and to discontinue therapy earlier (18.9% vs 12.8%; P = .006).19 Median hospital stay was also significantly longer in the IV group (14 days vs 11 days; P < .001).
SHORTEST EFFECTIVE DURATION: LESS MAY BE MORE
Optimization of antimicrobial duration has long been recognized as one of the key strategies in reducing unnecessary antimicrobial exposure, yet high-quality evidence on comparative effectiveness of duration in the setting of bacteremia has been limited until recently.20 The presence of bacteremia is often used as a justification for prolonged courses of antimicrobial regardless of infection source or clinical response. The Infectious Diseases Society of America guidelines suggest 7 to 14 days of treatment for intravascular catheter-associated gram-negative bacteremia, but the optimal duration for non–catheter-related gram-negative bacteremia is not addressed.21 This lack of clear guidance and the historical scarcity of robust data make it difficult to inform best practices, which leads to wide variability in clinical practice and 14 days being the most prescribed duration.22,23
Pooled clinical trials’ data from subsets of patients with bacteremia and those from observational studies have been the best available evidence for the treatment duration of GN-BSI until recently (Table 1).24-32 Two meta-analyses evaluating RCTs of adult and pediatric patients with pyelonephritis, UTI, peritonitis, and pneumonia found no differences in clinical failure, microbiologic cure, or survival between short and long courses of therapy in the subset of patient with associated bacteremia.24,25 Six heterogeneous RCTs of short vs long courses of therapy for complicated UTI or pyelonephritis reported no differences in clinical cure rates in the subset of patients with associated GN-BSI.2 The observational studies outlined in Table 1 are also consistent with RCT results supporting noninferiority in clinical cure and mortality outcomes between short and long courses of therapy.26-32 These findings may also be extrapolated to immunocompromised hosts given a considerable representation of 10% to 47% of the study population with immunosuppressive conditions.

Nelson and colleagues conducted the only retrospective study to date reporting conflicting results of higher risk of treatment failure (defined as composite endpoint of mortality or recurrent infection within 90 days of index BSI) in patients receiving a short course of therapy.27 However, the difference was driven by 90-day mortality (8.2% vs 3.3%; P = .04) not recurrent infection (6.7% vs 6.5%; P = 0.93). Giannella and colleagues also evaluated 90-day mortality as a primary endpoint in a much larger cohort of over 850 patients in Italy and found no difference in mortality rates between short and long courses of antimicrobials.30
Yahav and colleagues conducted the first well-designed open-label RCT comparing short and long courses of antimicrobials in uncomplicated GN-BSI.33 This noninferiority study randomized more than 600 hospitalized patients with adequate source control who were afebrile and hemodynamically stable for ≥48 hours to receive either 7 days or 14 days of therapy. The source of infections was predominantly urinary (68%), and the causative pathogens were 90% Enterobacteriacae, including 20% MDR strains. The primary outcome was a composite of 90-day all-cause mortality or clinical failure defined as either relapse of bacteremia, local or distant complications, readmission, or extended hospital stay >14 days. The authors reported no statistically significant differences in the primary outcome between short (45.8%) and long (48.3%) courses of treatment. In the prespecified post hoc analysis designed to evaluate infection-related outcomes at an earlier time frame, there were no observed differences in complications, relapses, or mortality between study groups at 14 and 28 days. Further subgroup analysis demonstrated similar results among patients with MDR pathogens, primarily extended-spectrum ß-lactamases (ESBL). Interestingly, there was a more rapid return to baseline activity and functional capacity among patients randomized to a short course of therapy. The authors acknowledged that the patients’ perception of illness while taking antimicrobials may have influenced self-reported well-being and functional performance. In exploratory analysis, prolonged hospitalization and readmission were excluded from the primary study endpoint to mirror outcomes assessed by Nelson and colleagues. There were no statistically significant differences in death, relapses, or complications between groups randomized to short (18.6%) or long (15.1%) courses of therapy, with a risk difference of 3.5% (95% CI, –2.5% to 9.5%) in this study population.
Patients with Pseudomonas aeruginosa BSI often have more chronic medical comorbidities, immunocompromised conditions, higher severity of illness, and more indwelling catheters than do patients with Enterobacteriaceae BSI.32 It is uncertain whether shorter duration of therapy is generalizable to this population, given that Pseudomonas accounted for a relatively low number (8%) of infections in the published RCT.33 Fabre and colleagues included high-risk patients with >65% of the cohort with severe immunocompromised conditions consisting of stem cell transplantation, recent chemotherapy, or neutropenia, and they reported no difference in 30-day mortality or recurrent infections among patients with pseudomonal BSI regardless of duration of therapy.32
ORAL TREATMENT: CHALLENGING TRADITIONAL DOGMA
It is a well-accepted standard of practice that BSI are treated with upfront IV antimicrobials that can rapidly achieve therapeutic serum concentration. Whether IV administration is warranted for the entire duration of therapy, though, remains controversial. Even in an era of highly bioavailable oral antimicrobials, clinicians often assume that IV antimicrobials are more potent and efficacious than oral antimicrobials.8,9 This belief has contributed to the dogma that IV therapy is necessary irrespective of the associated risks and costs. Oral antimicrobials are often overlooked as alternatives despite established benefits in avoiding complications associated with IV catheters, decreasing hospital LOS, and improving quality of life.34 There are promising clinical data in support of the efficacy and safety of transitioning from sequential-IV to highly bioavailable oral agents for the treatment of uncomplicated bacteremia caused by both gram-positive and gram-negative pathogens.2,35 Highly bioavailable oral antimicrobials are also increasingly integrated as sequential therapy for deep-seated infections in bone and joint infections, such as vertebral osteomyelitis.19,36 These findings have been confirmed in a recent RCT demonstrating noninferiority of oral antimicrobial combinations after satisfactory clinical responses to at least 10 days of IV therapy, compared with continued IV regimens, in left-sided infective endocarditis.36 While not a prespecified endpoint, hospital LOS was shorter among patients randomized to oral antimicrobials.
Although there are no large-scale RCTs sufficiently powered to address the role of oral antimicrobials in the treatment of uncomplicated GN-BSI, some insights can be gleaned from the existing literature (Table 2). In the RCT establishing noninferiority of short vs long courses of antimicrobials for uncomplicated GN-BSI, the majority of patients randomized to 7 days vs 14 days of therapy, 64% and 81%, respectively, were de-escalated to oral antimicrobials, with fluoroquinolones (FQs) being the predominant (>70%) oral regimen, followed by trimethoprim/sulfamethoxazole (T/S) and oral ß-lactams.33

Despite the Food and Drug Administration warnings of the potentially permanent adverse effects involving tendons, muscles, joints, nerves, and most recently, aortic aneurysms and ruptures,37 FQs remain a unique class of drugs with favorable pharmacodynamic and pharmacokinetic properties that achieve approximately equivalent serum and tissue concentration when administered either intravenously or orally. This advantage was recognized early on as a potential IV-sparing therapeutic option. A prospective RCT that evaluated oral vs IV ciprofloxacin as initial empiric therapy among 141 patients with pyelonephritis or complicated UTI (38% with secondary BSI) reported no significant differences in microbiological failure or clinical response between the two treatment groups.38 Two small RCTs have also demonstrated the safety and effectiveness of sequential-IV antimicrobial to oral FQs in the setting of GN-BSI secondary to urinary source and cholangitis.39,40 Oral ß-lactams, however, achieve substantially lower serum concentration than do their IV counterparts and, accordingly, may be less reliably effective.2
Five retrospective cohort studies have more directly investigated the role of oral antimicrobials in the setting of GN-BSI secondary to common focal infections (Table 2 and Table 3).41-45 Two observational studies reported no difference of treatment failure among patients who received IV-only therapy vs those who were switched to oral therapy in bacteremia secondary to UTIs.41,42 Catheter-associated complications were higher in the IV cohort (6.1% vs 0.4%; P = .03).42 In the largest multicenter cohort study to date, which included 1,478 patients with Enterobacteriaceae bacteremia, there was no difference in 30-day mortality or recurrent bacteremia between patients converted to oral step-down therapy and patients who received the full course of IV antimicrobials.43 Furthermore, the median hospital LOS was shorter (5 days vs 7 days; P < .001) among patients who were transitioned to oral therapy, a finding that is consistent with other studies.39-42 In their analysis, the oral antimicrobials were categorized as low-bioavailability (ß-lactams) or high-bioavailability (FQ and T/S), and there was no difference in outcomes when results were stratified by bioavailability. Mercuro and colleagues reported similar clinical success among patients who received oral ß-lactams and those who received FQs as step-down therapy.44 Notably, patients were more likely to tolerate ß-lactams without experiencing adverse effects than were those who received FQs (91.7% vs 82.1%; P = .049). In contrast, Kutob and colleagues compared step-down oral antimicrobials categorized as low bioavailability (ß-lactams), moderate bioavailability (ciprofloxacin and T/S), and high bioavailability (levofloxacin). They reported that treatment failures were significantly higher among patients who received low-bioavailability (14%) and moderate-bioavailability (12%) antimicrobials, compared with those who received the high-bioavailability agent (2%; P = .02).45 Interestingly, the bioavailability of ciprofloxacin reaches 85% and T/S approaches 90%, and they are often categorized as highly bioavailable agents in other studies.43,46 If they were reclassified as highly bioavailable agents, the study conclusions might differ. Nevertheless, the reported success with oral step-down therapy exceeded 85% in all five studies.41-45

It is important to acknowledge the possibility of unmeasured confounders in these retrospective, observational studies despite statistical adjustments and that they are likely underpowered to determine the clinical significance of oral bioavailability of antimicrobials. In a meta-analysis of published studies and abstracts that included 2,289 patients with Enterobacteriaceae bacteremia, all-cause mortality was similar between patients de-escalated to an oral FQ, T/S, or ß-lactam.46 Overall recurrence of infection (bacteremia or primary site) occurred more frequently in patients transitioned to oral ß-lactams than FQs, but relapse of bacteremia was not statistically different between comparator groups. Bioavailability of the oral agents may not be the sole determinant of higher recurrence; adherence may be poor because of the more frequent dosing required for oral ß-lactams to achieve targeted pharmacokinetics. Additionally, suboptimal dosing of oral ß-lactams noted in the studies may have also contributed to the increased recurrences.
After source control has been achieved and bacterial inoculum burden is sufficiently reduced with appropriate upfront IV therapy, the bioavailability of oral antimicrobials may become less important. However, existing observational data indicate clinical experience is most established with highly bioavailable oral agents, particularly FQs, though the risks vs benefits require careful consideration. For now, the preferred oral agent remains uncertain and selections should be individualized based on susceptibility, patient factors, and other clinical considerations. More importantly, if there are no contraindications or concerns of malabsorption, oral step-down therapy should be initiated as soon as source control and good clinical responses have been achieved.
TEST OF CURE: RECONSIDERING FOLLOW-UP BLOOD CULTURES
Routine follow-up blood cultures (FUBCs) are strongly recommended in Staphylococcus aureus bacteremia because of the propensity for endovascular and metastatic infection, which dictates clinical decision-making regarding duration of therapy. In contrast, GN-BSI secondary to focal infections is usually transient, and the need for confirmation of blood culture clearance is less clear. The low yield of FUBC in many clinical settings suggests that it may not be helpful.47-50 Despite the questionable impact on the clinical management of GN-BSI, FUBCs are routinely ordered in the hospital.1,47 Although there is no high-quality evidence addressing the utility of FUBC, several observational studies suggest clinicians should reconsider routinely ordering FUBC.
Canzoneri and colleagues retrospectively evaluated 383 episodes of bacteremia with at least one FUBC drawn after the initial blood culture.48 On average, 2.32 FUBC were performed per patient for GN-BSI episode, and only 8 patients (5.7%) had persistent bacteremia. Specifically, only 3% had documented positive FUBC among patients with urinary tract source of infection. It was estimated that 17 FUBCs are needed to yield one positive result for GN-BSI. This finding is consistent with results from another study that examined 1,801 episodes of bacteremia, 901 of which were gram-negative organisms, predominantly (67%) Escherichia coli and Klebsiella spp.49 Among GN-BSI episodes, FUBCs were performed in 247 cases, with 27 (10.9%) cases demonstrating persistent bacteremia. A nested case-control analysis between patients with cleared or persistent bacteremia found a lower yield in FUBC with gram-negative organisms and a genitourinary source of infection. Moreover, persistent bacteremia did not influence a change in antimicrobial regimen. Kang and colleagues investigated 1,068 episodes of Klebsiella pneumoniae bacteremia, with FUBCs performed in 862 (80.7%) cases despite only a 7.2% incidence of persistent bacteremia.50 The independent risk factors associated with persistent bacteremia were intra-abdominal infection, solid organ transplantation, high Charlson comorbidity index score, and unfavorable treatment responses, which suggests the need for FUBC may be individualized rather than routine.
In the setting of GN-BSI in which the probability of persistent bacteremia is relatively low, especially in genitourinary sources of infection, FUBCs are not warranted. It is uncomfortable for patients and exposes them to harms of false-positive results, leading to antimicrobial administration with possible adverse effects, which can be further compounded by unnecessary testing, potentially missed alternative diagnosis, and increasing hospital LOS.1,51 Given the low yield of FUBC in GN-BSI, and the lack of association of persistent bacteremia with change in antimicrobial therapy or clinical outcomes, we recommend avoiding FUBC as a test of cure. Documentation of gram-negative blood culture clearance should be reserved for situations in which there is concern for deeper or otherwise uncontrolled source of infection.
CONCLUSION
The optimal management of gram-negative bacteremia in hospitalized patients is evolving. There is a growing body of evidence supporting shorter duration for a total of 7 days with oral step-down therapy as safe and effective for patients with uncomplicated Enterobacteriaceae bacteremia who have achieved adequate source control and demonstrated clinical stability and improvement. Although comparative data regarding the optimal duration of therapy in the setting of MDR strains such as ESBL Enterobacteriaceae and pseudomonal BSI are limited, available data appear promising in favor of shorter treatment duration with oral step-down therapy. Routine follow-up blood culture is not cost-effective and may result in unnecessary healthcare resource utilization and inappropriate use of antimicrobials. Table 4 provides a framework for the clinical management of GN-BSI in the hospital. Taken together, these steps will facilitate antimicrobial stewardship, limit unnecessary antimicrobial exposure, and improve quality of patient care.

Disclosures
The authors have no conflicts of interest to disclose.
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
1. Cobrun B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood culture? JAMA . 2012;308(5):502-511. https://doi.org/10.1001/jama.2012.8262
2. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non- Staphylococcus aureus bacteremia. Open Forum Infect Dis . 2018;5(5):ofy087. https://doi.org/10.1093/ofid/ofy087
3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med . 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63(4):e61-e111. https://doi.org/10.1093/cid/ciw353
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis . 2014;59(2):e10-e52. https://doi.org/10.1093/cid/ciu296
6. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infections in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133-164. https://doi.org/10.1086/649554
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of American and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis . 2011;52(5):e103-e120. https://doi.org/10.1093/cid/ciq257
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother . 2014;5(2):83-87. https://doi.org/10.4103/0976-500X.130042
9. Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother . 2016;71(8):2295-2299. https://doi.org/10.1093/jac/dkw129
10. Spellberg B. The maturing antibiotic mantra: “shorter is still better.” J Hosp Med. 2018;13(5):361-362. https://doi.org/10.12788/jhm.2904
11. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med . 2017;177(9):1308-1315. https://doi.org/10.1001/jamainternmed.2017.1938
12. Lin RY, Nuruzzaman F, Shah SN. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J Hosp Med . 2009;4(2):E7-E15. https://doi.org/10.1002/jhm.414
13. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis . 2011;53(1):42-48. https://doi.org/10.1093/cid/cir301.
14. Vaughn VM, Scott FA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients with hospitalized pneumonia – a multihospital cohort study. Ann Intern Med . 2019;171(3):153-163. https://doi.org/10.7326/M18-3640
15. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal ß -lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy . 2019;39(3):261-270. https://doi.org/10.1002/phar.2201
16. Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs . 2012;35(2):84-91. https://doi.org/10.1097/NAN.0b013e31824237ce
17. Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis . 2019;69(3):414-420. https://doi.org/10.1093/cid/ciy863
18. Fallouh N, McGurik HM, Falnders SA, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med . 2015;128(7):722-738. https://doi.org/10.1016/j.amjmed.2015.01.027
19. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med . 2019;380:425-436. https://doi.org/10.1056/NEJMoa1710926
20. Hayashi Y, Peterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis . 2011;52(10):1232-1240. https://doi.org/10.1093/cid/cir063
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis . 2009;49(1):1-45. https://doi.org/10.1086/599376
22. Corona A, Bertolini G, Ricotta AM, Wilson A, Singer M. Variability of treatment duration for bacteraemia in the critically ill: a multinational survey. J Antimicrob Chemother . 2003;52(5):849-852. https://doi.org/10.1093/jac/dkg447
23. Diallo K, Thilly N, Luc A, et al. Management of bloodstream infections by infection specialists: an international ESCMID cross-sectional survey. Int J Antimicrob Agents . 2018;51(5):794-798. https://doi.org/10.1016/j.ijantimicag.2017.12.010
24. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection – 7 day or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother . 2013;68(10):2183-2191. https://doi.org/10.1093/jac/dkt177
25. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care . 2011;15(6):R267. https://doi.org/10.1186/cc10545
26. Daneman N, Rishu AH, Xiong W, et al. Duration of antimicrobial treatment for bacteremia in Canadian critically ill patients. Crit Care Med . 2016;44(2):256-264. https://doi.org/10.1097/CCM.0000000000001393
27. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection . 2017;45(5):613-620. https://doi.org/10.1007/s15010-017-1020-5
28. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis . 2018;66:172-177. https://doi.org/10.1093/cid/cix767
29. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect . 2018;24(11):1184-1189. https://doi.org/10.1016/j.cmi.2018.01.021
30. Giannella M, Pascale R, Toschi A, et al. Treatment duration of Escherichia coli bloodstream infection and outcomes: retrospective single-center study. Clin Microbiol Infect . 2018;24(10):1077-1083. https://doi.org/10.1016/j.cmi.2018.01.013
31. Sousa A, Perez-Rodriguez MT, Suarez M, et al. Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis . 2019;38(5):851-857. https://doi.org/10.1007/s10096-019-03467-5.
32. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis . 2019;69(11):2011-2014. https://doi.org/10.1093/cid/ciz223
33. Yahav D, Franceschini E, Koppel F, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis . 2019;69(7):1091-1098. https://doi.org/10.1093/cid/ciy1054
34. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? an evidence-based narrative review. J Hosp Med . 2018;13(5):328-335. https://doi.org/10.12788/jhm.2949
35. Al-Hasan MN, Rac H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. 2020;26(3):299-306. https://doi.org/10.1016/j.cmi.2019.05.012
36. Iversen K, Ihlemann N, Gill SU. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
37. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed October 30, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
38. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M. Oral vs intravenous ciprofloxacin in the initial empirical management of severe pyelonephritis or complicated urinary tract infections: a prospective randomized clinical trial. Arch Intern Med. 1999;159(1):53-58. https://doi.org/10.1001/archinte.159.1.53
39. Amodio-Groton M, Madu A, Madu CN, et al. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann Pharmacother. 1996;30(6):596-602. http://doi.org/10.1177/106002809603000605
40. Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59(11):2790-2796. https://doi.org/10.1007/s10620-014-3233-0
41. Rieger KL, Bosso JA, MacVane SH, Temple Z, Wahlquist A, Bohm N. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017;37(11):1479-1483. https://doi.org/10.1002/phar.2024
42. Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. https://doi.org/10.1016/j.jgar.2019.03.013
43. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179(3):316-323. https://doi.org/10.1001/jamainternmed.2018.6226
44. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdwon therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus ß-lactams. Int J Antimicrob Agents. 2018;51(5):687-692. https://doi.org/10.1016/j.ijantimicag.2017.12.007
45. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. https//doi.org/10.1016/j.ijantimicag.2016.07.013
46. Punjabi C, Tien V, Meng L, et al. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(10):ofz364. https://doi.org/10.1093/ofid/ofz364
47. Chen AI, Bilker WB, Hamilton KW. Blood culture utilization at an academic hospital: addressing a gap in benchmarking. Infect Control Hosp Epidemiol. 2018:39(11):1353-1359. http://doi.org/10.1017/ice.2018.231
48. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776-1779. https://doi:10.1093/cid/cix648
49. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016:16:286-295. https://doi.org/10.1186/s12879-016-1622-z
50. Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? a retrospective case-control study. BMC Infect Dis. 2013;13(1):365-372. https://doi.org/10.1186/1471-2334-13-365
51. Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265(3):365-369. https://doi:10.1001/jama.1991.03460030071031
© 2020 Society of Hospital Medicine
Hospitalized Medical Patients with Posttraumatic Stress Disorder (PTSD): Review of the Literature and a Roadmap for Improved Care
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.
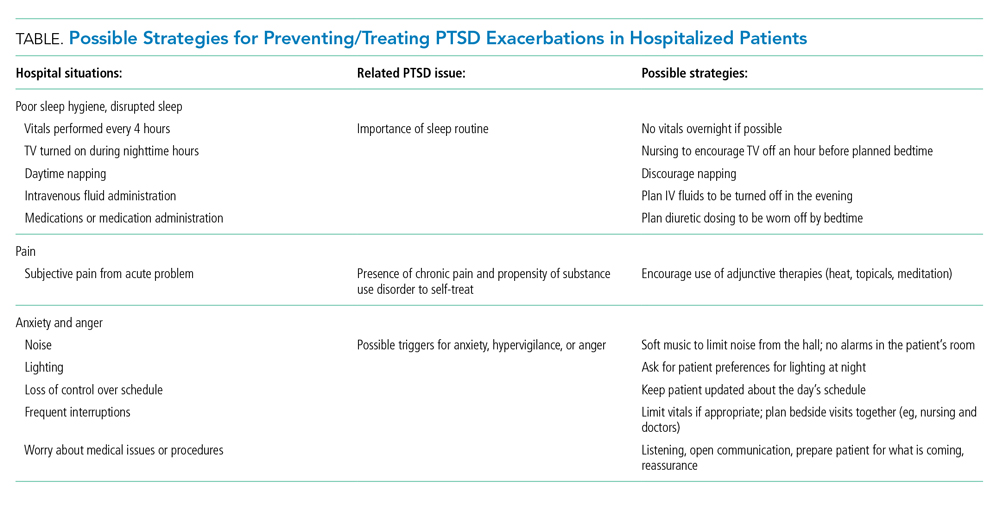
Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.

Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
Posttraumatic stress disorder (PTSD) is a syndrome that occurs after exposure to a significant traumatic event and is characterized by persistent, debilitating symptoms that fall into four “diagnostic clusters” as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Version V (DSM-V). Patients may experience intrusive thoughts, avoidance of distressing stimuli, persistent negative mood, and hypervigilance, all of which last longer than 1 month.1
A national survey of United States households conducted during 2001-2003 estimated the 12-month prevalence of PTSD among adults to be 3.5%.2 Lifetime prevalence has been found to be between 6.8%3 and 7.8%.4 PTSD is more common in veterans. The prevalence of PTSD in veterans differs depending on the conflict in which the veteran participated. Vietnam veterans have an estimated lifetime prevalence of approximately 30%,5,6 Gulf War veterans approximately 15%,7 and veterans of more recent conflicts in Afghanistan and Iraq of approximately 21%.8 With the MISSION Act moving more veteran care into the private sector, non-VA inpatient providers will need to become better versed in PTSD.9
Patients with PTSD have more contact with the healthcare system, even for non–mental health problems,8,10-13 and a significantly higher burden of medical comorbities,14 such as diabetes mellitus, liver disease, gastritis and gastric ulcers, HIV, arthritis,15 and coronary heart disease.16 Veterans with PTSD are hospitalized three times more often than are those with no mental health diagnoses,8 and patients with psychiatric comorbidities have higher lengths of stay.17 More than 1.4 million hospitalizations occurring during 2002-2011 had either a primary or secondary associated diagnosis of PTSD, with total inflation-adjusted charges of 34.9 billion dollars.18 In the inpatient sample from this study, greater than half were admitted for a primary diagnosis of mental diseases and disorders (Major Diagnostic Category [MDC] 19). Following mental illness, the most common primary diagnoses for men were MDC 5 (Circulatory System, 12.1%), MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 9.2%), and MDC 4 (Respiratory System, 7.4%), while the most common categories for women were MDC 20 (Alcohol/Drug Use or Induced Mental Disorder, 5.8%), MDC 21 (Injuries, Poison, and Toxic Effect of Drugs, 4.9%), and MDC 6 (Digestive System, 4.5%).18
In both the inpatient and outpatient settings, a fundamental challenge to comprehensive PTSD management is correctly diagnosing this condition.19 Confounding the difficulties in diagnosis are numerous comorbidities. In addition to the physical comorbidities described above, more than 70% of patients with PTSD have another psychological comorbidity such as affective disorders, anxiety disorders, or substance use disorder/dependency.20
Given that PTSD may be an underrecognized burden on the healthcare system, we sought to better understand how PTSD could affect hospitalized patients admitted for medical problems by conducting this narrative review. Additionally, three of the authors collaborated with the VA Employee Education Service to conduct a needs assessment of VA hospitalists in 2013. Respondents identified managing and educating patients and families about PTSD as a major educational need (unpublished data available upon request from the corresponding author). Therefore, our aims were to present a synthesis of existing literature, familiarize readers with the tenets of trauma-informed care as a framework to guide care for these patients, and generate ideas for changes that inpatient providers could implement now. We began by consulting a research librarian at the Clement J. Zablocki VA Medical Center in Milwaukee, Wisconsin, who searched the following databases: PsycInfo, CINAHL, MEDLINE, and PILOTS (a PTSD/trauma specific database). Search terms included hospital, hospitalized, and hospitalization, as well as traumatic stress, posttraumatic stress, and PTSD. Pertinent guidelines and the reference lists from included papers were examined. We focused on papers that described patients admitted for medical problems other than PTSD because those patients who are admitted for PTSD-related problems should be primarily managed by psychiatry (not hospitalists) with the primary focus of their hospitalization being their PTSD. We also excluded papers about patients developing PTSD secondary to hospitalization, which already has a well-developed literature.21-23
THE LITERATURE ABOUT PTSD IN HOSPITALIZED PATIENTS
The literature is sparse describing frequency or type of problems encountered by hospitalized medical patients with PTSD. A recent small survey study reported that 40% of patients anticipated triggers for their PTSD symptoms in the hospital; such triggers included loud noises and being shaken awake.24 Two papers describe case vignettes of patients who had exacerbations of their PTSD while in the Intensive Care Unit (ICU), although neither contain frequency or severity data.25,26 Approximately 8% of patients in VA ICUs have PTSD,27 and a published abstract suggests that they appear to require more sedation than do patients without PTSD.28 Another published case report describes a patient with recurrent PTSD symptoms (nightmares) after moving into a nursing home.29 These papers suggest other providers have recognized and are concerned about hospitalized patients with PTSD. At present, there are no data to quantify how often hospitalized patients have PTSD exacerbations or how troublesome such exacerbations are to these patients.
Given that there is little empiric literature to guide inpatient management of PTSD as a comorbidity in hospitalized medical patients, we extrapolate some information from the outpatient setting. PTSD is often underdiagnosed and underreported by individual patients in the outpatient setting.30 Failure to have an associated diagnosis of PTSD may lead to underrecognition and undertreatment of these patients by inpatient providers in the hospital setting. Additionally, the numerous psychological and physical comorbidities in PTSD can create unique challenges in properly managing any single problem in these patients.20 Armed with this knowledge, providers should be vigilant in the recognition, assessment, and treatment of PTSD.
INPATIENT MANAGEMENT OF PTSD
Trauma-Informed Care: A Conceptual Model
Trauma-informed care is a mindful and sensitive approach to caring for patients who have suffered trauma.31 It requires understanding that many people have suffered trauma in their lives and that the trauma continues to impact many aspects of their lives.32 Trauma-informed care has many advocates and has been implemented across myriad health and social services settings.33 Its principles can be applied in both the inpatient and outpatient hospital settings. While it is an appropriate approach to patients with PTSD, it is not specific to PTSD. People who have suffered sexual trauma, intimate partner violence, child abuse, or other exposures would also be included in the group of people for whom trauma-informed care is a suitable approach. There are four key assumptions to a trauma-informed approach to care (the 4 R’s): (1) realization that trauma affects an individual’s coping strategies, relationships, and health; (2) recognition of the signs of trauma; (3) having an appropriate, planned response to patients identified as having suffered a trauma; and (4) resisting retraumatization in the care setting.31,32
General Approach to Treating Medical Patients With PTSD in the Inpatient Setting
Recognition
Consistent with a trauma-informed care approach, inpatient providers should be able to recognize patients who may have PTSD. First, careful review of the past medical history may show some patients already carry this diagnosis. Second, patients with PTSD often have other comorbidities that could offer a clue that PTSD could be present as well; for example, risk for PTSD is increased when mood, anxiety, or substance use disorders are present.20 When PTSD is suspected, screening is a reasonable next step.
The Primary Care-PTSD-5 (PC-PTSD-5) is a validated screening tool used in the outpatient setting.34 It is easily administered and has good predictive validity (positive likelihood ratio [LR+] of 6.33 and LR– of 0.06). It begins with a question of whether the patient has ever experienced a trauma. A positive initial response triggers a series of five yes/no questions. Answering “yes” to three or more questions is a positive screen. A positive screen should result in consultation to psychiatry to conduct more formal evaluation and guide longer-term management.
Collaboration
Individual trauma-focused psychotherapy is the primary treatment of choice for PTSD with strong evidence supporting its practice.35 This treatment is administered by a psychiatrist or psychologist and will be limited in the inpatient medical setting. Current recommendations suggest pharmacotherapy only when individualized trauma-focused psychotherapy is not available, the patient declines it, or as an adjunct when psychotherapy alone is not effective.36 Therefore, inpatient providers may see patients who are prescribed selective serotonin reuptake inhibitors (eg, paroxetine, fluoxetine) or serotonin and norepinephrine reuptake inhibitors (eg, venlafaxine).36 In the past, PTSD-related nightmares were often treated with prazosin.37 However, a recent randomized controlled trial of prazosin in veterans with PTSD failed to show significant improvement in nightmares.38 Hence, current guidelines do not recommend prazosin as a first-line therapy.39 For hospitalized patients with PTSD symptoms refractory to the interventions outlined herein, particularly those patients with possible borderline personality traits (as suggested by severe anger and impulsivity), we strongly recommend partnering with psychiatry. Finally, given the high prevalence of substance use disorders (SUDs) in PTSD patients, awareness and treatment of comorbidities such as opioid and alcohol dependence must be concurrently addressed.
Individualizing Care
It is essential for the healthcare team to identify ways to meet each patient’s immediate needs. Many of the ideas proposed below are not specific to PTSD; many require an interprofessional approach to care.40 From a trauma-informed care standpoint, this is akin to having a planned response for patients who have suffered trauma. Assessing the individual’s needs and incorporating therapeutic modalities such as reflective listening, broadening safe opportunities for control, and providing complementary and integrative medicine (IM) therapies may help manage symptoms and establish rapport.41 Through reflective listening, a collaborative approach can be established to identify background, triggers, and a safe approach for managing PTSD and its comorbid conditions. Ensuring frequent communication and allowing the patient to be at the center of decision-making establishes a safe environment and promotes positive rapport between the patient and healthcare team.36 Providing a sense of control by involving the patients in their healthcare decisions and in the structure of care delivery may benefit the patients’ well-being. Furthermore, incorporating IM encourages rest and relaxation in the chaotic hospital environment. Suggested IM interventions include deep breathing, aromatherapy, guided imagery, muscle relaxation, and music therapy.42,43
Key Inpatient Issues Affecting PTSD
In the following sections, we outline common clinical situations that may exacerbate PTSD symptoms and propose some evidence-based responses (Table). In general, nonpharmacologic approaches are favored over pharmacologic approaches for patients with PTSD.

Sleep Hygiene
Sleep problems are very common in patients with PTSD, with nightmares occurring in more than 70% of patients and insomnia in 80%.44 In PTSD, sleep problems are linked to poor physical health and other health outcomes45,46 and may exacerbate other PTSD symptoms.4
Treating the sleep problems that occur with PTSD is an important aspect of PTSD care. Usually administered in the outpatient setting, the treatment of choice is cognitive-behavioral therapy (CBT).48 Sleep-specific CBT focuses, among other things, on strategies that encourage good sleep hygiene,49 which includes promoting regular sleep/wake-up times and specific bedtime routines, avoiding stimulation (eg, light, noise, TV) or excessive liquids before bed, refraining from daytime naps, and using relaxation techniques. Many of these recommendations seem at odds with hospital routines, which may contribute to decompensation of hospitalized patients with PTSD.
While starting sleep-specific CBT in the hospital may not be realistic, we suggest the following goals and strategies as a starting place for promoting healthy sleep for hospitalized patients with PTSD. To begin, factors affecting sleep hygiene should be addressed. Inpatient providers could pay more attention to intravenous (IV) fluid orders, perhaps adjusting them to run only during the daytime hours. Medications can be scheduled at times conducive to maintaining home routines. Avoiding the administration of diuretics close to bedtime may decrease the likelihood of frequent nighttime wakening. Grouping patient care activities, such as bathing or wound care, during daytime hours may allow more opportunities for rest at night. Incorporating uninterrupted sleep protocols, such as quiet hours between 10
Second, providers need to ask about established home bedtime routines and facilitate implementation in the hospital. Through collaboration with patients, providers can incorporate an individualized plan of care for sleep early in hospitalization.50 Partnering with nurses is also essential to creating a sleep-friendly environment that can improve patient experiences.51 Breathing exercises, meditating, listening to music and praying are all examples of “bedtime wind down” strategies recommended in sleep-specific CBT.49 Many of these could be successfully implemented in the hospital and may benefit other hospitalized patients too.52 In patients with PTSD and obstructive sleep apnea, continuous positive airway pressure (CPAP) reduces nightmares, and if inpatients are on CPAP at home, it should be continued in the hospital.53
Pain
If sleep disturbance is the hallmark of PTSD,47 chronic pain is its coconspirator.15 Uncontrolled pain can make it much more difficult to treat patients with PTSD, which in turn may lead to further decompensation from a mental health standpoint.54 SUDs such as alcohol or opioid dependencies are highly comorbid with PTSD45 and introduce a layer of complexity when managing painin these patients. Providers should be thoughtful when electing to treat acute or chronic pain with opioids and take particular care to establish realistic therapeutic goals if doing so. While patients with PTSD have a greater likelihood of having an SUD, undertreating pain risks exacerbating underlying PTSD symptoms.
Nonpharmacologic therapies, which include communicating, listening, and expressing compassion and understanding, should be utilized by inpatient providers as a first-line treatment in patients with PTSD who suffer from pain. Additionally, relaxation techniques, physical therapy, and physical activity55 can be offered. Pharmacologically, nonopioid medications such as acetaminophen or NSAIDs should always be considered first. Should the use of opioids be deemed necessary, inpatient providers should preferentially use oral over intravenous medications and consider establishing a fixed timeframe for short-term opioids, which should be limited to a few days. Providers should communicate clear expectations with their patients to maximize the desired effect of any specific treatment while minimizing the risk of medication side effects with the goal of agreeing on a short yet effective treatment course.
Anxiety and Anger
One of the most challenging situations for the inpatient provider is encountering a patient who is anxious, angry, or hypervigilant. Mismatch between actual and expected communication between the provider and the patient can lead to frustration and anxiety. A trauma-informed care approach would suggest that frequent and thorough communication with patients may prevent or ameliorate the stresses and anxieties of hospitalization that may manifest as anger because of retraumatization. Hospitalizations usually lead to disruption of normal routine (eg, unpredictable meal times or medication administration), interrupted sleep (eg, woken up for blood draws or provider evaluation), and lack of control of schedule (eg, unsure of exact time when a procedure may be occurring), any of which may trigger symptoms of anxiety and anger in patients with PTSD and lead to hypervigilance.
If situations involving patient anxiety do arise, employ compassion and communication. Extra time spent with the patient, while challenging in the hectic hospital environment, is critical, and nonpharmacological treatments should be the priority. Engaging patients by asking about their PTSD triggers24 may help prevent exacerbations. For example, some patients may specify how they prefer to be woken up to prevent startle reactions. PTSD triggers can be reduced via effective communication with the entire healthcare team. Some immediate yet effective strategies are listening, validation, and negotiation. Benzodiazepine or antipsychotic usage should be avoided.36 Inpatient social work and comanagement with psychiatry involvement may be helpful in more severe exacerbations. A small observational study of patients hospitalized for severe PTSD found an association between walking more during hospitalization and fewer PTSD symptoms,56 suggesting that staying active could be helpful for inpatients with PTSD who are able to safely ambulate.
SUMMARY
PTSD is a common comorbidity among hospitalized patients in the United States. Typical hospital routines may exacerbate symptoms of PTSD such as anxiety and anger. Inpatient providers can play an important role in making hospitalizations go more smoothly for these patients by using principles consistent with trauma-informed care. Specifically, partnering with patients to construct a plan that preserves their sleep routines and accounts for potential triggers for decompensation can improve the hospital experience for patients with PTSD. Some PTSD interventions require additional investment from the healthcare system to deploy, such as staff training in trauma-informed care and reflective listening techniques. Electronic health record–based protocols and order sets for patients with PTSD can leverage available resources. Further research should evaluate hospital outcomes that result from a more tailored approach to the care of patients with PTSD. More effective, patient-centered PTSD care could lower rates of leaving against medical advice and improve the inpatient experience for patients and providers alike.
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
1. DSM-5 Fact Sheet: Posttraumatic Stress Disorder. American Psychological Association. 2013. Accessed 30 July 2019. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-PTSD.pdf
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. https://doi.org/10.1001/archpsyc.62.6.617
3. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. https://doi.org/10.1001/archpsyc.62.6.593
4. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
5. Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5(3):365-376. https://doi.org/10.1002/jts.2490050304
6. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma And the Vietnam War Generation: Report of findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990.
7. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410. https://doi.org/10.1097/JOM.0b013e3181a2feeb
8. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24. https://doi.org/10.1007/s11606-009-1117-3
9. VA MISSION Act. Department of Veterans Affairs. 2019. Accessed February 2, 2020. https://missionact.va.gov/
10. Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398-407. https://doi.org/10.3122/jabfm.2008.05.070082
11. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
12. Dobie DJ, Maynard C, Kivlahan DR, et al. Posttraumatic stress disorder screening status is associated with increased VA medical and surgical utilization in women. J Gen Intern Med. 2006;21(Suppl 3):S58-S64. https://doi.org/10.1111/j.1525-1497.2006.00376.x
13. Calhoun PS, Bosworth HB, Grambow SC, Dudley TK, Beckham JC. Medical service utilization by veterans seeking help for posttraumatic stress disorder. Am J Psychiatry. 2002;159(12):2081-2086. https://doi.org/10.1176/appi.ajp.159.12.2081
14. Frayne SM, Chiu VY, Iqbal S, et al. Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med. 2011;26(1):33-39. https://doi.org/10.1007/s11606-010-1497-4
15. Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73(8):697-707. https://doi.org/10.1097/PSY.0b013e3182303775
16. Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970-978. https://doi.org/10.1016/j.jacc.2013.04.085
17. Bressi SK, Marcus SC, Solomon PL. The impact of psychiatric comorbidity on general hospital length of stay. Psychiatr Q. 2006;77(3):203-209. https://doi.org/10.1007/s11126-006-9007-x
18. Haviland MG, Banta JE, Sonne JL, Przekop P. Posttraumatic stress disorder-related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Men Dis. 2016;204(2):78-86. https://doi.org/10.1097/NMD.0000000000000432
19. Frommberger U, Angenendt J, Berger M. Post-traumatic stress disorder--a diagnostic and therapeutic challenge. Dtsch Arztebl Int. 2014;111(5):59-65. https://doi.com/10.3238/arztebl.2014.0059
20. Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry. 2014;59(9):460-467. https://doi.org/10.1177/070674371405900902
21. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421-434. https://doi.org/10.1016/j.genhosppsych.2008.05.006
22. Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506-1518. https://doi.org/10.1007/s00134-007-0730-z
23. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. https://doi.org/10.1097/CCM.0000000000000882
24. Fletcher KE, Collins J, Holzhauer B, Lewis F, Hendricks M. Medical patients with PTSD identify issues with hospitalization. J Gen Intern Med. 2020;35(6):1906-1907. https://doi.org/10.1007/s11606-019-05480-y
25. Struble LM, Sullivan BJ, Hartman LS. Psychiatric disorders impacting critical illness. Crit Care Nurs Clin North Am. 2014;26(1):115-138. https://doi.org/10.1016/j.ccell.2013.10.002
26. Baxter A. Posttraumatic stress disorder and the intensive care unit patient: implications for staff and advanced practice critical care nurses. Dimens Crit Care Nurs. 2004;23(4):145-150. http://doi.org/10.1097/00003465-200407000-00001
27. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Preexisting comorbid psychiatric conditions and mortality in nonsurgical intensive care patients. Am J Crit Care. 2010;19(3):241-249. https://doi.org/10.4037/ajcc2010967
28. Kebbe J, Lal A, El-Solh A, Jaoude P. Effects of PTSD on patient outcomes in the intensive care unit. Chest. 2015;148(4 Suppl):220A. https://doi.org/10.1378/chest.2274366
29. Johnson KG, Rosen J. Re-emergence of posttraumatic stress disorder nightmares with nursing home admission: treatment with prazosin. J Am Med Dir Assoc. 2013;14(2):130-131. https://doi.org/10.1016/j.jamda.2012.10.007
30. Zimmerman M, Mattia JI. Is posttraumatic stress disorder underdiagnosed in routine clinical settings? J Nerv Ment Dis. 1999;187(7):420-428. https://doi.org/10.1097/00005053-199907000-00005
31. Trauma-informed care. Agency for Healthcare Research and Quality. 2015. Accessed July 30, 2019. http://www.ahrq.gov/professionals/prevention-chronic-care/healthier-pregnancy/preventive/trauma.html
32. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Substance Abuse and Mental Health Administration, Department of Health & Human Services; 2014. HHS Publication No. SMA 14-4884. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf
33. DeCandia CJ, Guarino K. Trauma-informed care: an ecological response. J Child Youth Care Work. 2015;24:7-32.
34. Prins A, Bovin MJ, Smolenski DJ, et al. The PRIMARY CARE PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. https://doi.org/10.1007/s11606-016-3703-5
35. Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. https://doi.org/10.1002/da.22511
36. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Department of Veterans Affairs/Department of Defense. 2017. Accessed July 22, 2019. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGClinicianSummaryFinal.pdf
37. Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(4). https://doi.org/10.4088/PCC.16r01943
38. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517. https://doi.org/10.1056/NEJMoa1507598
39. El-Solh AA. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat Sci Sleep. 2018;10:409-420. https://doi.org/10.2147/NSS.S166089
40. What is ROVER? Treatment Services. VA. 2018. Accessed February 14, 2020. https://www.houston.va.gov/docs/ROVERBrochure.pdf
41. Moser DK, Chung ML, McKinley S, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276-288. https://doi.org/10.1016/s0964-3397(03)00061-2
42. Bulechek G, Butcher H, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC), 6th Ed. Elsevier; 2013.
43. Blanaru M, Bloch B, Vadas L, et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13. https://doi.org/10.4081/mi.2012.e13
44. Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449-452. https://doi.org/10.1016/s0022-3956(02)00025-0
45. Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. 2014;26(2):237-247. https://doi.org/10.3109/09540261.2014.901300
46. Clum GA, Nishith P, Resick PA. Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. J Nerv Ment Dis. 2001;189(9):618-622. https://doi.org/10.1097/00005053-200109000-00008
47. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382. https://doi.org/10.1176/appi.ajp.2012.12040432
48. Ho FYY, Chan CS, Tang KNS. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90-102. https://doi.org/10.1016/j.cpr.2015.09.005
49. Thompson KE, Franklin CL, Hubbard K. PTSD sleep therapy group: training your mind and body for better sleep: Therapist Manual. A product of the Department of Veterans Affairs South Central (VISN 16) Mental Illness Research, Education, and Clinical Center (MIRECC). Accessed July 22, 2019. https://www.mirecc.va.gov/VISN16/docs/Sleep_Therapy_Group_Therapist_Manual.pdf
50. Ye L, Keane K, Hutton Johnson S, Dykes PC. How do clinicians assess, communicate about, and manage patient sleep in the hospital? J Nurs Adm. 2013;43(6):342-347. https://doi.org/10.1097/NNA.0b013e3182942c8a
51. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
52. Gagner-Tjellesen D, Yurkovich EE, Gragert M. Use of music therapy and other ITNIs in acute care. J Psychosoc Nurs Ment Health Serv. 2001;39(10):26-37.
53. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636. https://doi.org/10.5664/jcsm.3786
54. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. 2015;51(4):295-304. https://doi.org/10.1111/ppc.12093
55. Bosch J, Weaver TL, Neylan TC, Herbst E, McCaslin SE. Impact of engagement in exercise on sleep quality among veterans with posttraumatic stress disorder symptoms. Mil Med. 2017;182(9):e1745-e1750. https://doi.org/10.7205/MILMED-D-16-00385
56. Rosenbaum S, Vancampfort D, Tiedemann A, et al. Among inpatients, posttraumatic stress disorder symptom severity is negatively associated with time spent walking. J Nerv Ment Dis. 2016;204(1):15-19. https://doi.org/10.1097/NMD.0000000000000415
© 2021 Society of Hospital Medicine
Left Out in the Cold
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
A previously healthy 4-year-old boy presented to his pediatrician for nasal congestion, left ear pain, and intermittent fevers, which he’d been experiencing for 2 days. His exam was consistent with acute otitis media. Cefdinir was prescribed given a rash allergy to amoxicillin. His fever, congestion, and otalgia improved the next day.
Three days later he developed abdominal pain, fever, and labored breathing; his mother brought him to the emergency department (ED). His temperature was 38.0 °C, heart rate 141 beats per minute, blood pressure 117/71 mm Hg, respiratory rate 22 breaths per minute; he had oxygen saturation of 96% on ambient air. Despite mild accessory muscle use, he appeared comfortable and interactive. His left tympanic membrane was bulging without erythema. His neck was supple and mucous membranes moist. He had neither cervical lymphadenopathy nor conjunctival pallor. The cardiopulmonary exam was normal except for tachycardia. His abdomen was soft and not distended without organomegaly or tenderness.
Upper respiratory tract symptoms are commonly encountered in pediatrics and most often result from self-limited viral processes. Evaluation of a child with upper respiratory tract symptoms aims to identify serious causes like meningitis, as well as assessing the need for antimicrobial therapy. Supportive management is often appropriate in otitis media. His new, more concerning symptoms portend either a progression of the original process causing his upper respiratory tract symptoms or a separate etiology. It is key to determine which signs and symptoms are associated with the primary process and which are compensatory or secondary. If he were to be more ill appearing, for example, it is possible that his respiratory distress may be related to an underlying systemic illness rather than a primary lung process. Respiratory distress, abdominal pain, and fever could be a result of sepsis from an intrabdominal process such as ruptured appendicitis, intussusception, or malrotation with volvulus. Other causes of sepsis, such as meningitis or severe mastoiditis, both rare complications of otitis media, should be considered, although he does not appear severely ill. Acute myelogenous leukemia or other malignancies and illnesses associated with immunodeficiency can present with sepsis and chloromas in the middle ear that can be misconstrued as otitis media.
A chest radiograph demonstrated left lower lobe patchy opacities concerning for pneumonia. Rapid respiratory syncytial virus and influenza antigen test results were negative. Laboratory testing for general bloodwork was not obtained. He was administered a single dose of intramuscular ceftriaxone, prescribed a 5-day course of azithromycin, and discharged home. The child’s breathing gradually improved, but he continued to have subjective fevers. Two days later, he developed dark red urine. His mother brought him back to the outpatient clinic.
At the time of the ED visit, a diagnosis of community-acquired pneumonia was plausible given fever, mildly increased work of breathing, and an opacification on chest radiography. Most community-acquired pneumonia is caused by viruses; common bacterial causes for his age include Streptococcus pneumoniae and Moraxella catarrhalis. The first-line treatment for uncomplicated community-acquired pneumonia in children is amoxicillin, but this was appropriately avoided given his allergy.
The persistent fevers are surprising. The improvement in breathing corresponds to the treatment (and resolution) of community-acquired pneumonia. However, the development of dark urine does not. Red urine—in the absence of ingested pigments (such as those found in beets)—usually results from hematuria, hemoglobinuria, or myoglobinuria. Gross hematuria can originate from the kidneys to the urethral meatus. Abdominal masses, kidney trauma, or underlying kidney disease may all present with gross hematuria (or microscopic hematuria, seen only on urinalysis). The urine should be examined for the presence of heme, protein, and for evidence of infection; microscopy should be performed to examine for cellular casts and dysmorphic red cells. Tests of renal function, a comprehensive metabolic panel, evaluation of hematologic indexes, and assessments of inflammatory markers should be performed.
The child lived with his parents and had no siblings. He experienced no physical trauma, and there was no family history of kidney disease or hematuria. His father had a persistent cough and fever for 1 month, but recovered around the time the patient began to experience his initial symptoms. This was the patient’s third diagnosis of pneumonia. He had not traveled and was up to date with immunizations. He attended day care.
The fact that this is not the first episode of “pneumonia” raises important possibilities. The most likely one is that the child has had multiple viral infections; however, he could have an underlying primary immunodeficiency (PI) that predisposes him to recurrent infections. More severe PIs often present with recurrent sepsis, bacteremia, and failure to thrive, none of which were present in this case. Less severe PIs (such as selective IgA deficiency) could be possible. Another possibility is that these recurrent episodes of pneumonia are a relapsing and remitting noninfectious process, such as an antineutrophil cytoplasmic antibodies–associated vasculitis or anti–glomerular basement membrane disease. The patient’s father’s recent prolonged respiratory symptoms may be suggestive of pertussis or a “walking pneumonia” potentially caused by Mycoplasma or another atypical bacterium.
His temperature was 36.9 °C, heart rate 107 beats per minute, blood pressure was 106/67 mm Hg, and respiratory rate was 24 breaths per minute with oxygen saturation of 100% on ambient air. He was well appearing. His mucous membranes were moist, and oropharynx was clear. He had scleral icterus. The cardiopulmonary exam was normal. He had no significant lymphadenopathy, hepatosplenomegaly, or rashes.
The finding of jaundice is an important diagnostic pivot point, especially when combined with hematuria. The next step is determining if the jaundice is resulting from unconjugated or conjugated hyperbilirubinemia; the former most often stems from hemolysis or impairment in conjugation, while the latter results from intrahepatic or extrahepatic biliary defects. Tests for hepatobiliary injury including evaluations of alanine and aspartate aminotransferases and alkaline phosphatase, as well as for hepatic function such as tests of coagulation, should be performed.
The patient was referred to the ED and admitted for further evaluation. A complete blood count revealed a white blood cell (WBC) count of 10,700/µL (61% polymorphonuclear neutrophils, 30% lymphocytes, 5% monocytes, 3% eosinophils, 1% basophils), hemoglobin count was 10.3 g/dL (reticulocyte 2% with absolute reticulocyte count 58,400/μL), and platelet count was 265,000/µL. Components of the basic metabolic panel were within reference ranges except for a mildly elevated blood urea nitrogen level of 14 mg/dL with normal creatinine level of 0.3 mg/dL. Total protein was 6.7 g/dL (reference range, 6.4-8.3) and albumin 3.9 g/dL (reference range, 3.4-4.8). Alkaline phosphatase level was 188 U/L (reference range, 44-147), aspartate aminotransferase level 76 U/L (reference range, 0-40), and alanine aminotransferase level 12 U/L (reference range, 7-40). Total bilirubin level was 2.4 mg/dL (reference range, less than 1.5) with direct bilirubin level of 0.4 mg/dL. His C-reactive protein level was 1.5 mg/mL (reference range, 0-0.75). Creatinine kinase (CK) level was 2,550 U/L (reference range, 2-198). International Normalized Ratio (INR) was 1.0. Urinalysis was notable for 2+ proteinuria, large hemoglobin pigment, and 6 red blood cells per high power field (reference range, 0-4).
His blood urea nitrogen is elevated, reflecting either prerenal azotemia or increased absorption of nitrogenous products. Unconjugated hyperbilirubinemia may result from impaired hepatic bilirubin uptake (such as in heart failure or portosystemic shunts), impaired bilirubin conjugation (resulting from genetic conditions or drugs), or excess bilirubin production (such as in hemolysis); his anemia and lack of other evidence of hepatic dysfunction point to hemolysis as the etiology. The reticulocyte production index is approximately 1%, which suggests that an increase in erythrocyte generation is present but inadequate. This, however, does not mean that an erythrocyte production abnormality is present since reticulocytosis can be delayed in many cases of acute hemolytic anemia. It is also possible that the same hemolytic process is affecting mature and immature erythrocytes. A peripheral blood smear should be reviewed for evidence of intravascular hemolysis and testing for autoimmune hemolysis should be performed. Notably, his white blood cell and platelet counts are preserved, which makes a bone marrow–involved malignancy or infiltrative process less likely. The alkaline phosphatase elevation may result from either intrahepatic or extrahepatic biliopathy; bone damage is also possible. The elevation of aspartate aminotransferase, CK, and potassium, along with marked urinary heme pigment, may indicate muscle damage; the most common myositis in children is benign acute childhood myositis resulting from viral infection. However, the moderate level of CK elevation seen in this case is nonspecific and can result from many different etiologies. A metabolic myopathy, such as carnitine palmitoyltransferase II deficiency, can be made worse by metabolic stress and result in rhabdomyolysis; the presentations of inborn errors of metabolism are varied and a planned-out, stepwise approach in evaluation is fundamental.
Lactic acid dehydrogenase (LDH) level was 1,457 U/L (reference range, 140-280), and haptoglobin level was less than 6 mg/dL (reference range, 30-200). Peripheral blood smear demonstrated occasional atypical, reactive-appearing lymphocytes with red cell clumping and agglutination, as well as rare target, burr, and fragmented red cells. Test results for urine myoglobin were negative. Results of urine culture were negative. No blood culture was collected.
The elevated LDH, decreased haptoglobin, and findings on the peripheral blood smear confirm hemolysis. The clumping of erythrocytes can be artifactual in the preparation of peripheral smears, but when considered in the context of hemolysis, may be clinically important. Clumping of erythrocytes on the peripheral smear indicates the binding of a protein to antigens on the erythrocyte membrane; when this occurs below body temperature, this is consistent with the presence of a “cold agglutinin,” usually an IgM antibody directed at erythrocyte surface antigens that causes agglutination and destruction, especially in cooler areas of the body. This is a well-known complication of Mycoplasma pneumoniae infections as well as Epstein-Barr virus (EBV) infections; it may also occur with lymphoid malignancies or autoimmune disease.
Direct Coombs IgG test findings were negative, direct Coombs C3 test was positive, and direct Coombs polyspecific test was positive. M pneumoniae IgG antibody level was 1.4 mg/dL (reference ranges: <0.9, negative; 0.91-1.09, equivocal; >1.1, positive); M pneumoniae IgM level was 529 U/mL (reference range: <770, negative). EBV capsid IgM and IgG levels were undetectable. EBV nuclear antigen IgG level was also undetectable. EBV viral load was fewer than 10 copies/mL. Antinuclear antibodies (ANA) level was negative. General IgE and IgM levels were normal, at 11 and 81 mg/dL, respectively. Repeat complete blood count showed WBC of 7,800/µL, hemoglobin of 8.7 g/dL, and platelet count of 341,000/µL. The patient’s hemoglobin remained stable during hospitalization.
This directed testing is helpful in further classifying the patient’s hemolytic anemia. Autoimmune hemolytic anemias are classified into warm antibody–mediated, cold antibody–mediated, and mixed-type forms; drug-induced and alloimmune hemolytic anemias also occur. In addition, both systemic lupus erythematosus and antiphospholipid antibody syndrome can have hemolytic anemia with variable Coombs testing results; neither fit well in this case. The absence of red blood cell–directed IgG antibodies substantially decreases the likelihood of warm antibody–mediated hemolytic anemia. In cold antibody–mediated hemolytic anemia, antibodies bind to the erythrocyte membrane and then adhere to complement C3, which leads to both intravascular and extravascular hemolysis. Important types of cold antibody–mediated hemolytic anemia in children are primary and secondary cold agglutinin disease, along with paroxysmal cold hemoglobinuria. The Donath-Landsteiner test can be helpful in differentiating these conditions. Antibodies to Mycoplasma may be delayed in response to acute infection, and a child who is reinfected may only produce IgG antibodies. Given the patient’s clinical stability and previous health, the most likely diagnosis is Mycoplasma-induced cold antibody–mediated hemolytic anemia. It may be helpful to check convalescent titers to Mycoplasma in 2 to 4 weeks.
Donath-Landsteiner (D-L) antibody test results were positive. Medication-derived hemolytic anemia testing was conducted, but the presence of positive D-L antibody makes the test results inconclusive. This ultimately led to a diagnosis of paroxysmal cold hemoglobinuria (PCH), presumably triggered by a viral syndrome. Convalescent titers to Mycoplasma were not checked given clinical improvement. Because the patient’s hemoglobin was stable during hospitalization, he was not treated with steroids. His parents were counseled on avoiding cold temperatures for several days. Within 1 month, his hemoglobin had recovered without further evidence of hemolysis.
DISCUSSION
Hemolytic anemia refers to the accelerated destruction of red blood cells and can be further classified as acquired or hereditary.1 Hereditary conditions causing hemolytic anemia include enzymopathies (eg, glucose-6-phosphate dehydrogenase deficiency), hemoglobinopathies (eg, sickle cell disease), and membrane abnormalities (eg, hereditary spherocytosis). Acquired pathologies include microangiopathic hemolytic anemia (MAHA), anemias directly caused by certain infections such as malaria, and immune-mediated (Coombs-positive) hemolytic anemias.
MAHA can sometimes be life-threatening and is therefore important to identify quickly. In the right clinical context, such processes may be rapidly recognized by the presence of schistocytes on blood smear in addition to an elevated serum LDH level. Schistocytes suggest mechanical destruction of erythrocytes in the vasculature, the hallmark of MAHA. Important MAHAs include thrombocytopenic purpura, hemolytic-uremic syndrome, and disseminated intravascular coagulation. Though this patient did have a mildly elevated LDH, MAHA was less likely because there were no schistocytes on the blood smear.
Autoimmune hemolytic anemias (AIHAs) are another important subset of acquired hemolytic anemias. AIHAs occur when there is antibody-mediated destruction of erythrocytes. The direct Coombs test evaluates for antibody- or complement-coated erythrocytes. After administration of anti-IgG and anti-C3 serum, the test evaluates for agglutination of the red cells caused by attached antibodies or complement. Coombs-positive AIHA can also be categorized by the temperature of agglutination. “Warm” hemolysis often involves IgG autoantibodies (ie, warm agglutinins), while “cold” antibodies, usually IgM autoantibodies, bind at colder temperatures (0-4 °C) and activate complements, including C3. In this patient, the Coombs C3 was positive while the Coombs IgG was negative, which is more suggestive of a cold complement–mediated pathway.
Cold AIHA can be further categorized into primary cold agglutinin disease, secondary cold agglutinin disease, and PCH. Primary cold agglutinin disease is an autoimmune disorder that mostly occurs in adults. Secondary cold AIHA can often be triggered by bacterial infection (commonly M pneumoniae) or viruses including EBV, measles, and mumps.2 Medications, including penicillin and cephalosporins, can also be implicated. Secondary cold AIHA is also linked with autoimmune diseases, such as systemic lupus erythematosus and lymphoproliferative disorders. PCH can be identified with the unique presence of a specific autoantibody (ie, D-L autoantibody) that agglutinates at cold temperatures but dissociates on subsequent rewarming.3 Complement remains affixed and activates hemolysis.
The D-L antibody responsible for PCH is an IgG antibody to the P-antigen present on the erythrocyte surface. Since the Coombs test is conducted at normal temperature, it will be positive for the affixed complement but not for IgG. The underlying mechanism for PCH was proposed by Julius Donath, MD, and Karl Landsteiner, MD, in 1904 and is considered to be the first description of autoimmune disease being precipitated by antibodies.4 The D-L antibody test itself is uncommonly performed and somewhat difficult to interpret, particularly in adults, and may lead to false-negative results.5
PCH is an acquired, cold AIHA more common to children6,7 and may account for up to 33% of pediatric AIHA cases.8 Typical presentation is after an upper respiratory tract illness; however, the trigger is often not identified. Implicated triggers include a number of viruses.9 Clinical presentation includes findings of intravascular hemolysis similar to those in our patient. The pathogenic IgG autoantibody is polyclonal and is likely formed because of immune stimulation, which is consistent with the predominance of nonmalignant triggers of this disease process.10 Hemolysis and associated symptoms are often exacerbated with cold exposure; both typically resolve within 2 weeks. In recurrent cases, which are a minority, immunosuppression may be considered.10
PCH remains an often-understated cause of hemolytic anemia particularly in children. Lacking obvious pathognomonic clinical symptoms, it may be overlooked for other forms of AIHA or MAHA. However, with a structured approach to evaluation, as with this patient who had hematuria and jaundice, early diagnosis can prevent an unnecessarily extensive workup and can provide reassurance to patient and parents. By understanding the basic categories of hemolytic anemia, the relevant blood testing available, and interpretation of Coombs test results, clinicians can ensure that PCH is a diagnosis that is not left out in the cold.
KEY TEACHING POINTS
- Examination for schistocytes on a blood smear can help identify life-threatening causes of hemolytic anemia.
- Characterization of cold AIHA includes defining the underlying etiology as primary cold agglutinin disease, secondary cold agglutinin disease, or PCH.
- PCH is a cold AIHA that is an underrecognized cause of hemolytic anemia in children. The diagnosis of PCH is made by testing for the presence of the D-L antibody.
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
1. Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
2. Djaldetti M. Paroxysmal cold hemoglobinuria. CRC Crit Rev Clin Lab Sci. 1978;9(1):49-83. https://doi.org/10.3109/10408367809150915
3. Levine P, Celano MJ, Falkowski F. The specificity of the antibody in paroxysmal cold hemoglobinuria (P.C.H.). Transfusion. 1963;3(4):278-280. https://doi.org/10.1111/j.1537-2995.1963.tb04643.x
4. Donath J, Landsteiner K. Uber Paroxysmale Hamoglobinurie. Munch Med Wochenschr. 1904;51:1590-1593
5. Zeller MP, Arnold DM, Al Habsi K, et al. Paroxysmal cold hemoglobinuria: a difficult diagnosis in adult patients. Transfusion. 2017;57(1):137-143. https://doi.org/10.1111/trf.13888
6. Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. a study of 22 cases. Vox Sang. 1990;58(4):281-286. https://doi.org/10.1111/j.1423-0410.1990.tb05000.x
7. Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4(2):137-164. https://doi.org/10.1080/10245332.1999.11746439
8. Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22(1):1-15. https://doi.org/10.1016/j.blre.2007.08.002
9. Leibrandt R, Angelino K, Vizel-Schwartz M, Shapira I. Paroxysmal cold hemoglobinuria in an adult with respiratory syncytial virus. Case Rep Hematol. 2018;2018:1-3. https://doi.org/10.1155/2018/7586719
10. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422-429. https://doi.org/10.1111/j.1365-2141.2007.06664.x
© 2020 Society of Hospital Medicine
Fool Me Twice: The Role for Hospitals and Health Systems in Fixing the Broken PPE Supply Chain
The story of the coronavirus disease 2019 (COVID-19) pandemic in the United States has been defined, in part, by a persistent shortage of medical supplies that has made it difficult and dangerous for healthcare workers to care for infected patients. States, health systems, and even individual hospitals are currently competing against one another—sometimes at auction—to obtain personal protective equipment (PPE). This “Wild West” scenario has resulted in bizarre stories involving attempts to obtain PPE. One health system recently described a James Bond–like pursuit of essential PPE, complete with a covert trip to an industrial warehouse, trucks filled with masks but labeled as food delivery vehicles, and an intervention by a United States congressman.1 Many states have experienced analogous, but still atypical, stories: masks flown in from China using the private jet of a professional sports team owner,2 widespread use of novel sterilization modalities to allow PPE reuse,3 and one attempt to purchase price-gouged PPE from the host of the show “Shark Tank.”4 In some cases, hospitals and healthcare workers have pleaded for PPE on fundraising and social media sites.5
These profound deviations from operations of contemporary health system supply chains would have seemed beyond belief just a few months ago. Instead, they now echo the collective experiences of healthcare stakeholders trying to obtain PPE to protect their frontline healthcare workers during the COVID-19 pandemic.
HEALTHCARE MARKETS DURING A PANDEMIC
How did we get into this situation? The manufacture of medical supplies like gowns and masks is a highly competitive business with very slim margins, and as a result, medical equipment manufacturers aim to match their supply with the market’s demand, with hospitals and health systems using just-in-time ordering to limit excess inventory.6 While this approach adds efficiency and reduces costs, it also renders manufacturers and customers vulnerable to supply disruptions and shortages when need surges. The COVID-19 pandemic represents perhaps the most extreme example of a massive, widespread surge in demand that occurred multifocally and in a highly compressed time frame. Unlike other industries (eg, consumer paper products), however, in which demand exceeding supply causes inconvenience, the lack of PPE has led to critical public health consequences, with lives of both healthcare workers and vulnerable patients lost because of these shortages of medical equipment.
THE SPECIAL CASE OF PPE
There are many reasons for the PPE crisis. As noted above, manufacturers have prioritized efficiency over the ability to quickly increase production. They adhere to just-in-time ordering rather than planning for a surge in demand with extra production capacity, all to avoid having warehouses filled with unsold products if surges never occur. This strategy, compounded by the fact that most PPE in the United States is imported from areas in Asia that were profoundly affected early on by COVID-19, led to the observed widespread shortages. When PPE became unavailable from usual suppliers, hospitals were unable to locate other sources of existing PPE because of a lack of transparency about where PPE could be found and how it could be obtained. The Food and Drug Administration and other federal regulatory agencies maintained strict regulations around PPE production and, despite the crisis, made few exceptions.7 The FDA did grant a few Emergency Use Authorizations (EUAs) for certain improvised, decontaminated, or alternative respirators (eg, the Chinese-made KN95), but it has only very infrequently issued EUAs to allow domestic manufacturers to ramp up production.8 These failures were accompanied by an serious increase in PPE use, leading to spikes in price, price gouging, and hoarding,9 problems that were further magnified as health systems and hospitals were forced to compete with nonhealthcare businesses for PPE.
LACK OF FEDERAL GOVERNMENT RESPONSE
The Defense Production Act (DPA) gives the federal government the power to increase production of goods needed during a crisis8 to offer purchasing guarantees, coordinate federal agencies, and regulate distribution and pricing. However, the current administration’s failure to mount a coordinated federal response has contributed to the observed market instability, medical supply shortages, and public health crisis we face. We have previously recommended that the federal government use the power of the DPA to reduce manufacturers’ risk of being uncompensated for excess supply, support temporary reductions in regulatory barriers, and create mandatory centralized reporting of PPE supply, including completed PPE and its components.10 We stand by these recommendations but also acknowledge that hospitals and health systems may be simultaneously considering how to best prepare for future crises and even surges in demand over the next 18 months as the COVID-19 pandemic continues.
RECOMMENDATIONS FOR HEALTH SYSTEMS AND HOSPITALS
1. Encourage mandates at the hospital, health system, and state level regarding minimum inventory levels for essential equipment. Stockpiles are essential for emergency preparedness. In the long term, these sorts of stockpiles are economically infeasible without government help to maintain them. In the near term, however, it is sensible that hospitals and health systems would maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases. However, a soon-to-published study suggests that over 40% of hospitals had a PPE stockpile of less than 2 weeks.11 Although this survey was conducted at the height of the shortage, it suggests that there is opportunity for improvement.
2. Coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution. The best example of this is the seven-state purchasing consortium announced by New York Governor Andrew Cuomo in early May.12 Unfortunately, since the announcement, there have been few details about whether the states were successful in their effort to reduce prices or to obtain PPE in bulk. Still, hospitals and health systems could join or emulate purchasing collaboratives to allow resources to be better allocated according to need. There are barriers to such collaboratives because the market is currently set up to encourage competition among health systems and hospitals. During the pandemic, however, cooperation has increasingly been favored over competition in science and healthcare delivery. There are also existing hospital purchasing collaboratives (eg, Premier, Inc13), which have taken steps to vet suppliers and improve access to PPE, but it is not clear how successful these efforts have been to date.
3. Advocate for strong federal leadership, including support for increased domestic manufacturing; replenishment and maintenance of state and health system stockpiles of PPE, ventilators, and medications; and development of a centrally coordinated PPE allocation and distribution process. While hospitals and health systems may favor remaining as apolitical as possible, the need for a federal response to stabilize the PPE market may be too urgent and necessary to ignore.
CONCLUSION
As hospitals and health systems prepare for continued surges in COVID-19 cases, they face challenges in providing PPE for frontline clinicians and staff. A federal plan to enhance nimbleness in responding to multifocal, geographic outbreaks and ensure awareness regarding inventory would improve our chances to successfully navigate the next pandemic and optimize the protection of our health workers, patients, and public health. In the absence of such a plan, hospitals should maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases and should continue to attempt to coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution.
1. Artenstein AW. In pursuit of PPE. N Engl J Med. 2020;382(18):e46. https://doi.org/10.1056/nejmc2010025
2. McGrane V, Ellement JR. A Patriots plane full of 1 million N95 masks from China arrived Thursday. Here’s how the plan came together. Boston Globe. Updated April 2, 2020. Accessed April 27, 2020. https://www.bostonglobe.com/2020/04/02/nation/kraft-family-used-patriots-team-plane-shuttle-protective-masks-china-boston-wsj-reports/
3. Kolodny L. California plans to decontaminate 80,000 masks a day for health workers amid the COVID-19 pandemic. CNBC. April 8, 2020. Updated April 9, 2020. Accessed April 27, 2020. https://www.cnbc.com/2020/04/08/california-plans-to-sanitize-80000-n95-masks-a-day-for-health-workers.html
4. Levenson M. Company questions deal by ‘Shark Tank’ star to sell N95 masks to Florida. New York Times. April 22, 2020. Accessed May 20, 2020. https://www.nytimes.com/2020/04/22/us/daymond-john-n95-masks-florida-3m.html
5. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. New York Times. March 19, 2020. Updated March 22, 2020. Accessed April 27, 2020. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html
6. Lee HL, Billington C. Managing supply chain inventory: pitfalls and opportunities. MIT Sloan Management Review. April 15, 1992. Accessed April 27, 2020. https://sloanreview.mit.edu/article/managing-supply-chain-inventory-pitfalls-and-opportunities/
7. Emergency Situations (Medical Devices): Emergency Use Authorizations. Food and Drug Administration. Accessed May 10, 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
8. Watney C, Stapp A. Masks for All: Using Purchase Guarantees and Targeted Deregulation to Boost Production of Essential Medical Equipment. Mercatus Center: George Mason University. April 8, 2020. Accessed June 23, 2020. https://www.mercatus.org/publications/covid-19-crisis-response/masks-all-using-purchase-guarantees-and-targeted-deregulation
9. Volkov M. DOJ hoarding and price gouging task force seizes critical medical supplies and distributes to New York and New Jersey hospitals. Corruption, Crime & Compliance blog. April 2, 2020. Accessed April 27, 2020. https://blog.volkovlaw.com/2020/04/doj-hoarding-and-price-gouging-task-force-seizes-critical-medical-supplies-and-distributes-to-new-york-and-new-jersey-hospitals/
10. Lagu T, Werner R, Artenstein AW. Why don’t hospitals have enough masks? Because coronavirus broke the market. Washington Post. May 21, 2020. Accessed May 25, 2020. https://www.washingtonpost.com/outlook/2020/05/21/why-dont-hospitals-have-enough-masks-because-coronavirus-broke-market/
11. Auerbach A, O’Leary KJ, Harrison JD, et al. Hospital ward adaptation during the COVID-19 Pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15:483-488.
12. Voytko L. NY will team up with 6 states to buy medical supplies, Cuomo says. Forbes. May 3, 2020. Accessed May 26, 2020. https://www.forbes.com/sites/lisettevoytko/2020/05/03/ny-will-team-up-with-6-states-to-buy-medical-supplies-cuomo-says/
13. Premier. Supply Chain Solutions. Accessed May 26, 2020. https://www.premierinc.com/solutions/supply-chain
The story of the coronavirus disease 2019 (COVID-19) pandemic in the United States has been defined, in part, by a persistent shortage of medical supplies that has made it difficult and dangerous for healthcare workers to care for infected patients. States, health systems, and even individual hospitals are currently competing against one another—sometimes at auction—to obtain personal protective equipment (PPE). This “Wild West” scenario has resulted in bizarre stories involving attempts to obtain PPE. One health system recently described a James Bond–like pursuit of essential PPE, complete with a covert trip to an industrial warehouse, trucks filled with masks but labeled as food delivery vehicles, and an intervention by a United States congressman.1 Many states have experienced analogous, but still atypical, stories: masks flown in from China using the private jet of a professional sports team owner,2 widespread use of novel sterilization modalities to allow PPE reuse,3 and one attempt to purchase price-gouged PPE from the host of the show “Shark Tank.”4 In some cases, hospitals and healthcare workers have pleaded for PPE on fundraising and social media sites.5
These profound deviations from operations of contemporary health system supply chains would have seemed beyond belief just a few months ago. Instead, they now echo the collective experiences of healthcare stakeholders trying to obtain PPE to protect their frontline healthcare workers during the COVID-19 pandemic.
HEALTHCARE MARKETS DURING A PANDEMIC
How did we get into this situation? The manufacture of medical supplies like gowns and masks is a highly competitive business with very slim margins, and as a result, medical equipment manufacturers aim to match their supply with the market’s demand, with hospitals and health systems using just-in-time ordering to limit excess inventory.6 While this approach adds efficiency and reduces costs, it also renders manufacturers and customers vulnerable to supply disruptions and shortages when need surges. The COVID-19 pandemic represents perhaps the most extreme example of a massive, widespread surge in demand that occurred multifocally and in a highly compressed time frame. Unlike other industries (eg, consumer paper products), however, in which demand exceeding supply causes inconvenience, the lack of PPE has led to critical public health consequences, with lives of both healthcare workers and vulnerable patients lost because of these shortages of medical equipment.
THE SPECIAL CASE OF PPE
There are many reasons for the PPE crisis. As noted above, manufacturers have prioritized efficiency over the ability to quickly increase production. They adhere to just-in-time ordering rather than planning for a surge in demand with extra production capacity, all to avoid having warehouses filled with unsold products if surges never occur. This strategy, compounded by the fact that most PPE in the United States is imported from areas in Asia that were profoundly affected early on by COVID-19, led to the observed widespread shortages. When PPE became unavailable from usual suppliers, hospitals were unable to locate other sources of existing PPE because of a lack of transparency about where PPE could be found and how it could be obtained. The Food and Drug Administration and other federal regulatory agencies maintained strict regulations around PPE production and, despite the crisis, made few exceptions.7 The FDA did grant a few Emergency Use Authorizations (EUAs) for certain improvised, decontaminated, or alternative respirators (eg, the Chinese-made KN95), but it has only very infrequently issued EUAs to allow domestic manufacturers to ramp up production.8 These failures were accompanied by an serious increase in PPE use, leading to spikes in price, price gouging, and hoarding,9 problems that were further magnified as health systems and hospitals were forced to compete with nonhealthcare businesses for PPE.
LACK OF FEDERAL GOVERNMENT RESPONSE
The Defense Production Act (DPA) gives the federal government the power to increase production of goods needed during a crisis8 to offer purchasing guarantees, coordinate federal agencies, and regulate distribution and pricing. However, the current administration’s failure to mount a coordinated federal response has contributed to the observed market instability, medical supply shortages, and public health crisis we face. We have previously recommended that the federal government use the power of the DPA to reduce manufacturers’ risk of being uncompensated for excess supply, support temporary reductions in regulatory barriers, and create mandatory centralized reporting of PPE supply, including completed PPE and its components.10 We stand by these recommendations but also acknowledge that hospitals and health systems may be simultaneously considering how to best prepare for future crises and even surges in demand over the next 18 months as the COVID-19 pandemic continues.
RECOMMENDATIONS FOR HEALTH SYSTEMS AND HOSPITALS
1. Encourage mandates at the hospital, health system, and state level regarding minimum inventory levels for essential equipment. Stockpiles are essential for emergency preparedness. In the long term, these sorts of stockpiles are economically infeasible without government help to maintain them. In the near term, however, it is sensible that hospitals and health systems would maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases. However, a soon-to-published study suggests that over 40% of hospitals had a PPE stockpile of less than 2 weeks.11 Although this survey was conducted at the height of the shortage, it suggests that there is opportunity for improvement.
2. Coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution. The best example of this is the seven-state purchasing consortium announced by New York Governor Andrew Cuomo in early May.12 Unfortunately, since the announcement, there have been few details about whether the states were successful in their effort to reduce prices or to obtain PPE in bulk. Still, hospitals and health systems could join or emulate purchasing collaboratives to allow resources to be better allocated according to need. There are barriers to such collaboratives because the market is currently set up to encourage competition among health systems and hospitals. During the pandemic, however, cooperation has increasingly been favored over competition in science and healthcare delivery. There are also existing hospital purchasing collaboratives (eg, Premier, Inc13), which have taken steps to vet suppliers and improve access to PPE, but it is not clear how successful these efforts have been to date.
3. Advocate for strong federal leadership, including support for increased domestic manufacturing; replenishment and maintenance of state and health system stockpiles of PPE, ventilators, and medications; and development of a centrally coordinated PPE allocation and distribution process. While hospitals and health systems may favor remaining as apolitical as possible, the need for a federal response to stabilize the PPE market may be too urgent and necessary to ignore.
CONCLUSION
As hospitals and health systems prepare for continued surges in COVID-19 cases, they face challenges in providing PPE for frontline clinicians and staff. A federal plan to enhance nimbleness in responding to multifocal, geographic outbreaks and ensure awareness regarding inventory would improve our chances to successfully navigate the next pandemic and optimize the protection of our health workers, patients, and public health. In the absence of such a plan, hospitals should maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases and should continue to attempt to coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution.
The story of the coronavirus disease 2019 (COVID-19) pandemic in the United States has been defined, in part, by a persistent shortage of medical supplies that has made it difficult and dangerous for healthcare workers to care for infected patients. States, health systems, and even individual hospitals are currently competing against one another—sometimes at auction—to obtain personal protective equipment (PPE). This “Wild West” scenario has resulted in bizarre stories involving attempts to obtain PPE. One health system recently described a James Bond–like pursuit of essential PPE, complete with a covert trip to an industrial warehouse, trucks filled with masks but labeled as food delivery vehicles, and an intervention by a United States congressman.1 Many states have experienced analogous, but still atypical, stories: masks flown in from China using the private jet of a professional sports team owner,2 widespread use of novel sterilization modalities to allow PPE reuse,3 and one attempt to purchase price-gouged PPE from the host of the show “Shark Tank.”4 In some cases, hospitals and healthcare workers have pleaded for PPE on fundraising and social media sites.5
These profound deviations from operations of contemporary health system supply chains would have seemed beyond belief just a few months ago. Instead, they now echo the collective experiences of healthcare stakeholders trying to obtain PPE to protect their frontline healthcare workers during the COVID-19 pandemic.
HEALTHCARE MARKETS DURING A PANDEMIC
How did we get into this situation? The manufacture of medical supplies like gowns and masks is a highly competitive business with very slim margins, and as a result, medical equipment manufacturers aim to match their supply with the market’s demand, with hospitals and health systems using just-in-time ordering to limit excess inventory.6 While this approach adds efficiency and reduces costs, it also renders manufacturers and customers vulnerable to supply disruptions and shortages when need surges. The COVID-19 pandemic represents perhaps the most extreme example of a massive, widespread surge in demand that occurred multifocally and in a highly compressed time frame. Unlike other industries (eg, consumer paper products), however, in which demand exceeding supply causes inconvenience, the lack of PPE has led to critical public health consequences, with lives of both healthcare workers and vulnerable patients lost because of these shortages of medical equipment.
THE SPECIAL CASE OF PPE
There are many reasons for the PPE crisis. As noted above, manufacturers have prioritized efficiency over the ability to quickly increase production. They adhere to just-in-time ordering rather than planning for a surge in demand with extra production capacity, all to avoid having warehouses filled with unsold products if surges never occur. This strategy, compounded by the fact that most PPE in the United States is imported from areas in Asia that were profoundly affected early on by COVID-19, led to the observed widespread shortages. When PPE became unavailable from usual suppliers, hospitals were unable to locate other sources of existing PPE because of a lack of transparency about where PPE could be found and how it could be obtained. The Food and Drug Administration and other federal regulatory agencies maintained strict regulations around PPE production and, despite the crisis, made few exceptions.7 The FDA did grant a few Emergency Use Authorizations (EUAs) for certain improvised, decontaminated, or alternative respirators (eg, the Chinese-made KN95), but it has only very infrequently issued EUAs to allow domestic manufacturers to ramp up production.8 These failures were accompanied by an serious increase in PPE use, leading to spikes in price, price gouging, and hoarding,9 problems that were further magnified as health systems and hospitals were forced to compete with nonhealthcare businesses for PPE.
LACK OF FEDERAL GOVERNMENT RESPONSE
The Defense Production Act (DPA) gives the federal government the power to increase production of goods needed during a crisis8 to offer purchasing guarantees, coordinate federal agencies, and regulate distribution and pricing. However, the current administration’s failure to mount a coordinated federal response has contributed to the observed market instability, medical supply shortages, and public health crisis we face. We have previously recommended that the federal government use the power of the DPA to reduce manufacturers’ risk of being uncompensated for excess supply, support temporary reductions in regulatory barriers, and create mandatory centralized reporting of PPE supply, including completed PPE and its components.10 We stand by these recommendations but also acknowledge that hospitals and health systems may be simultaneously considering how to best prepare for future crises and even surges in demand over the next 18 months as the COVID-19 pandemic continues.
RECOMMENDATIONS FOR HEALTH SYSTEMS AND HOSPITALS
1. Encourage mandates at the hospital, health system, and state level regarding minimum inventory levels for essential equipment. Stockpiles are essential for emergency preparedness. In the long term, these sorts of stockpiles are economically infeasible without government help to maintain them. In the near term, however, it is sensible that hospitals and health systems would maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases. However, a soon-to-published study suggests that over 40% of hospitals had a PPE stockpile of less than 2 weeks.11 Although this survey was conducted at the height of the shortage, it suggests that there is opportunity for improvement.
2. Coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution. The best example of this is the seven-state purchasing consortium announced by New York Governor Andrew Cuomo in early May.12 Unfortunately, since the announcement, there have been few details about whether the states were successful in their effort to reduce prices or to obtain PPE in bulk. Still, hospitals and health systems could join or emulate purchasing collaboratives to allow resources to be better allocated according to need. There are barriers to such collaboratives because the market is currently set up to encourage competition among health systems and hospitals. During the pandemic, however, cooperation has increasingly been favored over competition in science and healthcare delivery. There are also existing hospital purchasing collaboratives (eg, Premier, Inc13), which have taken steps to vet suppliers and improve access to PPE, but it is not clear how successful these efforts have been to date.
3. Advocate for strong federal leadership, including support for increased domestic manufacturing; replenishment and maintenance of state and health system stockpiles of PPE, ventilators, and medications; and development of a centrally coordinated PPE allocation and distribution process. While hospitals and health systems may favor remaining as apolitical as possible, the need for a federal response to stabilize the PPE market may be too urgent and necessary to ignore.
CONCLUSION
As hospitals and health systems prepare for continued surges in COVID-19 cases, they face challenges in providing PPE for frontline clinicians and staff. A federal plan to enhance nimbleness in responding to multifocal, geographic outbreaks and ensure awareness regarding inventory would improve our chances to successfully navigate the next pandemic and optimize the protection of our health workers, patients, and public health. In the absence of such a plan, hospitals should maintain a minimum of 2 weeks’ worth of PPE to prepare for expected regional spikes in COVID-19 cases and should continue to attempt to coordinate efforts among states and health systems to collect and report inventory, regionalize resources, and coordinate their distribution.
1. Artenstein AW. In pursuit of PPE. N Engl J Med. 2020;382(18):e46. https://doi.org/10.1056/nejmc2010025
2. McGrane V, Ellement JR. A Patriots plane full of 1 million N95 masks from China arrived Thursday. Here’s how the plan came together. Boston Globe. Updated April 2, 2020. Accessed April 27, 2020. https://www.bostonglobe.com/2020/04/02/nation/kraft-family-used-patriots-team-plane-shuttle-protective-masks-china-boston-wsj-reports/
3. Kolodny L. California plans to decontaminate 80,000 masks a day for health workers amid the COVID-19 pandemic. CNBC. April 8, 2020. Updated April 9, 2020. Accessed April 27, 2020. https://www.cnbc.com/2020/04/08/california-plans-to-sanitize-80000-n95-masks-a-day-for-health-workers.html
4. Levenson M. Company questions deal by ‘Shark Tank’ star to sell N95 masks to Florida. New York Times. April 22, 2020. Accessed May 20, 2020. https://www.nytimes.com/2020/04/22/us/daymond-john-n95-masks-florida-3m.html
5. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. New York Times. March 19, 2020. Updated March 22, 2020. Accessed April 27, 2020. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html
6. Lee HL, Billington C. Managing supply chain inventory: pitfalls and opportunities. MIT Sloan Management Review. April 15, 1992. Accessed April 27, 2020. https://sloanreview.mit.edu/article/managing-supply-chain-inventory-pitfalls-and-opportunities/
7. Emergency Situations (Medical Devices): Emergency Use Authorizations. Food and Drug Administration. Accessed May 10, 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
8. Watney C, Stapp A. Masks for All: Using Purchase Guarantees and Targeted Deregulation to Boost Production of Essential Medical Equipment. Mercatus Center: George Mason University. April 8, 2020. Accessed June 23, 2020. https://www.mercatus.org/publications/covid-19-crisis-response/masks-all-using-purchase-guarantees-and-targeted-deregulation
9. Volkov M. DOJ hoarding and price gouging task force seizes critical medical supplies and distributes to New York and New Jersey hospitals. Corruption, Crime & Compliance blog. April 2, 2020. Accessed April 27, 2020. https://blog.volkovlaw.com/2020/04/doj-hoarding-and-price-gouging-task-force-seizes-critical-medical-supplies-and-distributes-to-new-york-and-new-jersey-hospitals/
10. Lagu T, Werner R, Artenstein AW. Why don’t hospitals have enough masks? Because coronavirus broke the market. Washington Post. May 21, 2020. Accessed May 25, 2020. https://www.washingtonpost.com/outlook/2020/05/21/why-dont-hospitals-have-enough-masks-because-coronavirus-broke-market/
11. Auerbach A, O’Leary KJ, Harrison JD, et al. Hospital ward adaptation during the COVID-19 Pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15:483-488.
12. Voytko L. NY will team up with 6 states to buy medical supplies, Cuomo says. Forbes. May 3, 2020. Accessed May 26, 2020. https://www.forbes.com/sites/lisettevoytko/2020/05/03/ny-will-team-up-with-6-states-to-buy-medical-supplies-cuomo-says/
13. Premier. Supply Chain Solutions. Accessed May 26, 2020. https://www.premierinc.com/solutions/supply-chain
1. Artenstein AW. In pursuit of PPE. N Engl J Med. 2020;382(18):e46. https://doi.org/10.1056/nejmc2010025
2. McGrane V, Ellement JR. A Patriots plane full of 1 million N95 masks from China arrived Thursday. Here’s how the plan came together. Boston Globe. Updated April 2, 2020. Accessed April 27, 2020. https://www.bostonglobe.com/2020/04/02/nation/kraft-family-used-patriots-team-plane-shuttle-protective-masks-china-boston-wsj-reports/
3. Kolodny L. California plans to decontaminate 80,000 masks a day for health workers amid the COVID-19 pandemic. CNBC. April 8, 2020. Updated April 9, 2020. Accessed April 27, 2020. https://www.cnbc.com/2020/04/08/california-plans-to-sanitize-80000-n95-masks-a-day-for-health-workers.html
4. Levenson M. Company questions deal by ‘Shark Tank’ star to sell N95 masks to Florida. New York Times. April 22, 2020. Accessed May 20, 2020. https://www.nytimes.com/2020/04/22/us/daymond-john-n95-masks-florida-3m.html
5. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. New York Times. March 19, 2020. Updated March 22, 2020. Accessed April 27, 2020. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html
6. Lee HL, Billington C. Managing supply chain inventory: pitfalls and opportunities. MIT Sloan Management Review. April 15, 1992. Accessed April 27, 2020. https://sloanreview.mit.edu/article/managing-supply-chain-inventory-pitfalls-and-opportunities/
7. Emergency Situations (Medical Devices): Emergency Use Authorizations. Food and Drug Administration. Accessed May 10, 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
8. Watney C, Stapp A. Masks for All: Using Purchase Guarantees and Targeted Deregulation to Boost Production of Essential Medical Equipment. Mercatus Center: George Mason University. April 8, 2020. Accessed June 23, 2020. https://www.mercatus.org/publications/covid-19-crisis-response/masks-all-using-purchase-guarantees-and-targeted-deregulation
9. Volkov M. DOJ hoarding and price gouging task force seizes critical medical supplies and distributes to New York and New Jersey hospitals. Corruption, Crime & Compliance blog. April 2, 2020. Accessed April 27, 2020. https://blog.volkovlaw.com/2020/04/doj-hoarding-and-price-gouging-task-force-seizes-critical-medical-supplies-and-distributes-to-new-york-and-new-jersey-hospitals/
10. Lagu T, Werner R, Artenstein AW. Why don’t hospitals have enough masks? Because coronavirus broke the market. Washington Post. May 21, 2020. Accessed May 25, 2020. https://www.washingtonpost.com/outlook/2020/05/21/why-dont-hospitals-have-enough-masks-because-coronavirus-broke-market/
11. Auerbach A, O’Leary KJ, Harrison JD, et al. Hospital ward adaptation during the COVID-19 Pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15:483-488.
12. Voytko L. NY will team up with 6 states to buy medical supplies, Cuomo says. Forbes. May 3, 2020. Accessed May 26, 2020. https://www.forbes.com/sites/lisettevoytko/2020/05/03/ny-will-team-up-with-6-states-to-buy-medical-supplies-cuomo-says/
13. Premier. Supply Chain Solutions. Accessed May 26, 2020. https://www.premierinc.com/solutions/supply-chain
© 2020 Society of Hospital Medicine