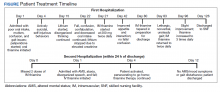
User login
Preprints During the COVID-19 Pandemic: Public Health Emergencies and Medical Literature
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
© 2020 Society of Hospital Medicine
Relationship of Hospital Star Ratings to Race, Education, and Community Income
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
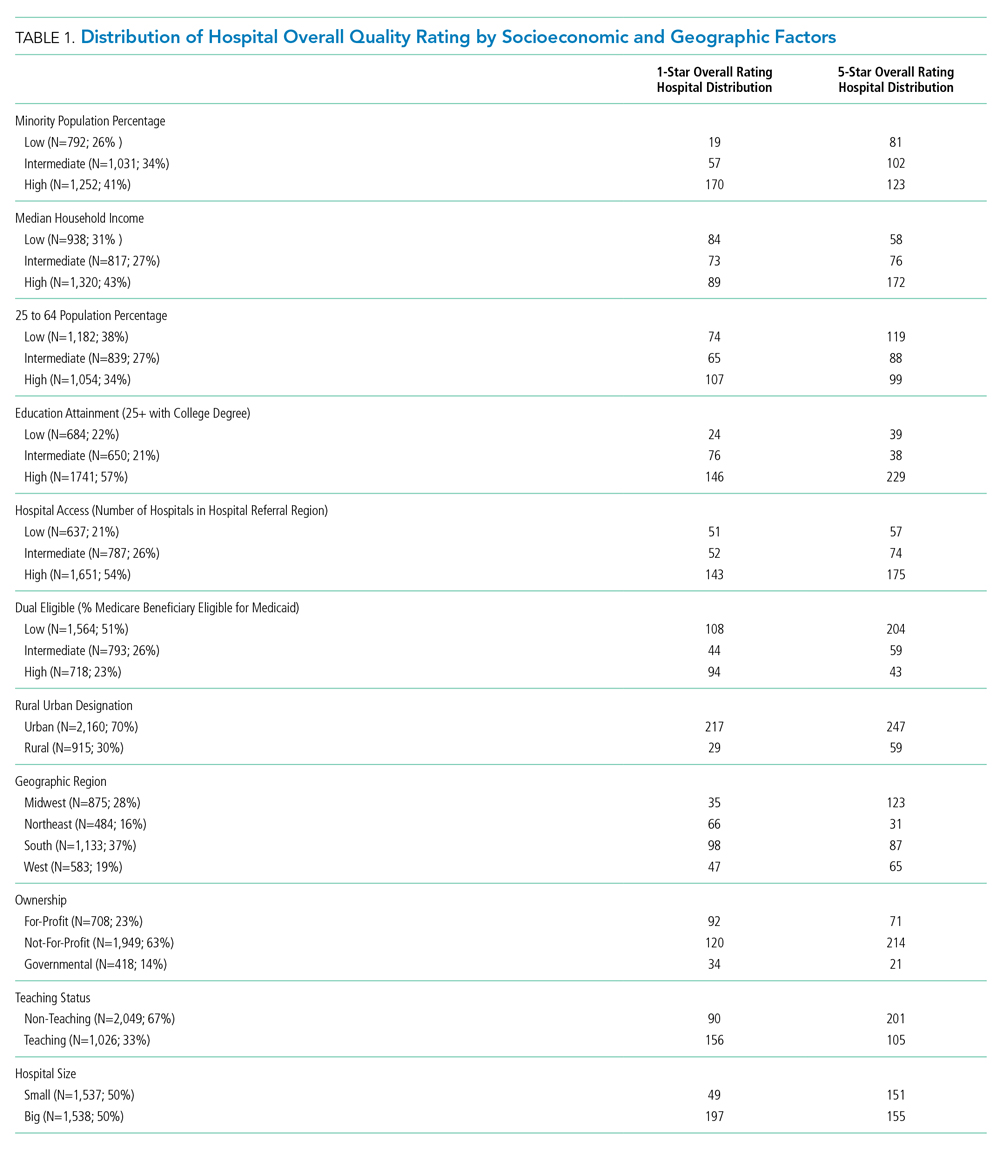
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.
To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.
RESULTS
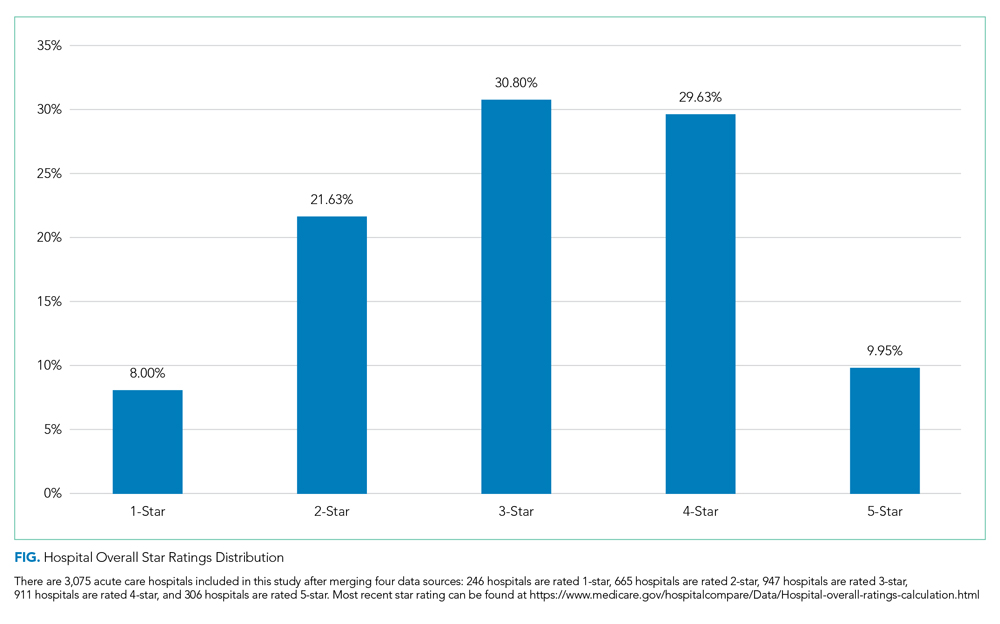
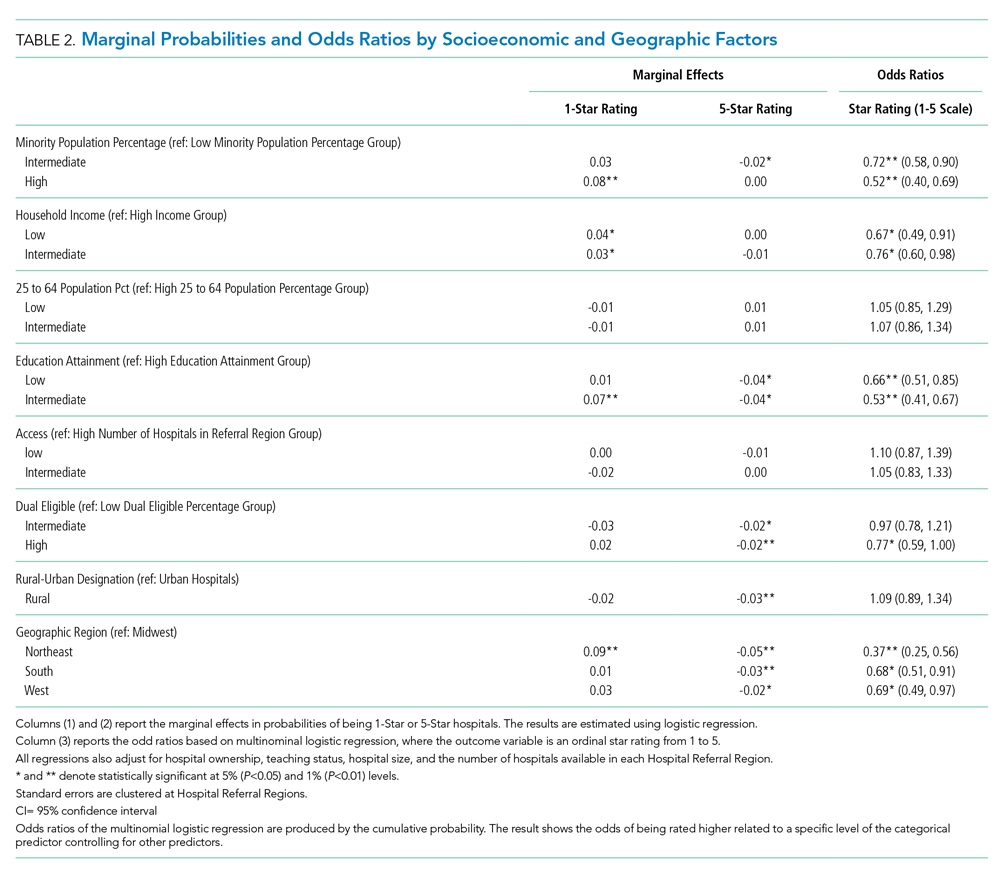
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.
Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.

To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.

RESULTS
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.

Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.

To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.

RESULTS
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.

Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
© 2020 Society of Hospital Medicine
COVID-19 Screening and Testing Among Patients With Neurologic Dysfunction: The Neuro-COVID-19 Time-out Process and Checklist
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
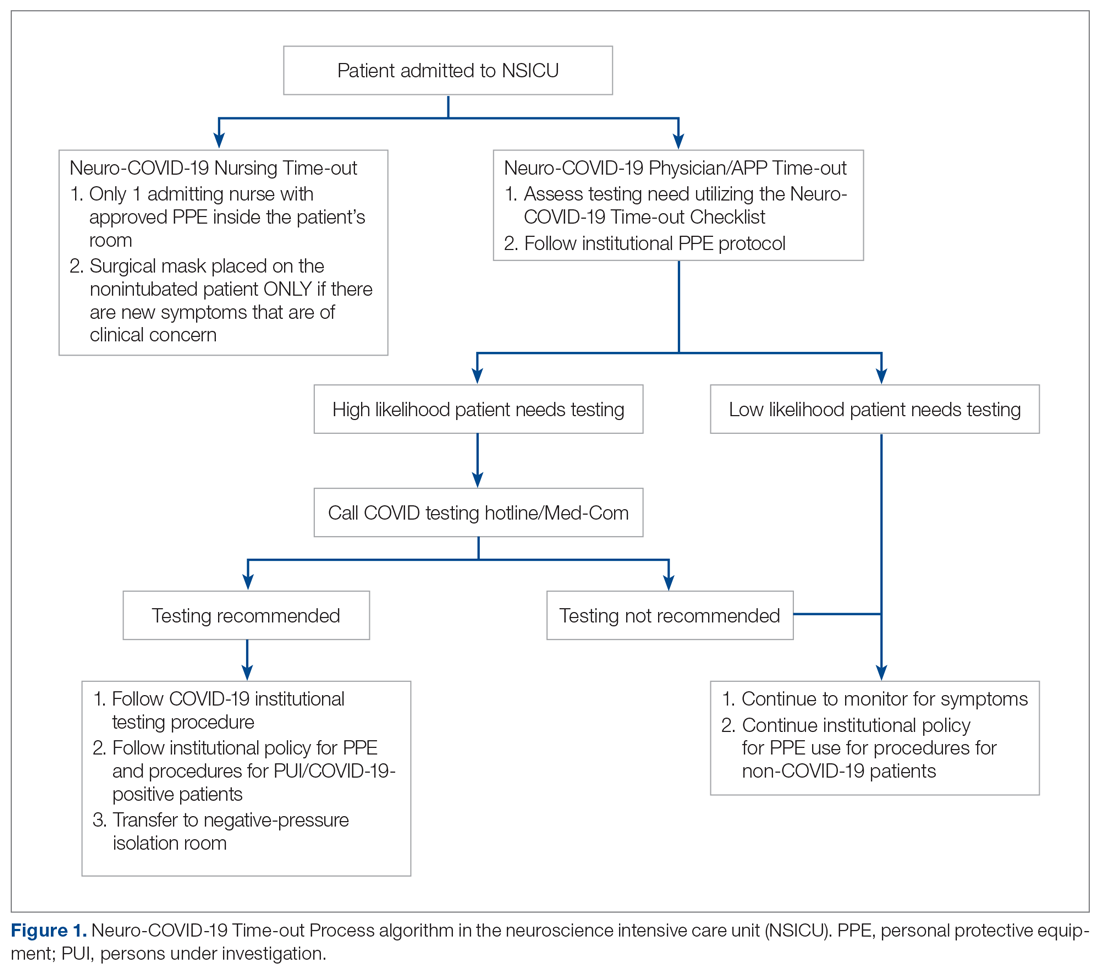
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
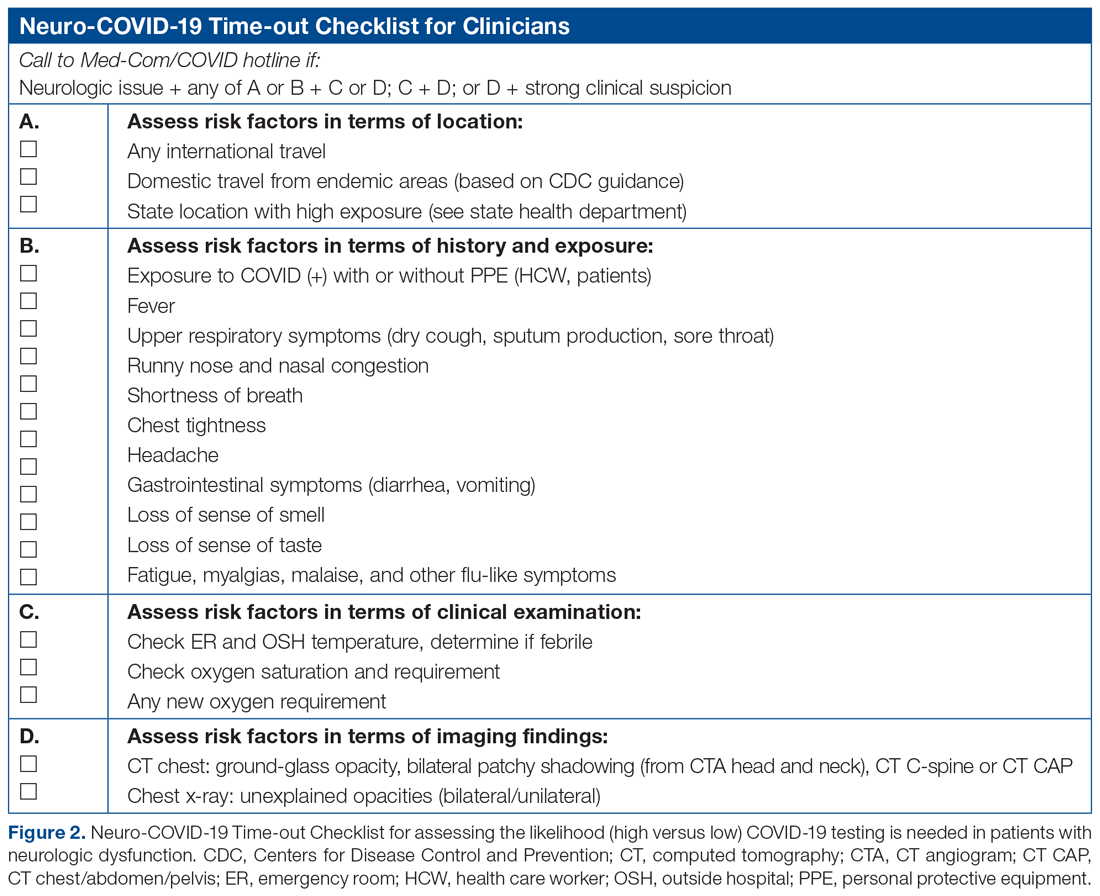
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
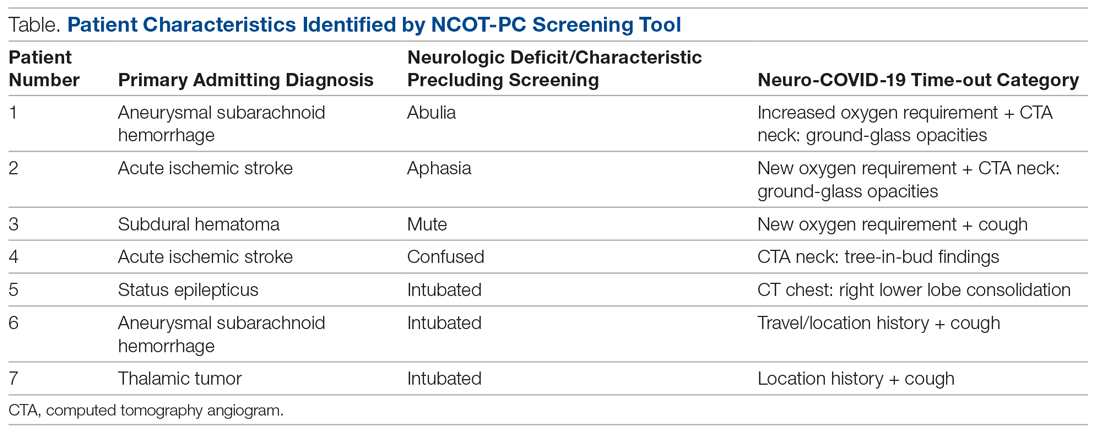
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
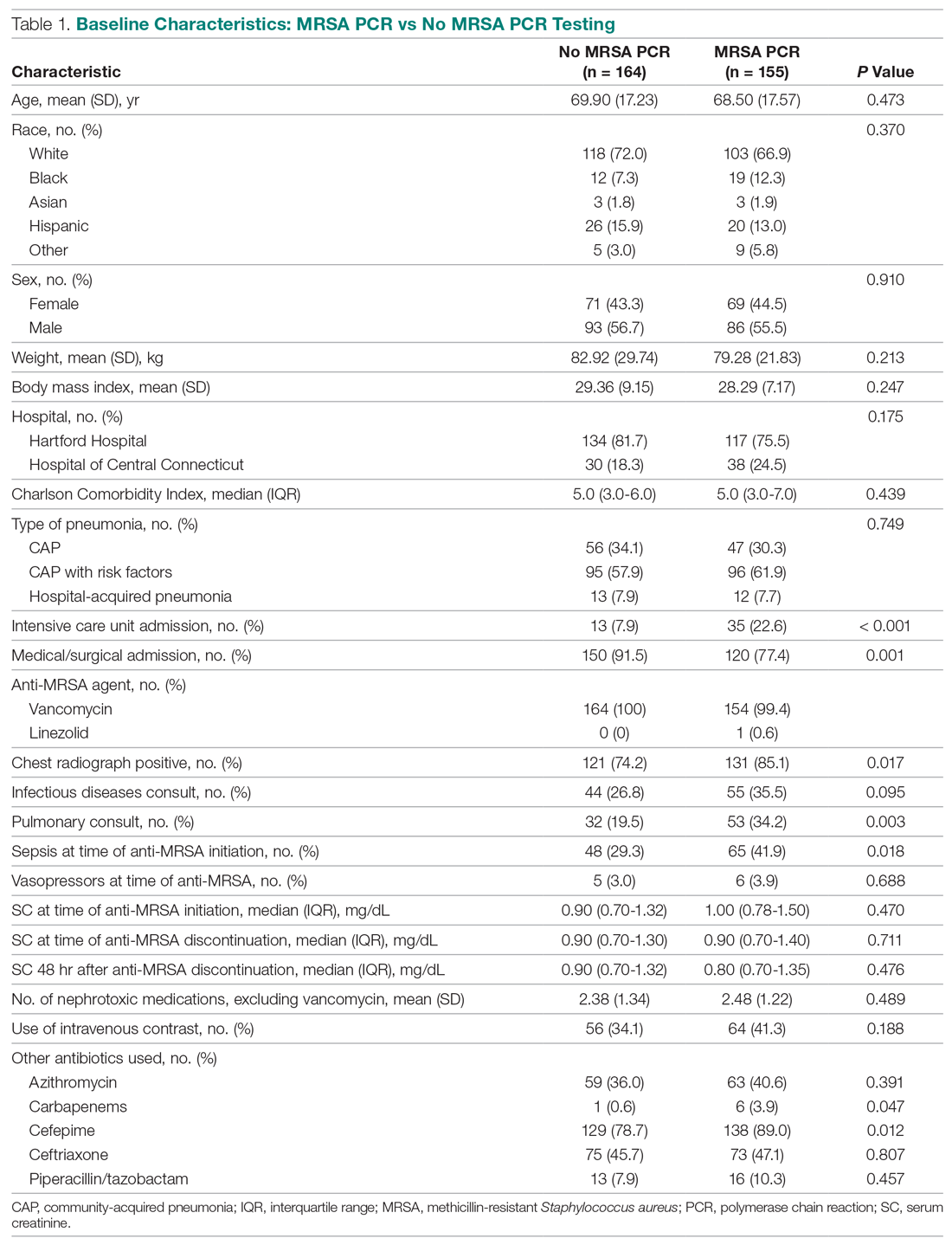
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
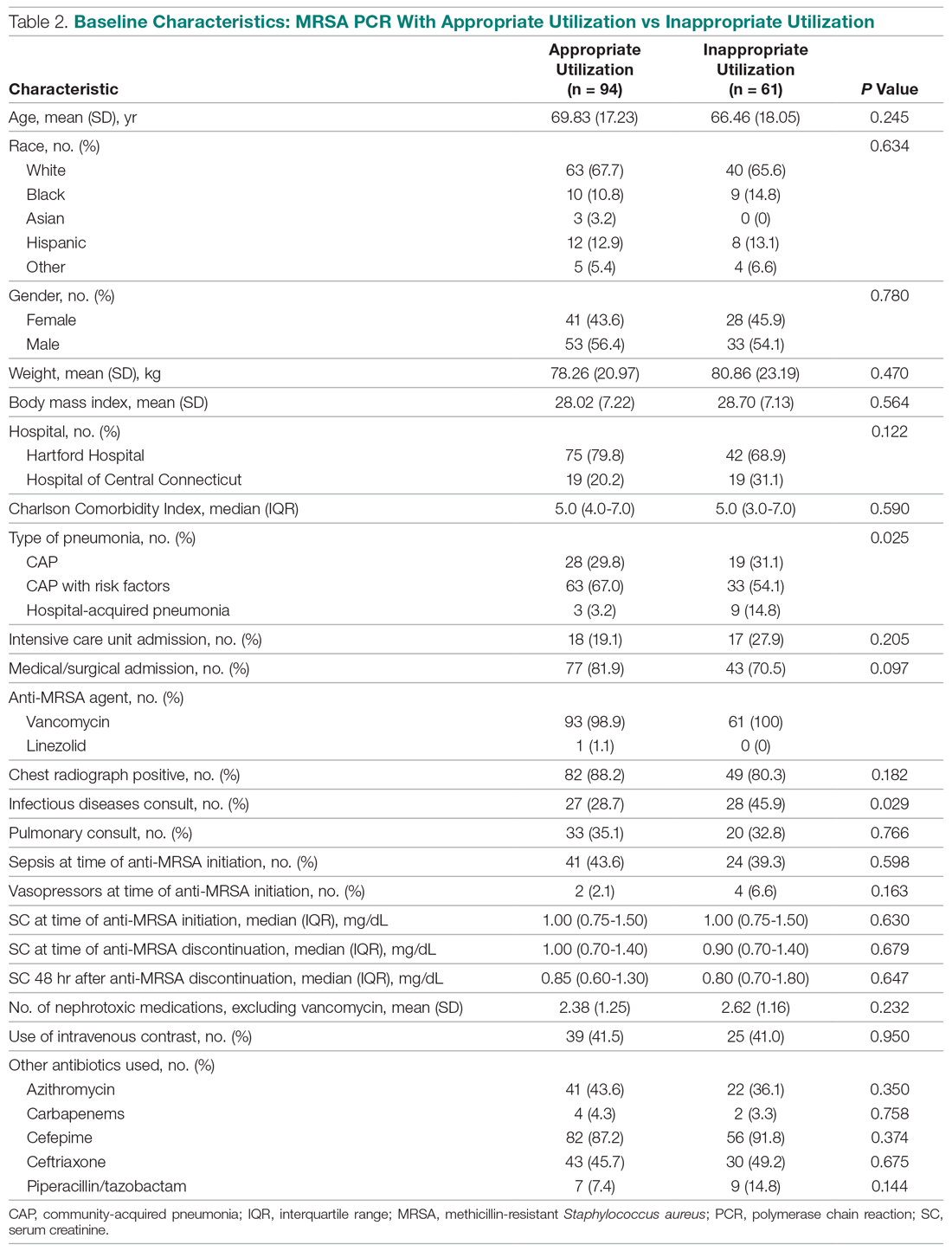
Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
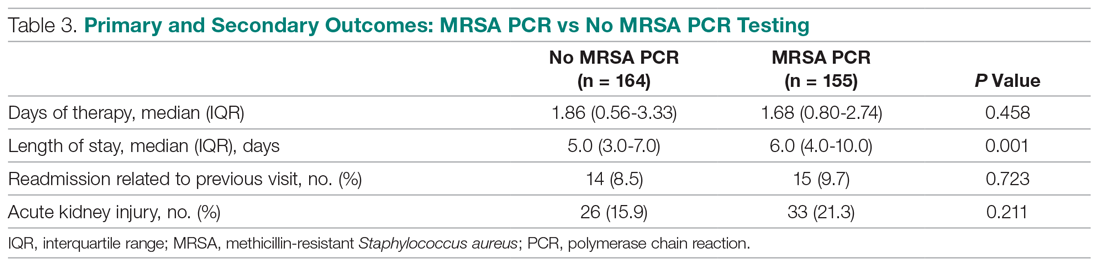
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
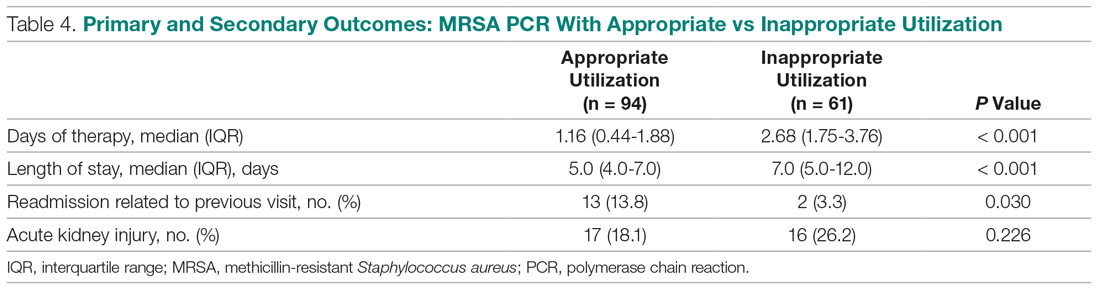
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
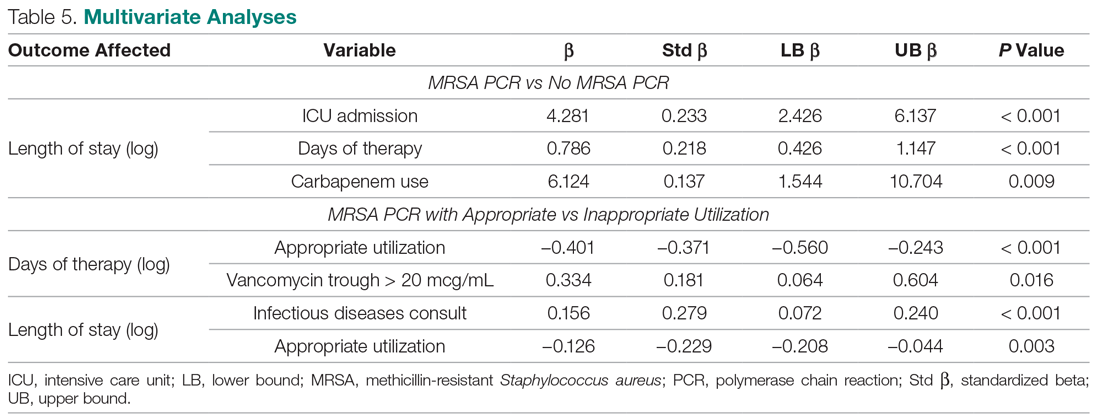
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
“I Really Didn’t Want To Come In”: The Unseen Effects of COVID-19 on Children
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
Systemic Corticosteroids in Critically Ill Patients With COVID-19
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Effect of a Smartphone App Plus an Accelerometer on Physical Activity and Functional Recovery During Hospitalization After Orthopedic Surgery
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
An Atypical Long-Term Thiamine Treatment Regimen for Wernicke Encephalopathy
Wernicke-Korsakoff syndrome is a cluster of symptoms attributed to a disorder of vitamin B1 (thiamine) deficiency, manifesting as a combined presentation of alcohol-induced Wernicke encephalopathy (WE) and Korsakoff syndrome (KS).1 While there is consensus on the characteristic presentation and symptoms of WE, there is a lack of agreement on the exact definition of KS. The classic triad describing WE consists of ataxia, ophthalmoplegia, and confusion; however, reports now suggest that a majority of patients exhibit only 1 or 2 of the elements of the triad. KS is often seen as a condition of chronic thiamine deficiency manifesting as memory impairment alongside a cognitive and behavioral decline, with no clear consensus on the sequence of appearance of symptoms. The typical relationship is thought to be a progression of WE to KS if untreated.
From a mental health perspective, WE presents with delirium and confusion whereas KS manifests with irreversible dementia and a cognitive deterioration. Though it is commonly taught that KS-induced memory loss is permanent due to neuronal damage (classically identified as damage to the mammillary bodies - though other structures have been implicated as well), more recent research suggest otherwise.2 A review published in 2018, for example, gathered several case reports and case series that suggest significant improvement in memory and cognition attributed to behavioral and pharmacologic interventions, indicating this as an area deserving of further study.3 About 20% of patients diagnosed with WE by autopsy exhibited none of the classical triad symptoms prior to death.4 Hence, these conditions are surmised to be significantly underdiagnosed and misdiagnosed.
Though consensus regarding the appropriate treatment regimen is lacking for WE, a common protocol consists of high-dose parenteral thiamine for 4 to 7 days.5 This is usually followed by daily oral thiamine repletion until the patient either achieves complete abstinence from alcohol (ideal) or decreases consumption. The goal is to allow thiamine stores to replete and maintain at minimum required body levels moving forward. In this case report, we highlight the utilization of a long-term, unconventional intramuscular (IM) thiamine repletion regimen to ensure maintenance of a patient’s mental status, highlighting discrepancies in our understanding of the mechanisms at play in WE and its treatment.
Case Presentation
A 65-year-old male patient with a more than 3-decade history of daily hard liquor intake, multiple psychiatric hospitalizations for WE, and a prior suicide attempt, presented to the emergency department (ED) with increased frequency of falls, poor oral intake, confabulation, and diminished verbal communication. A chart review revealed memory impairment alongside the diagnoses of schizoaffective disorder and WE, and confusion that was responsive to thiamine administration as well as a history of hypertension, hyperlipidemia, osteoarthritis, and urinary retention secondary to benign prostatic hyperplasia (BPH).
On examination the patient was found to be disoriented with a clouded sensorium. While the history of heavy daily alcohol use was clear in the chart and confirmed by other sources, it appeared unlikely that the patient had been using alcohol in the preceding month due to restricted access in his most recent living environment (a shared apartment with daily nursing assistance). He reported no lightheadedness, dizziness, palpitations, numbness, tingling, or any head trauma. He also negated the presence of active mood symptoms, auditory or visual hallucinations or suicidal ideation (SI)
The patient was admitted to the Internal Medicine Service and received a workup for the causes of delirium, including consideration of normal pressure hydrocephalus (NPH) and other neurologic conditions. Laboratory tests including a comprehensive metabolic panel, thyroid stimulating hormone, urinalysis, urine toxicology screen, and vitamin B12 and folate levels were in normal ranges. Although brain imaging revealed enlarged ventricles, NPH was considered unlikely because of the absence of ophthalmologic abnormalities, like gaze nystagmus, and urinary incontinence; conversely, there was some presence of urinary retention attributed to BPH and required an admission a few months prior. Moreover, magnetic resonance images showed that the ventricles were enlarged slightly out of proportion to the sulci, which can be seen with predominantly central volume loss compared with the pattern typically seen in NPH.
In light of concern for WE and the patient's history, treatment with IV thiamine and IV fluids was initiated and the Liaison Psychiatry Service was consulted for cognitive disability and treatment of his mood. Administration of IV thiamine rapidly restored his sensorium, but he became abruptly disorganized as the IV regimen graduated to an oral thiamine dose of 200 mg 3 times daily. Simultaneously, as medical stabilization was achieved, the patient was transferred to the inpatient psychiatry unit to address the nonresolving cognitive impairment and behavioral disorganization. This specifically involved newly emerging, impulsive, self-harming behaviors like throwing himself on the ground and banging his head on the floor. Such behaviors along with paucity of speech and decreased oral intake, ultimately warranted constant observation, which led to a decrease in self-harming activity. All this behavior was noted even though the patient was adherent to oral administration of thiamine. Throughout this time, the patient underwent several transfers back and forth between the Psychiatry and Internal Medicine services due to ongoing concern for the possibility of delirium or WE. However, the Neurology and Internal Medicine services did not feel that WE would explain the patient’s mental and behavioral status, in part due to his ongoing adherence with daily oral thiamine dosing that was not associated with improvement in mental status.
Recollecting the patient’s improvement with the parenteral thiamine regimen (IV and IM), the psychiatry unit tried a thiamine regimen of 200 mg IM and 100 mg oral 2 times daily. After about 2 weeks on this regimen, the patient subsequently achieved remarkable improvement in his cognitive and behavioral status, with resolution of selfharming behaviors. The patient was noted to be calmer, more linear, and more oriented, though he remained incompletely oriented throughout his hospitalization. As improvement in sensorium was established and the patient’s hospital stay prolonged (Figure), his mood symptoms began manifesting as guilt, low energy, decreased appetite, withdrawal, and passive SI. This was followed by a trial of lithium that was discontinued due to elevated creatine levels. As the patient continued to report depression, a multidrug regimen of divalproex, fluoxetine, and quetiapine was administered, which lead to remarkable improvement.
At this time, it was concluded that the stores of thiamine in the patient’s body may have been replenished, the alcohol intake completely ceased and that he needed to be weaned off of thiamine. The next step taken was reduction of the twice daily 200 mg IM thiamine dose to a once daily regimen, and oral thiamine was put on hold. Over the next 48 hours, the patient became less verbal, more withdrawn, incontinent of urine, and delirious. The twice daily IM 200 mg thiamine was restarted, but this time the patient demonstrated very slow improvement. After 2 weeks, the IM thiamine 200 mg was increased to 3 times daily, and the patient showed marked improvement in recall, mood, and effect.
Several attempts were made to reduce the IM thiamine burden on the patient and/ or transition to an exclusively oral regimen. However, he rapidly decompensated within hours of each attempt to taper the IM dose and required immediate reinstation. On the IM thiamine regimen, he eventually appeared to reach a stable cognitive and affective baseline marked by incomplete orientation but pleasant affect, he reported no mood complaints, behavioral stability, and an ability to comply with care needs and have simple conversations. Some speech content remained disorganized particularly if engaged beyond simple exchanges.
The patient was discharged to a skilled nursing facility after a month of 3 times daily IM administration of thiamine. Within the next 24 hours, the patient returned to the ED with the originally reported symptoms of ataxia, agitation, and confusion. On inquiry, it was revealed that the ordered vials of IM thiamine for injection had not arrived with him at the nursing facility and he had missed 2 doses. The blood laboratory results, scans, and all other parameters were otherwise found to be normal and the patient was adherent to his prescribed antipsychotics and antidepressants. As anticipated, restoration of the IM thiamine regimen revived his baseline within hours. While confusion and delirium resolved completely with treatment, the memory impairments persisted. This patient has been administered a 3 times daily IM dose of 200 mg thiamine for more than 2 years with a stable cognitive clinical picture.
Discussion
According to data from the 2016 National Survey on Drug Use and Health, 16 million individuals in the US aged ≥ 12 years reported heavy alcohol use, which is defined as binge drinking on ≥ 5 days in the past month.6,7 Thiamine deficiency is an alcoholrelated disorder that is frequently encountered in hospital settings. This deficiency can also occur in the context of malabsorption, malnutrition, a prolonged course of vomiting, and bariatric surgery.8,9
The deficiency in thiamine, which is sometimes known as WE, manifests rarely with all 3 of the classic triad of gait disturbances, abnormal eye movements, and mental status changes, with only 16.5% of patients displaying all of the triad.4 Moreover, there may be additional symptoms not listed in this triad, such as memory impairment, bilateral sixth nerve palsy, ptosis, hypotension, and hypothermia.10.11 This inconsistent presentation makes the diagnosis challenging and therefore requires a higher threshold for suspicion. If undiagnosed and/or untreated, WE can lead to chronic thiamine deficiency causing permanent brain damage in the guise of KS. This further increases the importance of timely diagnosis and treatment.
Our case highlights the utilization of an unconventional thiamine regimen that appeared to be temporally associated with mental status improvement. The patient’s clouded sensorium and confusion could not be attributed to metabolic, encephalopathic, or infectious pathologies due to the absence of supportive laboratory evidence. He responded to IV and IM doses of thiamine, but repeated attempts to taper the IM doses with the objective of transitioning to oral thiamine supplementation were followed by immediate decompensations in mental status. This was atypical of WE as the patient seemed adequately replete with thiamine, and missing a few doses should not be enough to deplete his stores. Thus, reflecting a unique case of thiamine-dependent chronically set WE when even a single missed dose of thiamine adversely affected the patient’s cognitive baseline. Interesting to note is this patient’s memory issue, as evident by clinical examination and dating back at least 5 years as per chart review. This premature amnestic component of his presentation indicates a likely parallel running KS component of his presentation. Conversely, the patient’s long history of alcohol use disorder, prior episodes of WE, and ideal response achieved only on parenteral thiamine repletion further supported the diagnosis of WE and our impression of the scenario.
Even though this patient had prior episodes of WE, there remained diagnostic uncertainty regarding his altered mental status for some time before the nonoral thiamine repletion treatment was implemented. Particularly in this admission, the patient’s mental status frequently waxed and waned and there was the additional confusion of whether a potential psychiatric etiology contributed to some of the elements of his presentation, such as his impulsive self-harm behaviors. This behavior led to recurrent transfers among the Psychiatry Service, Internal Medicine Service, and the ED.
The patient’s presentation did not reflect the classical triad of WE, and while this is consistent with the majority of clinical manifestations, various services were reluctant to attribute his symptoms to WE. Once the threshold of suspicion of thiamine deficiency was lowered and the deficit treated more aggressively, the patient seemed to improve tremendously. Presence of memory problems and confabulation, both of which this patient exhibited, are suggestive of KS and are not expected to recover with treatment, yet for this patient there did seem to be some improvement—though not complete resolution. This is consistent with newer evidence suggesting that some recovery from the deficits seen in KS is possible.3
Once diagnosed, the treatment objective is the replenishment of thiamine stores and optimization of the metabolic scenario of the body to prevent recurrence. For acute WE symptoms, many regimens call for 250 to 500 mg of IV thiamine supplementation 2 to 3 times daily for 3 to 5 days. High dose IV thiamine (≥ 500 mg daily) has been proposed to be efficacious and free of considerable adverse effects.12 A study conducted at the University of North Carolina described thiamine prescribing practices in a large academic hospital, analyzing data with the objective of assessing outcomes of ordering high-dose IV thiamine (HDIV, ≥ 200 mg IV twice daily) to patients with encephalopathy. 13 The researchers concluded that HDIV, even though rarely prescribed, was associated with decreased inpatient mortality in bivariable models. However, in multivariable analyses this decrease was found to be clinically insignificant. Our patient benefitted from both IV and IM delivery.
Ideally, after the initial IV thiamine dose, oral administration of thiamine 250 to 1,000 mg is continued until a reduction, if not abstinence, from alcohol use is achieved.5 Many patients are discharged on an oral maintenance dose of thiamine 100 mg. Oral thiamine is poorly absorbed and less effective in both prophylaxis and treatment of newly diagnosed WE; therefore, it is typically used only after IM or IV replenishment. It remains unclear why this patient required IM thiamine multiple times per day to maintain his mental status, and why he would present with selfinjurious behaviors after missing doses. The patient’s response can be attributed to late-onset defects in oral thiamine absorption at the carrier protein level of the brush border and basolateral membranes of his jejunum; however, an invasive procedure like a jejunal biopsy to establish the definitive etiology was neither necessary nor practical once treatment response was observed. 14 Other possible explanations include rapid thiamine metabolism, poor gastrointestinal absorption and a late-onset deficit in the thiamine diffusion mechanisms, and active transport systems (thiamine utilization depends on active transport in low availability states and passive transport when readily available). The nature of these mechanisms deserves further study. Less data have been reported on the administration and utility of IM thiamine for chronic WE; hence, our case report is one of the first illustrating the role of this method for sustained repletion.
Conclusions
This case presented a clinical dilemma because the conventional treatment regimen for WE didn’t yield the desired outcome until the mode and duration of thiamine administration were adjusted. It illustrates the utility of a sustained intensive thiamine regimen irrespective of sobriety status, as opposed to the traditional regimen of parenteral (primarily IV) thiamine for 3 to 7 days, followed by oral repletion until the patient achieves sustained abstinence. In this patient’s case, access to nursing care postdischarge facilitated his continued adherence to IM thiamine therapy.
The longitudinal time course of this case suggests a relationship between this route of administration and improvement in symptom burden and indicates that this patient may have a long-term need for IM thiamine to maintain his baseline mental status. Of great benefit in such patients would be the availability of a long-acting IM thiamine therapy. Risk of overdose is unlikely due to the water solubility of B group vitamins.
This case report highlights the importance of setting a high clinical suspicion for WE due to its ever-increasing incidence in these times. We also wish to direct researchers to consider other out-of-the-box treatment options in case of failure of the conventional regime. In documenting this patient report, we invite more medical providers to investigate and explore other therapeutic options for WE treatment with the aim of decreasing both morbidity and mortality secondary to the condition.
1. Lough ME. Wernicke’s encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012;22(2):181-194. doi:10.1007/s11065-012-9200-7
2. Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875- 2890. Published 2017 Nov 27. doi:10.2147/NDT.S130078
3. Johnson JM, Fox V. Beyond thiamine: treatment for cognitive impairment in Korsakoff’s syndrome. Psychosomatics. 2018;59(4):311-317. doi:10.1016/j.psym.2018.03.011
4. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-345. doi:10.1136/ jnnp.49.4.341
5. Xiong GL, Kenedl, CA. Wernicke-Korsakoff syndrome. https://emedicine.medscape.com/article/288379-overview. Updated May 16, 2018, Accessed July 24, 2020.
6. Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. Results from the 2016 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files /NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm. Accessed July 22, 2020.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. https://www.niaaa.nih.gov /alcohol-health/overview-alcohol-consumption/moderate -binge-drinking Accessed July 24, 2020.
8. Heye N, Terstegge K, Sirtl C, McMonagle U, Schreiber K, Meyer-Gessner M. Wernicke’s encephalopathy--causes to consider. Intensive Care Med. 1994;20(4):282-286. doi:10.1007/BF01708966
9. Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248(5):714-720. doi:10.1097/SLA.0b013e3181884308
10. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: FA Davis; 1989.
11. Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department [published correction appears in Alcohol Alcohol. 2003 May-Jun;38(3):291]. Alcohol Alcohol. 2002;37(6):513-521. doi:10.1093/alcalc/37.6.513
12. Nishimoto A, Usery J, Winton JC, Twilla J. High-dose parenteral thiamine in treatment of Wernicke’s encephalopathy: case series and review of the literature. In Vivo. 2017;31(1):121-124. doi:10.21873/invivo.11034
13. Nakamura ZM, Tatreau JR, Rosenstein DL, Park EM. Clinical characteristics and outcomes associated with highdose intravenous thiamine administration in patients with encephalopathy. Psychosomatics. 2018;59(4):379-387. doi:10.1016/j.psym.2018.01.004
14. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G23-G31. doi:10.1152/ajpgi.00132.2010
Wernicke-Korsakoff syndrome is a cluster of symptoms attributed to a disorder of vitamin B1 (thiamine) deficiency, manifesting as a combined presentation of alcohol-induced Wernicke encephalopathy (WE) and Korsakoff syndrome (KS).1 While there is consensus on the characteristic presentation and symptoms of WE, there is a lack of agreement on the exact definition of KS. The classic triad describing WE consists of ataxia, ophthalmoplegia, and confusion; however, reports now suggest that a majority of patients exhibit only 1 or 2 of the elements of the triad. KS is often seen as a condition of chronic thiamine deficiency manifesting as memory impairment alongside a cognitive and behavioral decline, with no clear consensus on the sequence of appearance of symptoms. The typical relationship is thought to be a progression of WE to KS if untreated.
From a mental health perspective, WE presents with delirium and confusion whereas KS manifests with irreversible dementia and a cognitive deterioration. Though it is commonly taught that KS-induced memory loss is permanent due to neuronal damage (classically identified as damage to the mammillary bodies - though other structures have been implicated as well), more recent research suggest otherwise.2 A review published in 2018, for example, gathered several case reports and case series that suggest significant improvement in memory and cognition attributed to behavioral and pharmacologic interventions, indicating this as an area deserving of further study.3 About 20% of patients diagnosed with WE by autopsy exhibited none of the classical triad symptoms prior to death.4 Hence, these conditions are surmised to be significantly underdiagnosed and misdiagnosed.
Though consensus regarding the appropriate treatment regimen is lacking for WE, a common protocol consists of high-dose parenteral thiamine for 4 to 7 days.5 This is usually followed by daily oral thiamine repletion until the patient either achieves complete abstinence from alcohol (ideal) or decreases consumption. The goal is to allow thiamine stores to replete and maintain at minimum required body levels moving forward. In this case report, we highlight the utilization of a long-term, unconventional intramuscular (IM) thiamine repletion regimen to ensure maintenance of a patient’s mental status, highlighting discrepancies in our understanding of the mechanisms at play in WE and its treatment.
Case Presentation
A 65-year-old male patient with a more than 3-decade history of daily hard liquor intake, multiple psychiatric hospitalizations for WE, and a prior suicide attempt, presented to the emergency department (ED) with increased frequency of falls, poor oral intake, confabulation, and diminished verbal communication. A chart review revealed memory impairment alongside the diagnoses of schizoaffective disorder and WE, and confusion that was responsive to thiamine administration as well as a history of hypertension, hyperlipidemia, osteoarthritis, and urinary retention secondary to benign prostatic hyperplasia (BPH).
On examination the patient was found to be disoriented with a clouded sensorium. While the history of heavy daily alcohol use was clear in the chart and confirmed by other sources, it appeared unlikely that the patient had been using alcohol in the preceding month due to restricted access in his most recent living environment (a shared apartment with daily nursing assistance). He reported no lightheadedness, dizziness, palpitations, numbness, tingling, or any head trauma. He also negated the presence of active mood symptoms, auditory or visual hallucinations or suicidal ideation (SI)
The patient was admitted to the Internal Medicine Service and received a workup for the causes of delirium, including consideration of normal pressure hydrocephalus (NPH) and other neurologic conditions. Laboratory tests including a comprehensive metabolic panel, thyroid stimulating hormone, urinalysis, urine toxicology screen, and vitamin B12 and folate levels were in normal ranges. Although brain imaging revealed enlarged ventricles, NPH was considered unlikely because of the absence of ophthalmologic abnormalities, like gaze nystagmus, and urinary incontinence; conversely, there was some presence of urinary retention attributed to BPH and required an admission a few months prior. Moreover, magnetic resonance images showed that the ventricles were enlarged slightly out of proportion to the sulci, which can be seen with predominantly central volume loss compared with the pattern typically seen in NPH.
In light of concern for WE and the patient's history, treatment with IV thiamine and IV fluids was initiated and the Liaison Psychiatry Service was consulted for cognitive disability and treatment of his mood. Administration of IV thiamine rapidly restored his sensorium, but he became abruptly disorganized as the IV regimen graduated to an oral thiamine dose of 200 mg 3 times daily. Simultaneously, as medical stabilization was achieved, the patient was transferred to the inpatient psychiatry unit to address the nonresolving cognitive impairment and behavioral disorganization. This specifically involved newly emerging, impulsive, self-harming behaviors like throwing himself on the ground and banging his head on the floor. Such behaviors along with paucity of speech and decreased oral intake, ultimately warranted constant observation, which led to a decrease in self-harming activity. All this behavior was noted even though the patient was adherent to oral administration of thiamine. Throughout this time, the patient underwent several transfers back and forth between the Psychiatry and Internal Medicine services due to ongoing concern for the possibility of delirium or WE. However, the Neurology and Internal Medicine services did not feel that WE would explain the patient’s mental and behavioral status, in part due to his ongoing adherence with daily oral thiamine dosing that was not associated with improvement in mental status.
Recollecting the patient’s improvement with the parenteral thiamine regimen (IV and IM), the psychiatry unit tried a thiamine regimen of 200 mg IM and 100 mg oral 2 times daily. After about 2 weeks on this regimen, the patient subsequently achieved remarkable improvement in his cognitive and behavioral status, with resolution of selfharming behaviors. The patient was noted to be calmer, more linear, and more oriented, though he remained incompletely oriented throughout his hospitalization. As improvement in sensorium was established and the patient’s hospital stay prolonged (Figure), his mood symptoms began manifesting as guilt, low energy, decreased appetite, withdrawal, and passive SI. This was followed by a trial of lithium that was discontinued due to elevated creatine levels. As the patient continued to report depression, a multidrug regimen of divalproex, fluoxetine, and quetiapine was administered, which lead to remarkable improvement.
At this time, it was concluded that the stores of thiamine in the patient’s body may have been replenished, the alcohol intake completely ceased and that he needed to be weaned off of thiamine. The next step taken was reduction of the twice daily 200 mg IM thiamine dose to a once daily regimen, and oral thiamine was put on hold. Over the next 48 hours, the patient became less verbal, more withdrawn, incontinent of urine, and delirious. The twice daily IM 200 mg thiamine was restarted, but this time the patient demonstrated very slow improvement. After 2 weeks, the IM thiamine 200 mg was increased to 3 times daily, and the patient showed marked improvement in recall, mood, and effect.
Several attempts were made to reduce the IM thiamine burden on the patient and/ or transition to an exclusively oral regimen. However, he rapidly decompensated within hours of each attempt to taper the IM dose and required immediate reinstation. On the IM thiamine regimen, he eventually appeared to reach a stable cognitive and affective baseline marked by incomplete orientation but pleasant affect, he reported no mood complaints, behavioral stability, and an ability to comply with care needs and have simple conversations. Some speech content remained disorganized particularly if engaged beyond simple exchanges.
The patient was discharged to a skilled nursing facility after a month of 3 times daily IM administration of thiamine. Within the next 24 hours, the patient returned to the ED with the originally reported symptoms of ataxia, agitation, and confusion. On inquiry, it was revealed that the ordered vials of IM thiamine for injection had not arrived with him at the nursing facility and he had missed 2 doses. The blood laboratory results, scans, and all other parameters were otherwise found to be normal and the patient was adherent to his prescribed antipsychotics and antidepressants. As anticipated, restoration of the IM thiamine regimen revived his baseline within hours. While confusion and delirium resolved completely with treatment, the memory impairments persisted. This patient has been administered a 3 times daily IM dose of 200 mg thiamine for more than 2 years with a stable cognitive clinical picture.
Discussion
According to data from the 2016 National Survey on Drug Use and Health, 16 million individuals in the US aged ≥ 12 years reported heavy alcohol use, which is defined as binge drinking on ≥ 5 days in the past month.6,7 Thiamine deficiency is an alcoholrelated disorder that is frequently encountered in hospital settings. This deficiency can also occur in the context of malabsorption, malnutrition, a prolonged course of vomiting, and bariatric surgery.8,9
The deficiency in thiamine, which is sometimes known as WE, manifests rarely with all 3 of the classic triad of gait disturbances, abnormal eye movements, and mental status changes, with only 16.5% of patients displaying all of the triad.4 Moreover, there may be additional symptoms not listed in this triad, such as memory impairment, bilateral sixth nerve palsy, ptosis, hypotension, and hypothermia.10.11 This inconsistent presentation makes the diagnosis challenging and therefore requires a higher threshold for suspicion. If undiagnosed and/or untreated, WE can lead to chronic thiamine deficiency causing permanent brain damage in the guise of KS. This further increases the importance of timely diagnosis and treatment.
Our case highlights the utilization of an unconventional thiamine regimen that appeared to be temporally associated with mental status improvement. The patient’s clouded sensorium and confusion could not be attributed to metabolic, encephalopathic, or infectious pathologies due to the absence of supportive laboratory evidence. He responded to IV and IM doses of thiamine, but repeated attempts to taper the IM doses with the objective of transitioning to oral thiamine supplementation were followed by immediate decompensations in mental status. This was atypical of WE as the patient seemed adequately replete with thiamine, and missing a few doses should not be enough to deplete his stores. Thus, reflecting a unique case of thiamine-dependent chronically set WE when even a single missed dose of thiamine adversely affected the patient’s cognitive baseline. Interesting to note is this patient’s memory issue, as evident by clinical examination and dating back at least 5 years as per chart review. This premature amnestic component of his presentation indicates a likely parallel running KS component of his presentation. Conversely, the patient’s long history of alcohol use disorder, prior episodes of WE, and ideal response achieved only on parenteral thiamine repletion further supported the diagnosis of WE and our impression of the scenario.
Even though this patient had prior episodes of WE, there remained diagnostic uncertainty regarding his altered mental status for some time before the nonoral thiamine repletion treatment was implemented. Particularly in this admission, the patient’s mental status frequently waxed and waned and there was the additional confusion of whether a potential psychiatric etiology contributed to some of the elements of his presentation, such as his impulsive self-harm behaviors. This behavior led to recurrent transfers among the Psychiatry Service, Internal Medicine Service, and the ED.
The patient’s presentation did not reflect the classical triad of WE, and while this is consistent with the majority of clinical manifestations, various services were reluctant to attribute his symptoms to WE. Once the threshold of suspicion of thiamine deficiency was lowered and the deficit treated more aggressively, the patient seemed to improve tremendously. Presence of memory problems and confabulation, both of which this patient exhibited, are suggestive of KS and are not expected to recover with treatment, yet for this patient there did seem to be some improvement—though not complete resolution. This is consistent with newer evidence suggesting that some recovery from the deficits seen in KS is possible.3
Once diagnosed, the treatment objective is the replenishment of thiamine stores and optimization of the metabolic scenario of the body to prevent recurrence. For acute WE symptoms, many regimens call for 250 to 500 mg of IV thiamine supplementation 2 to 3 times daily for 3 to 5 days. High dose IV thiamine (≥ 500 mg daily) has been proposed to be efficacious and free of considerable adverse effects.12 A study conducted at the University of North Carolina described thiamine prescribing practices in a large academic hospital, analyzing data with the objective of assessing outcomes of ordering high-dose IV thiamine (HDIV, ≥ 200 mg IV twice daily) to patients with encephalopathy. 13 The researchers concluded that HDIV, even though rarely prescribed, was associated with decreased inpatient mortality in bivariable models. However, in multivariable analyses this decrease was found to be clinically insignificant. Our patient benefitted from both IV and IM delivery.
Ideally, after the initial IV thiamine dose, oral administration of thiamine 250 to 1,000 mg is continued until a reduction, if not abstinence, from alcohol use is achieved.5 Many patients are discharged on an oral maintenance dose of thiamine 100 mg. Oral thiamine is poorly absorbed and less effective in both prophylaxis and treatment of newly diagnosed WE; therefore, it is typically used only after IM or IV replenishment. It remains unclear why this patient required IM thiamine multiple times per day to maintain his mental status, and why he would present with selfinjurious behaviors after missing doses. The patient’s response can be attributed to late-onset defects in oral thiamine absorption at the carrier protein level of the brush border and basolateral membranes of his jejunum; however, an invasive procedure like a jejunal biopsy to establish the definitive etiology was neither necessary nor practical once treatment response was observed. 14 Other possible explanations include rapid thiamine metabolism, poor gastrointestinal absorption and a late-onset deficit in the thiamine diffusion mechanisms, and active transport systems (thiamine utilization depends on active transport in low availability states and passive transport when readily available). The nature of these mechanisms deserves further study. Less data have been reported on the administration and utility of IM thiamine for chronic WE; hence, our case report is one of the first illustrating the role of this method for sustained repletion.
Conclusions
This case presented a clinical dilemma because the conventional treatment regimen for WE didn’t yield the desired outcome until the mode and duration of thiamine administration were adjusted. It illustrates the utility of a sustained intensive thiamine regimen irrespective of sobriety status, as opposed to the traditional regimen of parenteral (primarily IV) thiamine for 3 to 7 days, followed by oral repletion until the patient achieves sustained abstinence. In this patient’s case, access to nursing care postdischarge facilitated his continued adherence to IM thiamine therapy.
The longitudinal time course of this case suggests a relationship between this route of administration and improvement in symptom burden and indicates that this patient may have a long-term need for IM thiamine to maintain his baseline mental status. Of great benefit in such patients would be the availability of a long-acting IM thiamine therapy. Risk of overdose is unlikely due to the water solubility of B group vitamins.
This case report highlights the importance of setting a high clinical suspicion for WE due to its ever-increasing incidence in these times. We also wish to direct researchers to consider other out-of-the-box treatment options in case of failure of the conventional regime. In documenting this patient report, we invite more medical providers to investigate and explore other therapeutic options for WE treatment with the aim of decreasing both morbidity and mortality secondary to the condition.
Wernicke-Korsakoff syndrome is a cluster of symptoms attributed to a disorder of vitamin B1 (thiamine) deficiency, manifesting as a combined presentation of alcohol-induced Wernicke encephalopathy (WE) and Korsakoff syndrome (KS).1 While there is consensus on the characteristic presentation and symptoms of WE, there is a lack of agreement on the exact definition of KS. The classic triad describing WE consists of ataxia, ophthalmoplegia, and confusion; however, reports now suggest that a majority of patients exhibit only 1 or 2 of the elements of the triad. KS is often seen as a condition of chronic thiamine deficiency manifesting as memory impairment alongside a cognitive and behavioral decline, with no clear consensus on the sequence of appearance of symptoms. The typical relationship is thought to be a progression of WE to KS if untreated.
From a mental health perspective, WE presents with delirium and confusion whereas KS manifests with irreversible dementia and a cognitive deterioration. Though it is commonly taught that KS-induced memory loss is permanent due to neuronal damage (classically identified as damage to the mammillary bodies - though other structures have been implicated as well), more recent research suggest otherwise.2 A review published in 2018, for example, gathered several case reports and case series that suggest significant improvement in memory and cognition attributed to behavioral and pharmacologic interventions, indicating this as an area deserving of further study.3 About 20% of patients diagnosed with WE by autopsy exhibited none of the classical triad symptoms prior to death.4 Hence, these conditions are surmised to be significantly underdiagnosed and misdiagnosed.
Though consensus regarding the appropriate treatment regimen is lacking for WE, a common protocol consists of high-dose parenteral thiamine for 4 to 7 days.5 This is usually followed by daily oral thiamine repletion until the patient either achieves complete abstinence from alcohol (ideal) or decreases consumption. The goal is to allow thiamine stores to replete and maintain at minimum required body levels moving forward. In this case report, we highlight the utilization of a long-term, unconventional intramuscular (IM) thiamine repletion regimen to ensure maintenance of a patient’s mental status, highlighting discrepancies in our understanding of the mechanisms at play in WE and its treatment.
Case Presentation
A 65-year-old male patient with a more than 3-decade history of daily hard liquor intake, multiple psychiatric hospitalizations for WE, and a prior suicide attempt, presented to the emergency department (ED) with increased frequency of falls, poor oral intake, confabulation, and diminished verbal communication. A chart review revealed memory impairment alongside the diagnoses of schizoaffective disorder and WE, and confusion that was responsive to thiamine administration as well as a history of hypertension, hyperlipidemia, osteoarthritis, and urinary retention secondary to benign prostatic hyperplasia (BPH).
On examination the patient was found to be disoriented with a clouded sensorium. While the history of heavy daily alcohol use was clear in the chart and confirmed by other sources, it appeared unlikely that the patient had been using alcohol in the preceding month due to restricted access in his most recent living environment (a shared apartment with daily nursing assistance). He reported no lightheadedness, dizziness, palpitations, numbness, tingling, or any head trauma. He also negated the presence of active mood symptoms, auditory or visual hallucinations or suicidal ideation (SI)
The patient was admitted to the Internal Medicine Service and received a workup for the causes of delirium, including consideration of normal pressure hydrocephalus (NPH) and other neurologic conditions. Laboratory tests including a comprehensive metabolic panel, thyroid stimulating hormone, urinalysis, urine toxicology screen, and vitamin B12 and folate levels were in normal ranges. Although brain imaging revealed enlarged ventricles, NPH was considered unlikely because of the absence of ophthalmologic abnormalities, like gaze nystagmus, and urinary incontinence; conversely, there was some presence of urinary retention attributed to BPH and required an admission a few months prior. Moreover, magnetic resonance images showed that the ventricles were enlarged slightly out of proportion to the sulci, which can be seen with predominantly central volume loss compared with the pattern typically seen in NPH.
In light of concern for WE and the patient's history, treatment with IV thiamine and IV fluids was initiated and the Liaison Psychiatry Service was consulted for cognitive disability and treatment of his mood. Administration of IV thiamine rapidly restored his sensorium, but he became abruptly disorganized as the IV regimen graduated to an oral thiamine dose of 200 mg 3 times daily. Simultaneously, as medical stabilization was achieved, the patient was transferred to the inpatient psychiatry unit to address the nonresolving cognitive impairment and behavioral disorganization. This specifically involved newly emerging, impulsive, self-harming behaviors like throwing himself on the ground and banging his head on the floor. Such behaviors along with paucity of speech and decreased oral intake, ultimately warranted constant observation, which led to a decrease in self-harming activity. All this behavior was noted even though the patient was adherent to oral administration of thiamine. Throughout this time, the patient underwent several transfers back and forth between the Psychiatry and Internal Medicine services due to ongoing concern for the possibility of delirium or WE. However, the Neurology and Internal Medicine services did not feel that WE would explain the patient’s mental and behavioral status, in part due to his ongoing adherence with daily oral thiamine dosing that was not associated with improvement in mental status.
Recollecting the patient’s improvement with the parenteral thiamine regimen (IV and IM), the psychiatry unit tried a thiamine regimen of 200 mg IM and 100 mg oral 2 times daily. After about 2 weeks on this regimen, the patient subsequently achieved remarkable improvement in his cognitive and behavioral status, with resolution of selfharming behaviors. The patient was noted to be calmer, more linear, and more oriented, though he remained incompletely oriented throughout his hospitalization. As improvement in sensorium was established and the patient’s hospital stay prolonged (Figure), his mood symptoms began manifesting as guilt, low energy, decreased appetite, withdrawal, and passive SI. This was followed by a trial of lithium that was discontinued due to elevated creatine levels. As the patient continued to report depression, a multidrug regimen of divalproex, fluoxetine, and quetiapine was administered, which lead to remarkable improvement.
At this time, it was concluded that the stores of thiamine in the patient’s body may have been replenished, the alcohol intake completely ceased and that he needed to be weaned off of thiamine. The next step taken was reduction of the twice daily 200 mg IM thiamine dose to a once daily regimen, and oral thiamine was put on hold. Over the next 48 hours, the patient became less verbal, more withdrawn, incontinent of urine, and delirious. The twice daily IM 200 mg thiamine was restarted, but this time the patient demonstrated very slow improvement. After 2 weeks, the IM thiamine 200 mg was increased to 3 times daily, and the patient showed marked improvement in recall, mood, and effect.
Several attempts were made to reduce the IM thiamine burden on the patient and/ or transition to an exclusively oral regimen. However, he rapidly decompensated within hours of each attempt to taper the IM dose and required immediate reinstation. On the IM thiamine regimen, he eventually appeared to reach a stable cognitive and affective baseline marked by incomplete orientation but pleasant affect, he reported no mood complaints, behavioral stability, and an ability to comply with care needs and have simple conversations. Some speech content remained disorganized particularly if engaged beyond simple exchanges.
The patient was discharged to a skilled nursing facility after a month of 3 times daily IM administration of thiamine. Within the next 24 hours, the patient returned to the ED with the originally reported symptoms of ataxia, agitation, and confusion. On inquiry, it was revealed that the ordered vials of IM thiamine for injection had not arrived with him at the nursing facility and he had missed 2 doses. The blood laboratory results, scans, and all other parameters were otherwise found to be normal and the patient was adherent to his prescribed antipsychotics and antidepressants. As anticipated, restoration of the IM thiamine regimen revived his baseline within hours. While confusion and delirium resolved completely with treatment, the memory impairments persisted. This patient has been administered a 3 times daily IM dose of 200 mg thiamine for more than 2 years with a stable cognitive clinical picture.
Discussion
According to data from the 2016 National Survey on Drug Use and Health, 16 million individuals in the US aged ≥ 12 years reported heavy alcohol use, which is defined as binge drinking on ≥ 5 days in the past month.6,7 Thiamine deficiency is an alcoholrelated disorder that is frequently encountered in hospital settings. This deficiency can also occur in the context of malabsorption, malnutrition, a prolonged course of vomiting, and bariatric surgery.8,9
The deficiency in thiamine, which is sometimes known as WE, manifests rarely with all 3 of the classic triad of gait disturbances, abnormal eye movements, and mental status changes, with only 16.5% of patients displaying all of the triad.4 Moreover, there may be additional symptoms not listed in this triad, such as memory impairment, bilateral sixth nerve palsy, ptosis, hypotension, and hypothermia.10.11 This inconsistent presentation makes the diagnosis challenging and therefore requires a higher threshold for suspicion. If undiagnosed and/or untreated, WE can lead to chronic thiamine deficiency causing permanent brain damage in the guise of KS. This further increases the importance of timely diagnosis and treatment.
Our case highlights the utilization of an unconventional thiamine regimen that appeared to be temporally associated with mental status improvement. The patient’s clouded sensorium and confusion could not be attributed to metabolic, encephalopathic, or infectious pathologies due to the absence of supportive laboratory evidence. He responded to IV and IM doses of thiamine, but repeated attempts to taper the IM doses with the objective of transitioning to oral thiamine supplementation were followed by immediate decompensations in mental status. This was atypical of WE as the patient seemed adequately replete with thiamine, and missing a few doses should not be enough to deplete his stores. Thus, reflecting a unique case of thiamine-dependent chronically set WE when even a single missed dose of thiamine adversely affected the patient’s cognitive baseline. Interesting to note is this patient’s memory issue, as evident by clinical examination and dating back at least 5 years as per chart review. This premature amnestic component of his presentation indicates a likely parallel running KS component of his presentation. Conversely, the patient’s long history of alcohol use disorder, prior episodes of WE, and ideal response achieved only on parenteral thiamine repletion further supported the diagnosis of WE and our impression of the scenario.
Even though this patient had prior episodes of WE, there remained diagnostic uncertainty regarding his altered mental status for some time before the nonoral thiamine repletion treatment was implemented. Particularly in this admission, the patient’s mental status frequently waxed and waned and there was the additional confusion of whether a potential psychiatric etiology contributed to some of the elements of his presentation, such as his impulsive self-harm behaviors. This behavior led to recurrent transfers among the Psychiatry Service, Internal Medicine Service, and the ED.
The patient’s presentation did not reflect the classical triad of WE, and while this is consistent with the majority of clinical manifestations, various services were reluctant to attribute his symptoms to WE. Once the threshold of suspicion of thiamine deficiency was lowered and the deficit treated more aggressively, the patient seemed to improve tremendously. Presence of memory problems and confabulation, both of which this patient exhibited, are suggestive of KS and are not expected to recover with treatment, yet for this patient there did seem to be some improvement—though not complete resolution. This is consistent with newer evidence suggesting that some recovery from the deficits seen in KS is possible.3
Once diagnosed, the treatment objective is the replenishment of thiamine stores and optimization of the metabolic scenario of the body to prevent recurrence. For acute WE symptoms, many regimens call for 250 to 500 mg of IV thiamine supplementation 2 to 3 times daily for 3 to 5 days. High dose IV thiamine (≥ 500 mg daily) has been proposed to be efficacious and free of considerable adverse effects.12 A study conducted at the University of North Carolina described thiamine prescribing practices in a large academic hospital, analyzing data with the objective of assessing outcomes of ordering high-dose IV thiamine (HDIV, ≥ 200 mg IV twice daily) to patients with encephalopathy. 13 The researchers concluded that HDIV, even though rarely prescribed, was associated with decreased inpatient mortality in bivariable models. However, in multivariable analyses this decrease was found to be clinically insignificant. Our patient benefitted from both IV and IM delivery.
Ideally, after the initial IV thiamine dose, oral administration of thiamine 250 to 1,000 mg is continued until a reduction, if not abstinence, from alcohol use is achieved.5 Many patients are discharged on an oral maintenance dose of thiamine 100 mg. Oral thiamine is poorly absorbed and less effective in both prophylaxis and treatment of newly diagnosed WE; therefore, it is typically used only after IM or IV replenishment. It remains unclear why this patient required IM thiamine multiple times per day to maintain his mental status, and why he would present with selfinjurious behaviors after missing doses. The patient’s response can be attributed to late-onset defects in oral thiamine absorption at the carrier protein level of the brush border and basolateral membranes of his jejunum; however, an invasive procedure like a jejunal biopsy to establish the definitive etiology was neither necessary nor practical once treatment response was observed. 14 Other possible explanations include rapid thiamine metabolism, poor gastrointestinal absorption and a late-onset deficit in the thiamine diffusion mechanisms, and active transport systems (thiamine utilization depends on active transport in low availability states and passive transport when readily available). The nature of these mechanisms deserves further study. Less data have been reported on the administration and utility of IM thiamine for chronic WE; hence, our case report is one of the first illustrating the role of this method for sustained repletion.
Conclusions
This case presented a clinical dilemma because the conventional treatment regimen for WE didn’t yield the desired outcome until the mode and duration of thiamine administration were adjusted. It illustrates the utility of a sustained intensive thiamine regimen irrespective of sobriety status, as opposed to the traditional regimen of parenteral (primarily IV) thiamine for 3 to 7 days, followed by oral repletion until the patient achieves sustained abstinence. In this patient’s case, access to nursing care postdischarge facilitated his continued adherence to IM thiamine therapy.
The longitudinal time course of this case suggests a relationship between this route of administration and improvement in symptom burden and indicates that this patient may have a long-term need for IM thiamine to maintain his baseline mental status. Of great benefit in such patients would be the availability of a long-acting IM thiamine therapy. Risk of overdose is unlikely due to the water solubility of B group vitamins.
This case report highlights the importance of setting a high clinical suspicion for WE due to its ever-increasing incidence in these times. We also wish to direct researchers to consider other out-of-the-box treatment options in case of failure of the conventional regime. In documenting this patient report, we invite more medical providers to investigate and explore other therapeutic options for WE treatment with the aim of decreasing both morbidity and mortality secondary to the condition.
1. Lough ME. Wernicke’s encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012;22(2):181-194. doi:10.1007/s11065-012-9200-7
2. Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875- 2890. Published 2017 Nov 27. doi:10.2147/NDT.S130078
3. Johnson JM, Fox V. Beyond thiamine: treatment for cognitive impairment in Korsakoff’s syndrome. Psychosomatics. 2018;59(4):311-317. doi:10.1016/j.psym.2018.03.011
4. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-345. doi:10.1136/ jnnp.49.4.341
5. Xiong GL, Kenedl, CA. Wernicke-Korsakoff syndrome. https://emedicine.medscape.com/article/288379-overview. Updated May 16, 2018, Accessed July 24, 2020.
6. Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. Results from the 2016 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files /NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm. Accessed July 22, 2020.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. https://www.niaaa.nih.gov /alcohol-health/overview-alcohol-consumption/moderate -binge-drinking Accessed July 24, 2020.
8. Heye N, Terstegge K, Sirtl C, McMonagle U, Schreiber K, Meyer-Gessner M. Wernicke’s encephalopathy--causes to consider. Intensive Care Med. 1994;20(4):282-286. doi:10.1007/BF01708966
9. Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248(5):714-720. doi:10.1097/SLA.0b013e3181884308
10. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: FA Davis; 1989.
11. Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department [published correction appears in Alcohol Alcohol. 2003 May-Jun;38(3):291]. Alcohol Alcohol. 2002;37(6):513-521. doi:10.1093/alcalc/37.6.513
12. Nishimoto A, Usery J, Winton JC, Twilla J. High-dose parenteral thiamine in treatment of Wernicke’s encephalopathy: case series and review of the literature. In Vivo. 2017;31(1):121-124. doi:10.21873/invivo.11034
13. Nakamura ZM, Tatreau JR, Rosenstein DL, Park EM. Clinical characteristics and outcomes associated with highdose intravenous thiamine administration in patients with encephalopathy. Psychosomatics. 2018;59(4):379-387. doi:10.1016/j.psym.2018.01.004
14. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G23-G31. doi:10.1152/ajpgi.00132.2010
1. Lough ME. Wernicke’s encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012;22(2):181-194. doi:10.1007/s11065-012-9200-7
2. Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875- 2890. Published 2017 Nov 27. doi:10.2147/NDT.S130078
3. Johnson JM, Fox V. Beyond thiamine: treatment for cognitive impairment in Korsakoff’s syndrome. Psychosomatics. 2018;59(4):311-317. doi:10.1016/j.psym.2018.03.011
4. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-345. doi:10.1136/ jnnp.49.4.341
5. Xiong GL, Kenedl, CA. Wernicke-Korsakoff syndrome. https://emedicine.medscape.com/article/288379-overview. Updated May 16, 2018, Accessed July 24, 2020.
6. Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. Results from the 2016 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files /NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm. Accessed July 22, 2020.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. https://www.niaaa.nih.gov /alcohol-health/overview-alcohol-consumption/moderate -binge-drinking Accessed July 24, 2020.
8. Heye N, Terstegge K, Sirtl C, McMonagle U, Schreiber K, Meyer-Gessner M. Wernicke’s encephalopathy--causes to consider. Intensive Care Med. 1994;20(4):282-286. doi:10.1007/BF01708966
9. Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248(5):714-720. doi:10.1097/SLA.0b013e3181884308
10. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: FA Davis; 1989.
11. Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department [published correction appears in Alcohol Alcohol. 2003 May-Jun;38(3):291]. Alcohol Alcohol. 2002;37(6):513-521. doi:10.1093/alcalc/37.6.513
12. Nishimoto A, Usery J, Winton JC, Twilla J. High-dose parenteral thiamine in treatment of Wernicke’s encephalopathy: case series and review of the literature. In Vivo. 2017;31(1):121-124. doi:10.21873/invivo.11034
13. Nakamura ZM, Tatreau JR, Rosenstein DL, Park EM. Clinical characteristics and outcomes associated with highdose intravenous thiamine administration in patients with encephalopathy. Psychosomatics. 2018;59(4):379-387. doi:10.1016/j.psym.2018.01.004
14. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G23-G31. doi:10.1152/ajpgi.00132.2010
Reconsidering Discharge Criteria in Children With Neurologic Impairment and Acute Respiratory Infections
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
© 2020 Society of Hospital Medicine
Is It Time to Revisit Pediatric Postdischarge Home Visits for Readmissions Reduction?
Despite concerted national efforts to decrease pediatric readmissions, recent data suggest that preventable and all-cause readmission rates of hospitalized children remain unchanged.1 Because some readmissions may be caused by inadequate postdischarge follow-up, nurse (RN) home visits offer the prospect of addressing unresolved clinical issues after discharge and ameliorating patient and family concerns that may otherwise prompt re-presentation for acute care. Yet a recent trial of this approach, the Hospital to Home Outcomes (H2O) trial,2 found the opposite to be true: participants receiving home nurse visits had higher reutilization rates than did participants in the control group. This raises interesting questions: Is it time to revisit postdischarge outreach as an intervention to reduce pediatric readmissions—and even pediatric readmissions altogether as an outcome metric?
In this issue of the Journal of Hospital Medicine, Riddle et al3 explored the perspectives of key stakeholders to understand the factors driving increased reutilization after postdischarge home visits in the H2O trial and obtained feedback for improving potential interventions. The investigators used a qualitative approach that consisted of telephone interviews with 33 parents who were enrolled in the H2O trial and in-person focus groups with 10 home care RNs involved in the trial, 12 hospital medicine physicians, and 7 primary care physicians (PCPs). Inductive thematic analysis was used to analyze responses to open-ended questions through a rigorous, iterative and multidisciplinary process. Key themes elicited from stakeholders included questions about the clinical appropriateness of reutilization episodes; the influence of insufficiently contextualized “red flag,” or warning sign, instructions given to parents in facilitating reutilization; the potential for hospital-employed home care nurses to inadvertently promote emergency department rather than PCP follow-up; and escalation of care exceeding that expected in a PCP office. Stakeholders suggested the intervention could be improved by enhancing postdischarge communication between home care RNs, hospital medicine physicians, and PCPs; tailoring home visits to specific clinical, patient, and family scenarios; and more clearly framing “red flags.”
We welcome the work of Riddle and colleagues in exposing the elements of home visits that may have led to increased utilization, and their proposed next steps to improve the intervention—enhancing contact with PCP offices and focusing interventions on specific populations—unquestionably have merit. We agree that this may be particularly true in children with medical complexity (a population that was excluded from this study), who have unique discharge needs and account for over half of pediatric readmissions.4 However, we suggest that the instinct to refine the design of the study intervention should be weighed against alternative possibilities: that postdischarge interventions are simply not effective in decreasing reutilization or, at the very least, that the findings of the H2O trial should not lead us to invest the resources required to further discern the efficacy of postdischarge interventions.
This counter-intuitive possibility is only compounded by the fact that reutilization rates were not improved in the study group’s H2O II trial, a follow-up study that focused on postdischarge nurse telephone calls as the intervention of interest5; and indeed, the results of these two, well-designed negative trials have been previously cited to propose postdischarge nurse contact as a potential target of deimplementation efforts.6 In the pediatric population, in which caregivers rather than patients themselves are generally responsible for seeking out care, postdischarge outreach may inevitably escalate concerning findings that will result in reutilization. Instead, perhaps the H2O study findings should prompt a broader exploration for alternative solutions to pediatric readmission reduction. One such solution could build on the finding by Riddle et al that stakeholders perceive ambiguity in whether discharging physicians, or rather PCPs, have ownership of clinical issues after discharge. Rather than asking visiting RNs to triangulate between inpatient and outpatient physicians, developing systems to directly integrate PCPs in the hospital discharge process for select patients—for instance, through leveraging the rapid expansion of telemedicine services during the COVID-19 crisis—may promote shared understanding of a patient’s illness trajectory and follow-up needs.
Importantly, the authors also noted that despite the findings of increased reutilization, parents who received home visits expressed their wishes to receive home visits in the future. While not a central finding of the study, this validates a hypothesis expressed in prior work by the H2O study group: “Hospital quality readmission metrics may not be well aligned with family desires for improved postdischarge transitions.”5 Given that efforts to reduce pediatric readmission have been largely unsuccessful and that readmission events are relatively uncommon in the general pediatric population,4 the parental wishes resonate with existing calls in the literature to consider looking beyond readmissions reduction in isolation as a quality metric. In contrast to the increasing presence of hospital reimbursement penalties among state Medicaid agencies for readmissions, a shift in focus toward outcome measures that are patient- and family-centered is imperative.1,7 If home visits are not ultimately a solution to pediatric reutilization reduction, they may nonetheless still enable families to effectively manage the concerns that families endorse following discharge, including medication safety and social hardships.8
In summary, Riddle et al not only provided important context for the unexpected outcome of a well-designed randomized clinical trial but also provided a rich source of qualitative data that furthers our understanding of a child’s discharge home from the hospital through the perspective of multiple stakeholders. While the authors offer well-reasoned next steps in narrowing the intervention population of interest and enhancing connections of families with PCP care, it may be time to broadly revisit postdischarge interventions and outcomes to identify new approaches and redefine quality measures for hospital-to-home transitions of children and their families.
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the hospital to home outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2019-0092
3. Riddle SW, Sherman SN, Moore MJ, et al. A qualitative study of increased pediatric reutilization after a postdischarge home nurse visit. J Hosp Med. 2020;15:518-525. https://doi.org/10.12788/jhm.3370
4. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
5. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
6. Bonafide CP, Keren R. Negative studies and the science of deimplementation. JAMA Pediatr. 2018;172(9):807-809. https://doi.org/ 10.1001/jamapediatrics.2018.2077
7. Leyenaar JK, Lagu T, Lindenauer PK. Are pediatric readmission reduction efforts falling flat? J Hosp Med. 2019;14(10):644-645. https://doi.org/10.12788/jhm.3269
8. Tubbs-Cooley HL, Riddle SW, Gold JM, et al. Paediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period. J Adv Nurs. 2020;76(6):1394-1403. https://doi.org/10.1111/jan.14341
Despite concerted national efforts to decrease pediatric readmissions, recent data suggest that preventable and all-cause readmission rates of hospitalized children remain unchanged.1 Because some readmissions may be caused by inadequate postdischarge follow-up, nurse (RN) home visits offer the prospect of addressing unresolved clinical issues after discharge and ameliorating patient and family concerns that may otherwise prompt re-presentation for acute care. Yet a recent trial of this approach, the Hospital to Home Outcomes (H2O) trial,2 found the opposite to be true: participants receiving home nurse visits had higher reutilization rates than did participants in the control group. This raises interesting questions: Is it time to revisit postdischarge outreach as an intervention to reduce pediatric readmissions—and even pediatric readmissions altogether as an outcome metric?
In this issue of the Journal of Hospital Medicine, Riddle et al3 explored the perspectives of key stakeholders to understand the factors driving increased reutilization after postdischarge home visits in the H2O trial and obtained feedback for improving potential interventions. The investigators used a qualitative approach that consisted of telephone interviews with 33 parents who were enrolled in the H2O trial and in-person focus groups with 10 home care RNs involved in the trial, 12 hospital medicine physicians, and 7 primary care physicians (PCPs). Inductive thematic analysis was used to analyze responses to open-ended questions through a rigorous, iterative and multidisciplinary process. Key themes elicited from stakeholders included questions about the clinical appropriateness of reutilization episodes; the influence of insufficiently contextualized “red flag,” or warning sign, instructions given to parents in facilitating reutilization; the potential for hospital-employed home care nurses to inadvertently promote emergency department rather than PCP follow-up; and escalation of care exceeding that expected in a PCP office. Stakeholders suggested the intervention could be improved by enhancing postdischarge communication between home care RNs, hospital medicine physicians, and PCPs; tailoring home visits to specific clinical, patient, and family scenarios; and more clearly framing “red flags.”
We welcome the work of Riddle and colleagues in exposing the elements of home visits that may have led to increased utilization, and their proposed next steps to improve the intervention—enhancing contact with PCP offices and focusing interventions on specific populations—unquestionably have merit. We agree that this may be particularly true in children with medical complexity (a population that was excluded from this study), who have unique discharge needs and account for over half of pediatric readmissions.4 However, we suggest that the instinct to refine the design of the study intervention should be weighed against alternative possibilities: that postdischarge interventions are simply not effective in decreasing reutilization or, at the very least, that the findings of the H2O trial should not lead us to invest the resources required to further discern the efficacy of postdischarge interventions.
This counter-intuitive possibility is only compounded by the fact that reutilization rates were not improved in the study group’s H2O II trial, a follow-up study that focused on postdischarge nurse telephone calls as the intervention of interest5; and indeed, the results of these two, well-designed negative trials have been previously cited to propose postdischarge nurse contact as a potential target of deimplementation efforts.6 In the pediatric population, in which caregivers rather than patients themselves are generally responsible for seeking out care, postdischarge outreach may inevitably escalate concerning findings that will result in reutilization. Instead, perhaps the H2O study findings should prompt a broader exploration for alternative solutions to pediatric readmission reduction. One such solution could build on the finding by Riddle et al that stakeholders perceive ambiguity in whether discharging physicians, or rather PCPs, have ownership of clinical issues after discharge. Rather than asking visiting RNs to triangulate between inpatient and outpatient physicians, developing systems to directly integrate PCPs in the hospital discharge process for select patients—for instance, through leveraging the rapid expansion of telemedicine services during the COVID-19 crisis—may promote shared understanding of a patient’s illness trajectory and follow-up needs.
Importantly, the authors also noted that despite the findings of increased reutilization, parents who received home visits expressed their wishes to receive home visits in the future. While not a central finding of the study, this validates a hypothesis expressed in prior work by the H2O study group: “Hospital quality readmission metrics may not be well aligned with family desires for improved postdischarge transitions.”5 Given that efforts to reduce pediatric readmission have been largely unsuccessful and that readmission events are relatively uncommon in the general pediatric population,4 the parental wishes resonate with existing calls in the literature to consider looking beyond readmissions reduction in isolation as a quality metric. In contrast to the increasing presence of hospital reimbursement penalties among state Medicaid agencies for readmissions, a shift in focus toward outcome measures that are patient- and family-centered is imperative.1,7 If home visits are not ultimately a solution to pediatric reutilization reduction, they may nonetheless still enable families to effectively manage the concerns that families endorse following discharge, including medication safety and social hardships.8
In summary, Riddle et al not only provided important context for the unexpected outcome of a well-designed randomized clinical trial but also provided a rich source of qualitative data that furthers our understanding of a child’s discharge home from the hospital through the perspective of multiple stakeholders. While the authors offer well-reasoned next steps in narrowing the intervention population of interest and enhancing connections of families with PCP care, it may be time to broadly revisit postdischarge interventions and outcomes to identify new approaches and redefine quality measures for hospital-to-home transitions of children and their families.
Despite concerted national efforts to decrease pediatric readmissions, recent data suggest that preventable and all-cause readmission rates of hospitalized children remain unchanged.1 Because some readmissions may be caused by inadequate postdischarge follow-up, nurse (RN) home visits offer the prospect of addressing unresolved clinical issues after discharge and ameliorating patient and family concerns that may otherwise prompt re-presentation for acute care. Yet a recent trial of this approach, the Hospital to Home Outcomes (H2O) trial,2 found the opposite to be true: participants receiving home nurse visits had higher reutilization rates than did participants in the control group. This raises interesting questions: Is it time to revisit postdischarge outreach as an intervention to reduce pediatric readmissions—and even pediatric readmissions altogether as an outcome metric?
In this issue of the Journal of Hospital Medicine, Riddle et al3 explored the perspectives of key stakeholders to understand the factors driving increased reutilization after postdischarge home visits in the H2O trial and obtained feedback for improving potential interventions. The investigators used a qualitative approach that consisted of telephone interviews with 33 parents who were enrolled in the H2O trial and in-person focus groups with 10 home care RNs involved in the trial, 12 hospital medicine physicians, and 7 primary care physicians (PCPs). Inductive thematic analysis was used to analyze responses to open-ended questions through a rigorous, iterative and multidisciplinary process. Key themes elicited from stakeholders included questions about the clinical appropriateness of reutilization episodes; the influence of insufficiently contextualized “red flag,” or warning sign, instructions given to parents in facilitating reutilization; the potential for hospital-employed home care nurses to inadvertently promote emergency department rather than PCP follow-up; and escalation of care exceeding that expected in a PCP office. Stakeholders suggested the intervention could be improved by enhancing postdischarge communication between home care RNs, hospital medicine physicians, and PCPs; tailoring home visits to specific clinical, patient, and family scenarios; and more clearly framing “red flags.”
We welcome the work of Riddle and colleagues in exposing the elements of home visits that may have led to increased utilization, and their proposed next steps to improve the intervention—enhancing contact with PCP offices and focusing interventions on specific populations—unquestionably have merit. We agree that this may be particularly true in children with medical complexity (a population that was excluded from this study), who have unique discharge needs and account for over half of pediatric readmissions.4 However, we suggest that the instinct to refine the design of the study intervention should be weighed against alternative possibilities: that postdischarge interventions are simply not effective in decreasing reutilization or, at the very least, that the findings of the H2O trial should not lead us to invest the resources required to further discern the efficacy of postdischarge interventions.
This counter-intuitive possibility is only compounded by the fact that reutilization rates were not improved in the study group’s H2O II trial, a follow-up study that focused on postdischarge nurse telephone calls as the intervention of interest5; and indeed, the results of these two, well-designed negative trials have been previously cited to propose postdischarge nurse contact as a potential target of deimplementation efforts.6 In the pediatric population, in which caregivers rather than patients themselves are generally responsible for seeking out care, postdischarge outreach may inevitably escalate concerning findings that will result in reutilization. Instead, perhaps the H2O study findings should prompt a broader exploration for alternative solutions to pediatric readmission reduction. One such solution could build on the finding by Riddle et al that stakeholders perceive ambiguity in whether discharging physicians, or rather PCPs, have ownership of clinical issues after discharge. Rather than asking visiting RNs to triangulate between inpatient and outpatient physicians, developing systems to directly integrate PCPs in the hospital discharge process for select patients—for instance, through leveraging the rapid expansion of telemedicine services during the COVID-19 crisis—may promote shared understanding of a patient’s illness trajectory and follow-up needs.
Importantly, the authors also noted that despite the findings of increased reutilization, parents who received home visits expressed their wishes to receive home visits in the future. While not a central finding of the study, this validates a hypothesis expressed in prior work by the H2O study group: “Hospital quality readmission metrics may not be well aligned with family desires for improved postdischarge transitions.”5 Given that efforts to reduce pediatric readmission have been largely unsuccessful and that readmission events are relatively uncommon in the general pediatric population,4 the parental wishes resonate with existing calls in the literature to consider looking beyond readmissions reduction in isolation as a quality metric. In contrast to the increasing presence of hospital reimbursement penalties among state Medicaid agencies for readmissions, a shift in focus toward outcome measures that are patient- and family-centered is imperative.1,7 If home visits are not ultimately a solution to pediatric reutilization reduction, they may nonetheless still enable families to effectively manage the concerns that families endorse following discharge, including medication safety and social hardships.8
In summary, Riddle et al not only provided important context for the unexpected outcome of a well-designed randomized clinical trial but also provided a rich source of qualitative data that furthers our understanding of a child’s discharge home from the hospital through the perspective of multiple stakeholders. While the authors offer well-reasoned next steps in narrowing the intervention population of interest and enhancing connections of families with PCP care, it may be time to broadly revisit postdischarge interventions and outcomes to identify new approaches and redefine quality measures for hospital-to-home transitions of children and their families.
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the hospital to home outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2019-0092
3. Riddle SW, Sherman SN, Moore MJ, et al. A qualitative study of increased pediatric reutilization after a postdischarge home nurse visit. J Hosp Med. 2020;15:518-525. https://doi.org/10.12788/jhm.3370
4. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
5. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
6. Bonafide CP, Keren R. Negative studies and the science of deimplementation. JAMA Pediatr. 2018;172(9):807-809. https://doi.org/ 10.1001/jamapediatrics.2018.2077
7. Leyenaar JK, Lagu T, Lindenauer PK. Are pediatric readmission reduction efforts falling flat? J Hosp Med. 2019;14(10):644-645. https://doi.org/10.12788/jhm.3269
8. Tubbs-Cooley HL, Riddle SW, Gold JM, et al. Paediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period. J Adv Nurs. 2020;76(6):1394-1403. https://doi.org/10.1111/jan.14341
1. Auger KA, Harris JM, Gay JC, et al. Progress (?) toward reducing pediatric readmissions. J Hosp Med. 2019;14(10):618-621. https://doi.org/10.12788/jhm.3210
2. Auger KA, Simmons JM, Tubbs-Cooley HL, et al. Postdischarge nurse home visits and reuse: the hospital to home outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2019-0092
3. Riddle SW, Sherman SN, Moore MJ, et al. A qualitative study of increased pediatric reutilization after a postdischarge home nurse visit. J Hosp Med. 2020;15:518-525. https://doi.org/10.12788/jhm.3370
4. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
5. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
6. Bonafide CP, Keren R. Negative studies and the science of deimplementation. JAMA Pediatr. 2018;172(9):807-809. https://doi.org/ 10.1001/jamapediatrics.2018.2077
7. Leyenaar JK, Lagu T, Lindenauer PK. Are pediatric readmission reduction efforts falling flat? J Hosp Med. 2019;14(10):644-645. https://doi.org/10.12788/jhm.3269
8. Tubbs-Cooley HL, Riddle SW, Gold JM, et al. Paediatric clinical and social concerns identified by home visit nurses in the immediate postdischarge period. J Adv Nurs. 2020;76(6):1394-1403. https://doi.org/10.1111/jan.14341
© 2020 Society of Hospital Medicine