User login
A Curriculum for Training Medical Faculty to Teach Mental Health Care—and Their Responses to the Learning
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.
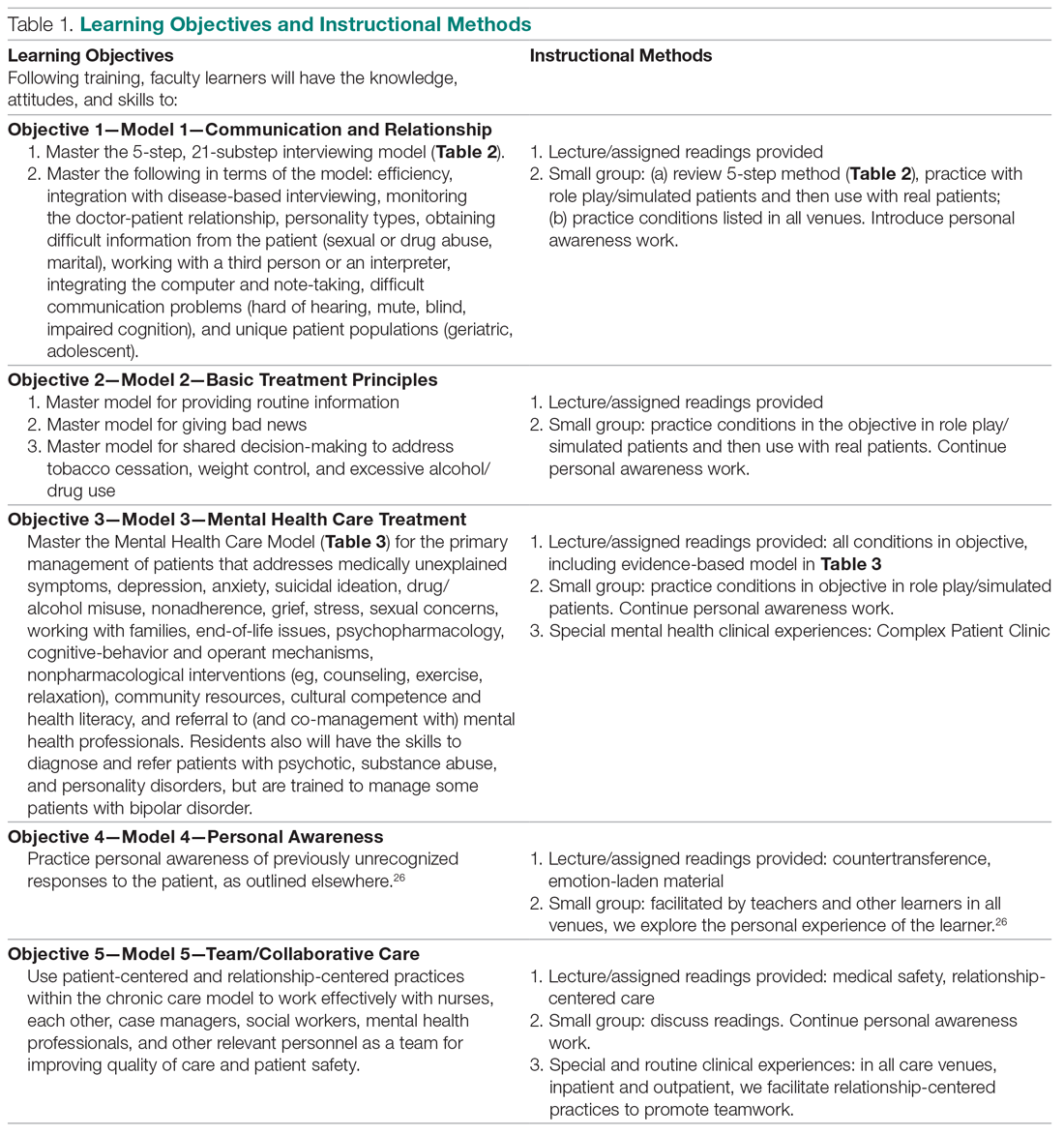
First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
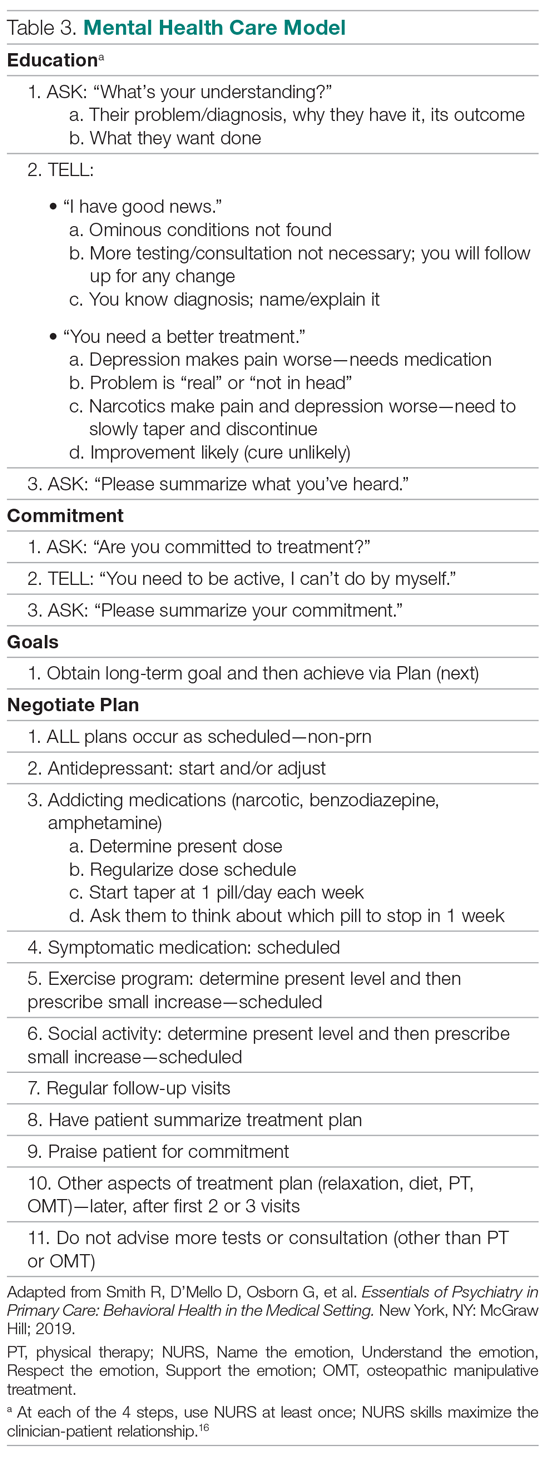
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21
Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.

First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21

Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.

First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21

Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
Remdesivir Reduces Time to Recovery in Adults Hospitalized With COVID-19: A Meaningful Step in Therapeutic Discovery
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
Study Overview
Objective. To assess the clinical efficacy and safety of remdesivir in hospitalized adults with laboratory-confirmed COVID-19 and with evidence of lower respiratory tract involvement.
Design. Double-blinded, randomized, placebo-controlled, multicenter trial.
Setting and participants. Enrollment for the study took place between February 21, 2020, and April 19, 2020, at 60 trial sites and 13 subsites in the United States, Denmark, the United Kingdom, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore. Study participants included patients aged ≥ 18 years who were hospitalized and had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as determined by a positive reverse transcription polymerase chain reaction assay on a respiratory specimen. Participants had evidence of lower respiratory tract infection at the time of enrollment; this was defined as radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Exclusion criteria for study participation included abnormal liver enzymes (alanine aminotransferase, aspartate aminotransferase) more than 5 times the upper limit of normal range; impaired renal function or need for hemodialysis or hemofiltration; pregnancy or breastfeeding; or anticipated hospital discharge or transfer to another hospital within 72 hours of enrollment.
Intervention. Participants were randomized in a 1:1 ratio to the remdesivir group or the placebo group and were administered either intravenous infusions of remdesivir (200-mg loading dose on day 1, followed by a 100-mg maintenance dose daily on days 2 through 10, or until hospital discharge or death) or placebo for up to 10 days. Blinding was maintained by masking infusions with an opaque bag and tubing. Randomization was stratified by study site and disease severity at enrollment. Supportive care was delivered to all participants according to the standard of care at each trial site hospital. Clinical status, determined using an 8-category ordinal scale and the National Early Warning Score, was assessed daily for each participant while hospitalized (day 1 through day 29).
Blood samples for safety laboratory tests were collected, and oropharyngeal or nasopharyngeal swab testing was performed for viral RNA detection and quantification on days 1, 3, 5, 8, and 11. All serious adverse events (AEs) and grade 3/4 AEs that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity reactions associated with the study drug or placebo administration were recorded.
Main outcome measures. The primary endpoint measure of this study was time to recovery, defined as the first day during the 28 days after enrollment on which a participant satisfied category 1 (ie, not hospitalized, no limitations of activities), 2 (ie, not hospitalized, limitation of activities, home oxygen requirement, or both), or 3 (ie, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care; hospitalization was extended for infection-control reason) on the 8-category ordinal scale. Secondary outcomes included all-cause mortality at 14 and 28 days after enrollment and grade 3/4 AEs and serious AEs that occurred during trial participation. Analysis of the primary outcome was performed using a log-rank test of the time to recovery comparing remdesivir with placebo group, stratified by disease severity.
The study’s primary outcome was initially defined as a difference in clinical status as ascertained by the 8-category ordinal scale between groups of participants who were administered remdesivir versus placebo on day 15. Because of new knowledge gained external to the study about a more protracted COVID-19 clinical course than previously recognized, a change in primary outcome to time to recovery was proposed by trial statisticians, who were unaware of treatment assignments (72 participants had been enrolled) or outcome data (no interim data) on March 22, 2020, with subsequent amendment approval on April 2, 2020. On April 27, 2020, the Data and Safety Monitoring Board (DSMB) reviewed the interim study analysis (with data cutoff date of April 22, 2020) and recommended the report and mortality data to be provided to trial team members from the National Institute of Allergy and Infectious Diseases; these findings were subsequently made public.
Main results. A total of 1107 patients were assessed for eligibility, of whom 1063 underwent randomization, with 541 assigned to remdesivir and 522 to placebo. Results were unblinded early at the recommendation of DSMB due to findings from the interim analysis that showed reduced time to recovery in the group that received remdesivir. As of April 28, 2020, a total of 391 participants in the remdesivir group and 340 participants in the placebo group had completed the trial (day 29), recovered, or died. The mean age of participants was 58.9 ± 15.0 years, the majority were men (64.3%) and were White (53.2%), and the most common prespecified coexisting conditions were hypertension (49.6%), obesity (37.0%), and type 2 diabetes mellitus (29.7%). The vast majority of participants (88.7%) had severe COVID-19 disease at enrollment, defined as requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥ 24 breaths per minute).
Based on available data from 1059 participants (538 from the remdesivir group and 521 from the placebo group), those in the remdesivir group had a shorter median recovery time of 11 days (95% confidence interval [CI], 9-12) as compared to 15 days (95% CI, 13-19) in the placebo group, with a rate ratio for recovery of 1.32 (95% CI, 1.12-1.55; P < 0.001). Moreover, the odds of improvement on day 15 in the 8-category ordinal scale score were higher in the remdesivir group, compared to the placebo group (proportional odds model; odds ratio, 1.50; 95% CI, 1.18-1.91; P = 0.001; 844 participants).
Mortality rate by 14 days was numerically lower in the remdesivir group (7.1%) compared to the placebo group (11.9%), but the difference was not statistically significant (Kaplan-Meier, hazard ratio for death, 0.70; 95% CI, 0.47-1.04). Serious AEs were reported in 114 of the 541 (21.1%) participants in the remdesivir group and 141 of the 522 (27.0%) participants in the placebo group. Moreover, grade 3/4 AEs occurred in 156 (28.8%) participants in the remdesivir group and in 172 (33.0%) in the placebo group.
Conclusion. The study found that remdesivir, compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.
Commentary
Since the initial reporting of a cluster of cases of pneumonia in Wuhan, China, on December 31, 2019, SARS-CoV-2 has been identified as the cause of this new disease (COVID-19), and to-date SARS-CoV-2 infection has affected more than 15.2 million people globally, with more than 3.9 million cases in the United States alone.1 Despite an unprecedented global research effort, as well as public-private research partnerships, both in terms of scale and scope, an effective pharmacologic therapy for COVID-19 has so far eluded the scientific and medical community. Early trials of hydroxychloroquine and lopinavir-ritonavir did not demonstrate a clinical benefit in patients with COVID-19.2,3 Moreover, the first randomized controlled trial of remdesivir in COVID-19, a nucleoside analogue prodrug and a broad-spectrum antiviral agent previously shown to have inhibitory effects on pathogenic coronaviruses, was an underpowered study, and thus inconclusive.4 Thus, given the persistence of the COVID-19 pandemic and a current lack of effective vaccines or curative treatments, the study reported by Beigel and colleagues is timely and provides much needed knowledge in developing potential therapies for COVID-19.
The present report described the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACCT-1), which aimed to evaluate the clinical efficacy and safety of intravenous remdesivir, as compared to placebo, in hospitalized adults with laboratory-confirmed COVID-19. The study itself was well-designed and conducted. The successful enrollment of more than 1000 participants randomized in a 1:1 ratio within a 2-month recruitment window, involving 60 international trial sites, shortly after the emergence of a new global pandemic was remarkable. This study provided the first evidence that remdesivir, an antiviral, can shorten time to recovery by approximately 31% compared to placebo in COVID-19 patients with lower respiratory tract involvement.
Interestingly, this beneficial effect of remdesivir on time to recovery was primarily observed in participants within the severe disease stratum (those requiring supplemental oxygen) at baseline (12 days in remdesivir group versus 18 days in placebo group), but not in those with mild-moderate disease at the time of study enrollment (5 days in either remdesivir or placebo group). Moreover, the beneficial effects of remdesivir on reducing time to recovery was not observed in participants who required mechanical ventilation or ECMO at enrollment. Thus, these preliminary results suggest that COVID-19 disease severity and timing, particularly in patients who require supplemental oxygen but prior to disease progression towards requiring mechanical ventilation, may present a window of opportunity to initiate remdesivir treatment in order to improve outcomes. Further analysis utilizing data from the entire cohort, including outcomes data from the full 28-day follow-up period, may better delineate the subgroup of hospitalized COVID-19 patients who may benefit most from remdesivir. Last, safety data from the present study, along with that reported by Wang and colleagues,4 provides evidence that intravenous remdesivir administration is likely safe in adults during the treatment period.
The preliminary results from the ACCT-1 provide early evidence that remdesivir shortens time to recovery in adult patients hospitalized for COVID-19 with pulmonary involvement. In light of these results, the US Food and Drug Administration issued an emergency use authorization for remdesivir on May 1, 2020, for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease.5 In addition, remdesivir has also recently been approved as a therapy for COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates, and has received conditional approval for use by the European Commission.6
Although these are encouraging developments in the race to identify effective therapeutics for COVID-19, a number of unanswered questions regarding the administration of remdesivir in the treatment of this disease remain. For instance, in an open-label, randomized, multicenter trial of patients with severe COVID-19 not requiring mechanical ventilation, treatment with a 5-day course versus a 10-day course of intravenous remdesivir did not result in a significant difference in efficacy.7 Thus, more studies are needed to better determine the shortest effective duration of remdesivir therapy in COVID-19 patients with different disease severity. Also, the mortality rate in COVID-19 patients who were treated with remdesivir remained high in the current study. Therefore, there is ample opportunity to evaluate treatment strategies, including multidrug interventions with remdesivir, to reduce mortality and improve clinical outcomes in patients hospitalized with COVID-19.
Applications for Clinical Practice
Remdesivir shortens time to recovery in adult patients hospitalized with COVID-19 who require supplemental oxygen therapy. While much needs to be learned in order to optimize treatment of COVID-19, preliminary findings from the current study provide an important first step towards these discoveries.
–Fred Ko, MD, MS
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
1. Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 16, 2020.
2. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial [published online April 14, 2020]. medRxiv 2020; doi:10.1101/2020.04.10.20060558.
3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787-1799.
4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578.
5. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed July 16, 2020.
6. Gilead’s COVID-19 antiviral remdesivir gets conditional EU clearance. www.reuters.com/article/us-health-coronavirus-eu-remdesivir/gileads-covid-19-antiviral-remdesivir-gets-conditional-eu-clearance-idUSKBN2441GK. Accessed July 6, 2020.
7. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020 May 27.doi: 10.1056/NEJMoa2015301. Online ahead of print.
US News releases latest top hospitals list, adds COVID heroes
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
Non–COVID-19 VA Hospital Admissions Drop During the Pandemic
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.
Anecdotal reports have suggested that people have been less likely to go to the hospital for emergencies during the COVID-19 pandemic. Findings from a study by 2 physicians at Mount Sinai in New York now provide support for that: Between March 11 and April 21, 2020, 42% fewer patients were admitted to US Department of Veterans Affairs (VA) inpatient facilities when compared with the preceding 6 weeks.
The researchers analyzed data from the VA Corporate Data Warehouse and examined at trends during the first 16 weeks of 2019 and 2020 for 6 common emergency conditions: stroke, myocardial infarction, heart failure, chronic obstructive pulmonary disease (COPD), appendicitis, and pneumonia. Strikingly, the number of patients admitted dropped from 77,624 in weeks 5 to 10 of 2020 to 45,155 in weeks 11 to 16.
The number of patients admitted for stroke declined by 52%; myocardial infarction, 40%; COPD, 48%; heart failure, 49%; and appendicitis, 57%. By contrast, the number of patients admitted overall and for each condition did not decline during the same weeks in 2019. Admissions for pneumonia dropped during weeks 11 to 16 by 14% in 2019 and 28% in 2020. When patients who tested positive for COVID-19 were excluded, however, pneumonia admissions decreased by 46%. Of patients who were admitted during weeks 11 to 16 of 2020, 2,458 had tested positive for COVID-19 during weeks 5 to 10.
The authers contend that the marked drop in admissions is unlikely to be attributable to a reduction in disease incidence. Rather, they theorize that many patients may be avoiding hospitals out of fear of becoming infected with SARS-CoV-2. These data “should raise serious concerns,” the authors say, about the well-being and health outcomes of the patients who aren’t getting the emergency or inpatient care they need.
Internists’ use of ultrasound can reduce radiology referrals
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.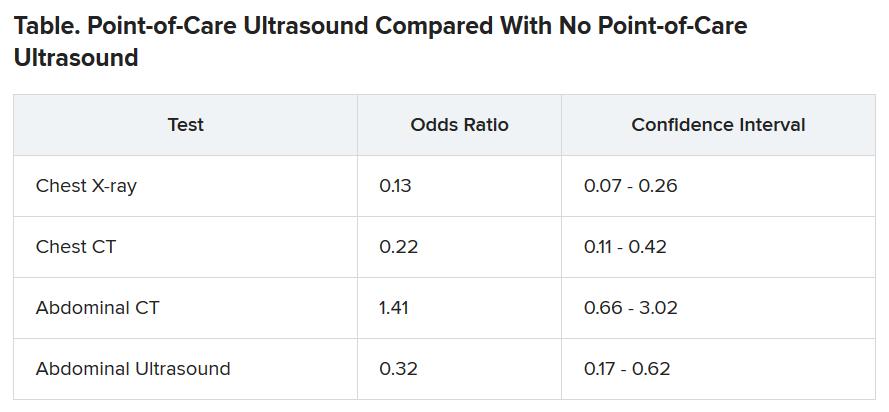
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Leadership & Professional Development: Dis-Missed: Cultural and Gender Barriers to Graceful Self-Promotion
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
© 2020 Society of Hospital Medicine
Comanagement of Hip Fracture Patients
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
© 2020 Society of Hospital Medicine
Surgical Comanagement for Hip Fracture: Time for a Randomized Trial
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
© 2020 Society of Hospital Medicine
Improving Healthcare Value: COVID-19 Emergency Regulatory Relief and Implications for Post-Acute Skilled Nursing Facility Care
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
© 2020 Society of Hospital Medicine
Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.
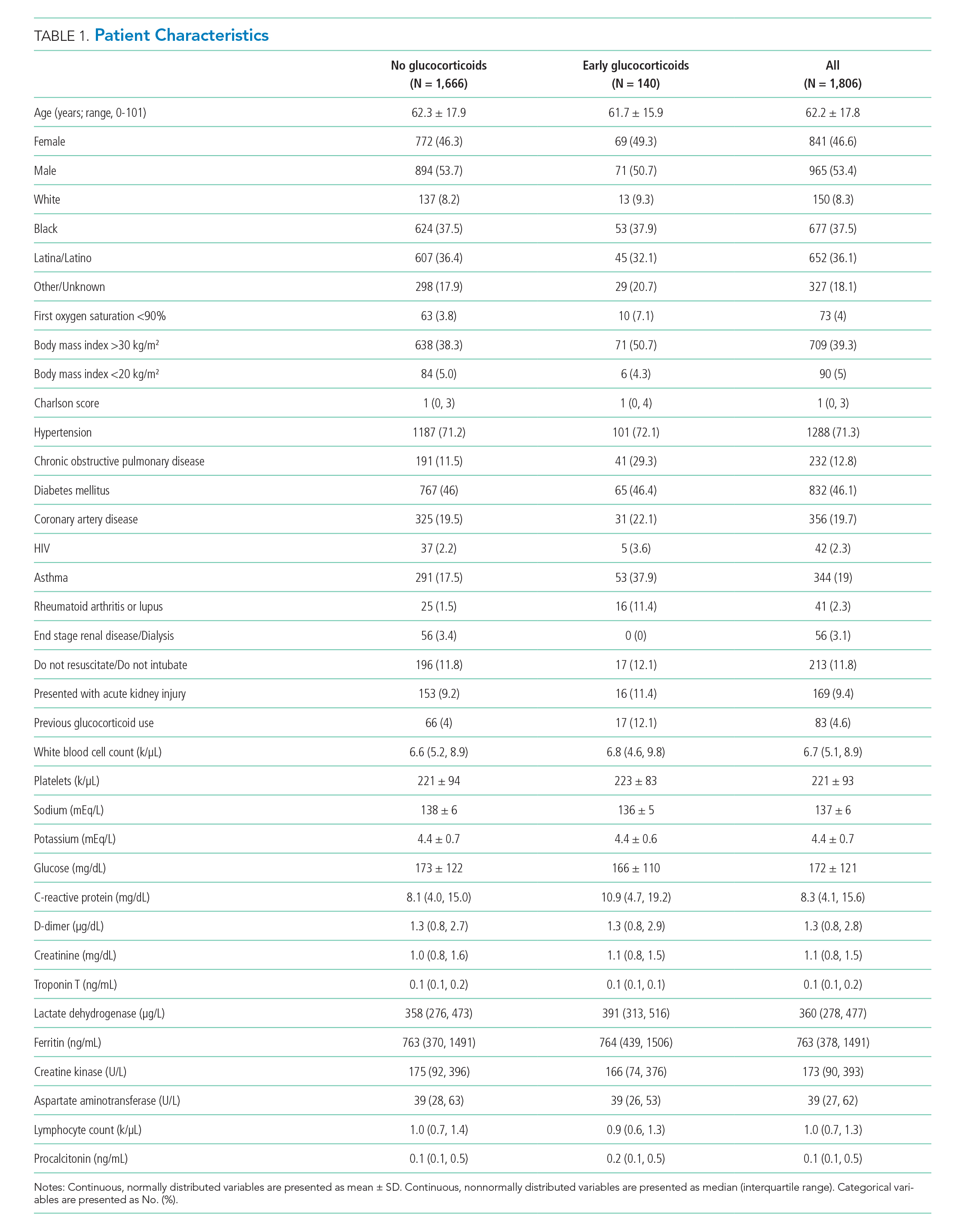
RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.
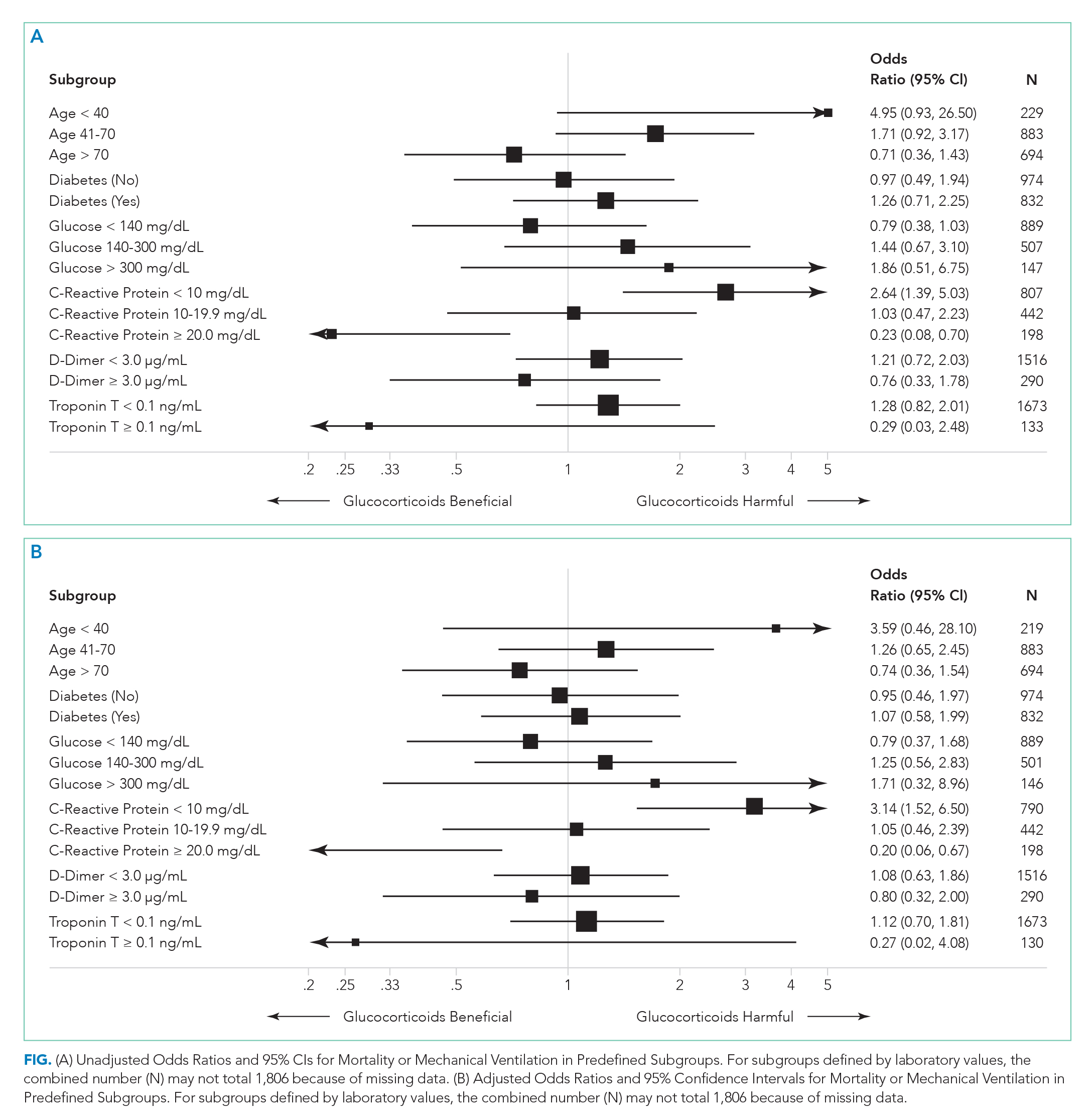
There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.
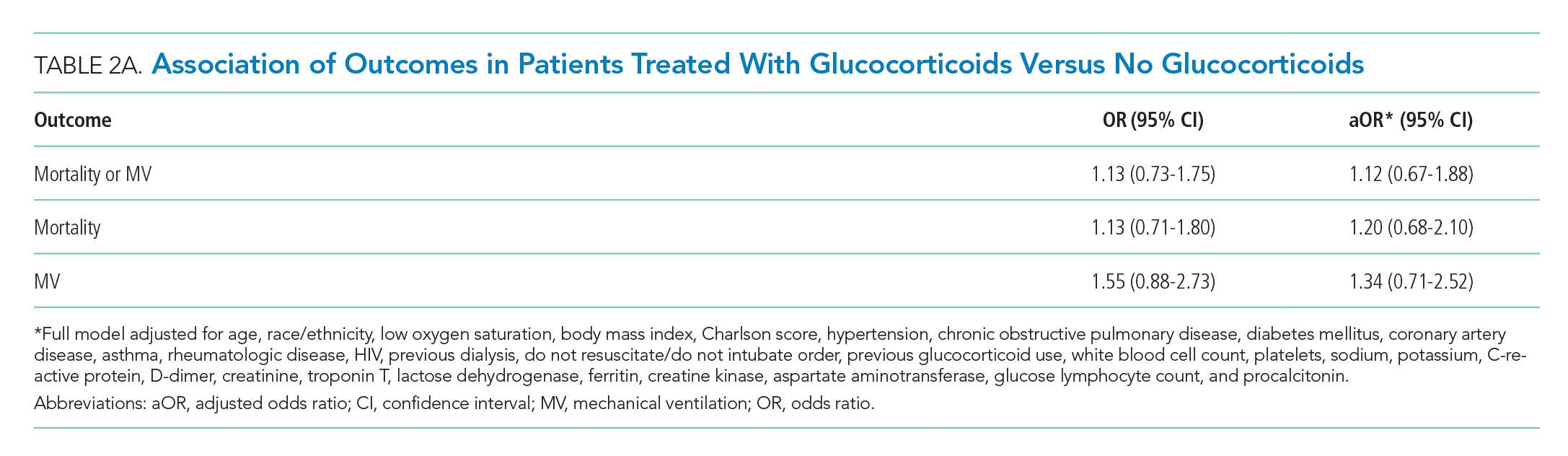
DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.
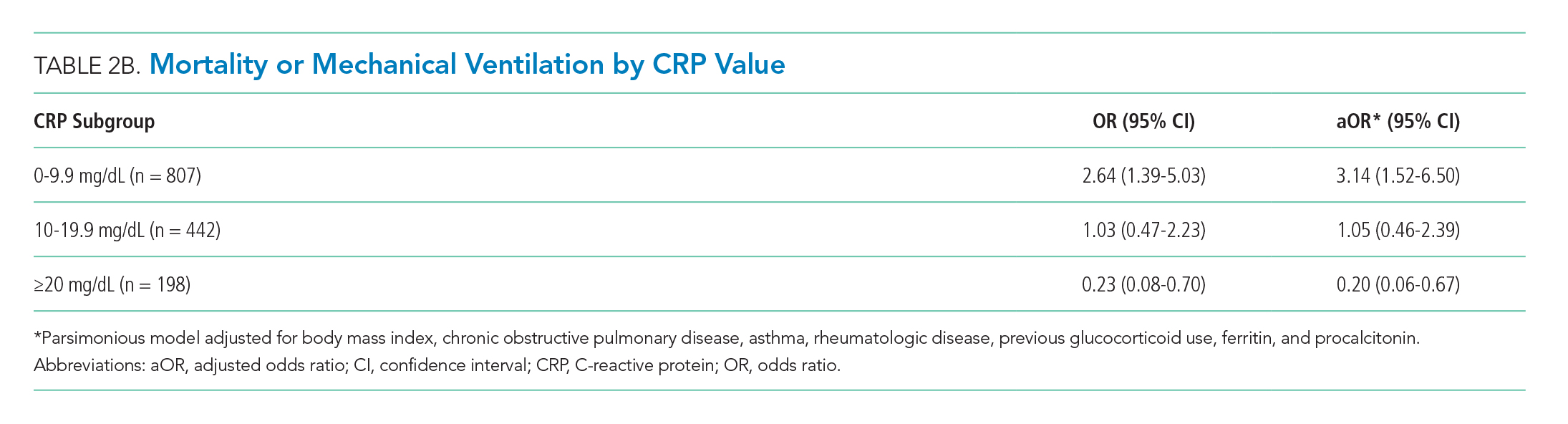
Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
© 2020 Society of Hospital Medicine
