User login
Establishing an Orthopedic Excess Hospital Days in Acute Care Program
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
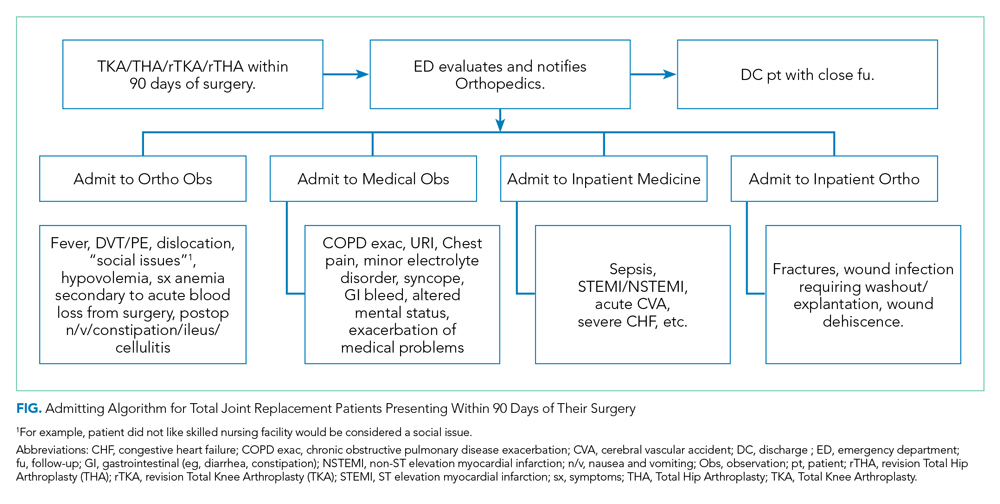
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).
Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
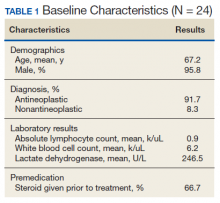
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
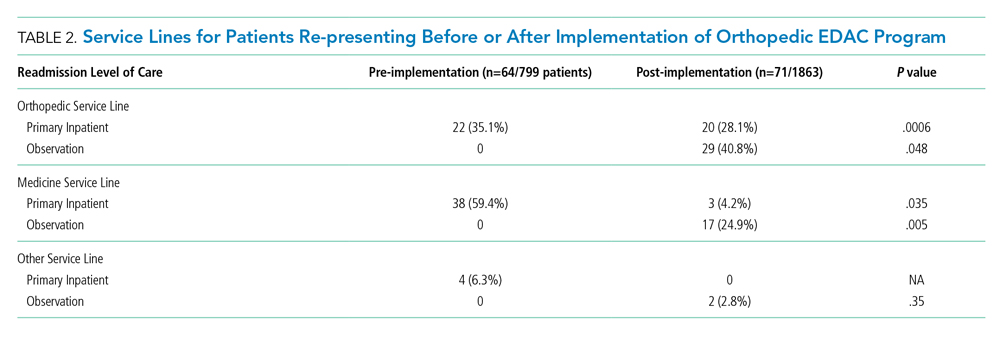
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.
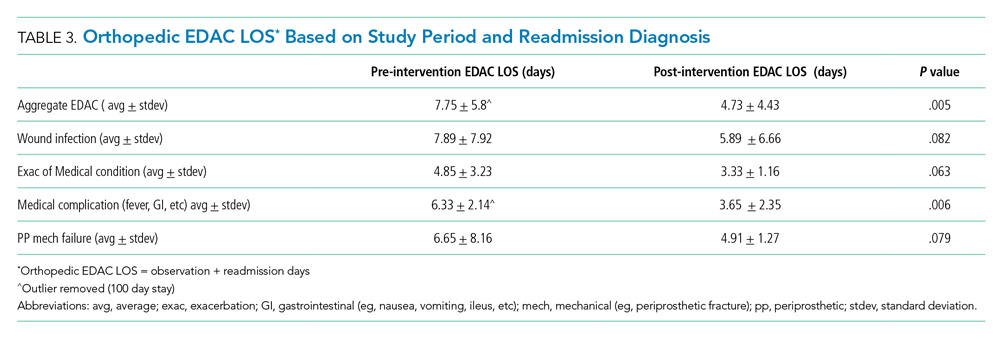
Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.
Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
© 2020 Society of Hospital Medicine
Strategies of Female Teaching Attending Physicians to Navigate Gender-Based Challenges: An Exploratory Qualitative Study
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).
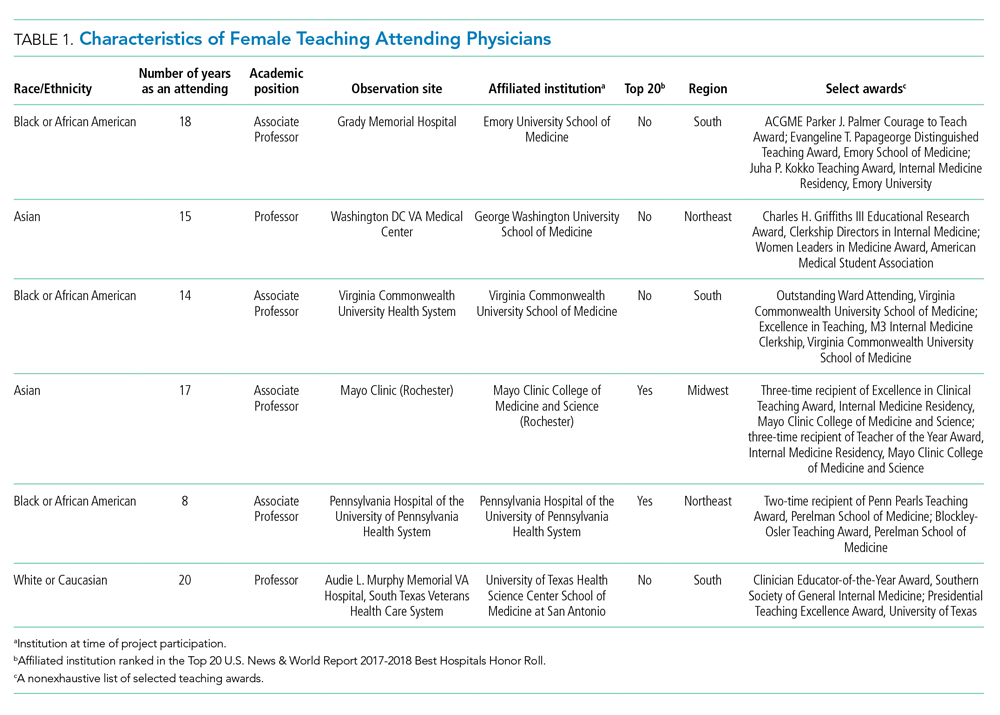
The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.
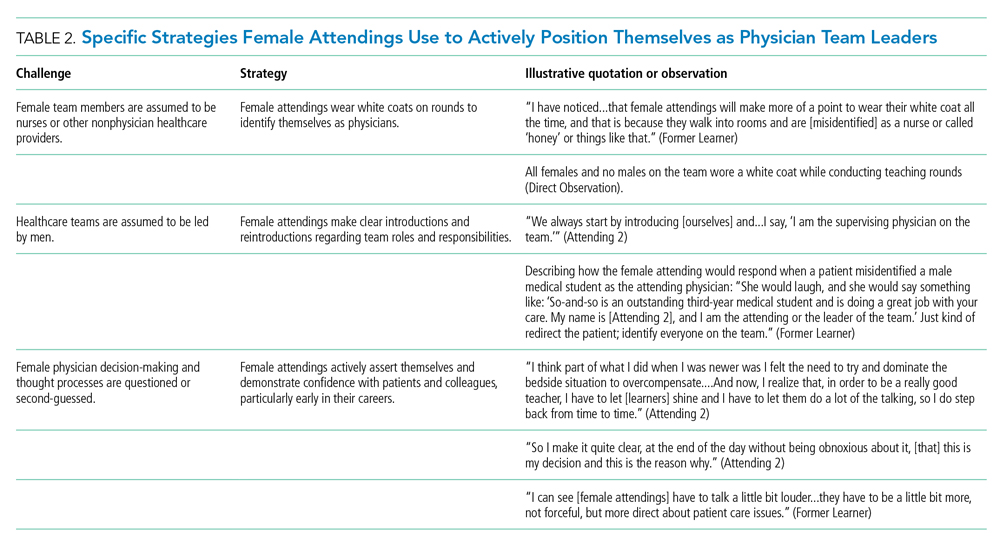
We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.
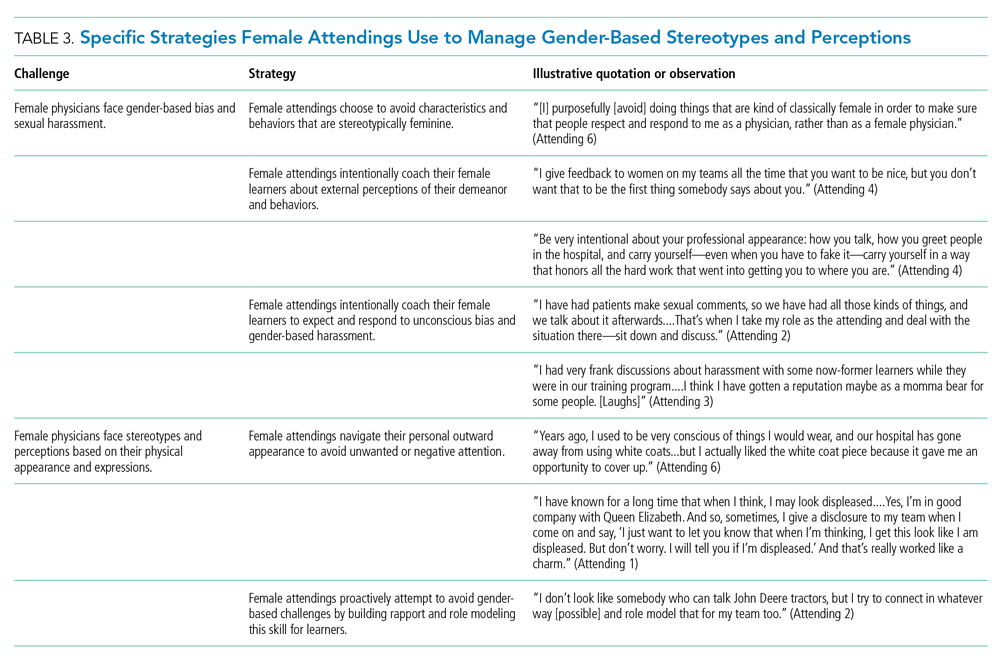
Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”
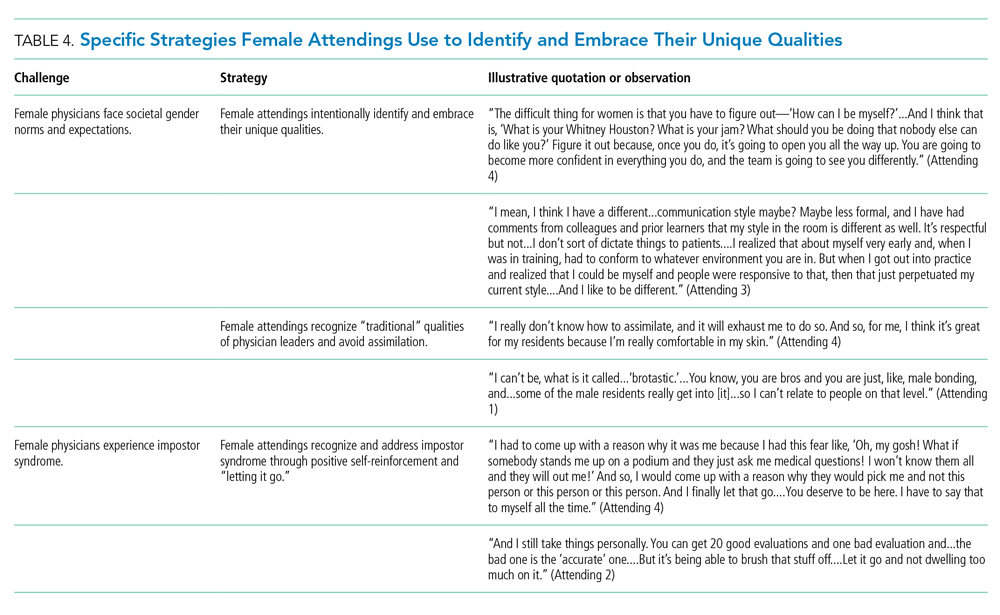
Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
© 2020 Society of Hospital Medicine
Pooled Testing for SARS-CoV-2 in Hospitalized Patients
Viral testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of all patients admitted to the hospital is an appealing objective given the recognition of asymptomatic or minimally symptomatic infections. Yet such testing requires that all admitted patients be classified as persons under investigation (PUIs) until their test results are known. If an outside laboratory is used for the SARS-CoV-2 testing, the delay in obtaining results for these PUIs may cause significant personal protective equipment (PPE) use, postpone some care for non-coronavirus disease 2019 (COVID-19) conditions, block beds, and produce anxiety among staff and other patients. Rapid in-house testing of all admitted patients may resolve these issues but may be limited by the supply of reagents. To address this challenge, we piloted a pooled testing strategy for patients at low risk for SARS-CoV-2 admitted to a community hospital.
METHODS
From April 17, 2020, to May 11, 2020, we implemented a pooled testing strategy using the GeneXpert® System (Cepheid, Sunnyvale, California) at Saratoga Hospital, a 171-bed community hospital in upstate New York. Under normal procedures for this system, a single patient swab is placed in a vial containing viral transport media (VTM). An aliquot of this media is then transferred into a Xpert® Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert® instrument in our laboratory. Obtaining immediate results allowed us to assign admitted patients to either a COVID-19 or a non–COVID-19 unit, improving the issues associated with PUIs. Unfortunately, we did not have enough test cartridges to sustain this strategy of rapid individual testing of all admitted patients, and supply lines have remained uncertain.
We sought to conserve our limited Xpert Xpress SARS CoV-2 test cartridges using the strategy of pooled testing, a technique reported in Germany and by the University of Nebraska.1,2 In this method, variable numbers of tests are pooled for a single analysis. If the test from the pooled vial is negative, these patients are all considered negative. If the pooled test is positive, all those patients need individual testing. This pooling method has been theorized to preserve test cartridges when the expected frequency of positive results is low.3
All patients admitted or placed on observation underwent SARS-CoV-2 PCR testing. The Emergency Department (ED) staff stratified patients into high or low risk to determine if they would be tested in a single send-out test (high risk) or a rapid in-house pooled group (low risk). High-risk patients were those with compatible history, physical exam, laboratory markers, and radiographic studies for COVID-19 disease. This often included increased supplemental oxygen requirement, multiple elevated inflammatory markers (including D-dimer, C-reactive protein, erythrocyte sedimentation rate, and ferritin levels), lymphopenia, and findings on chest radiograph or computed tomography scan including ground glass changes, multifocal pneumonia, or pneumonia. High-risk patients were admitted to the COVID unit or intensive care unit, had a send-out SARS-CoV-2 polymerase chain reaction (PCR) test, and were treated as a PUI until the results of their testing was known and correlated with their clinical course. Low-risk patients were those without complaints suggestive of COVID-19 infection and who may have had negative inflammatory markers, no significant lymphopenia, and negative imaging.
The samples from 3 admitted patients thought to be at low-risk for COVID-19 using the clinical judgement of our ED staff were pooled for testing. All samples were obtained using nasopharyngeal swabs by experienced staff. The swabs from these patients were placed into a single vial of 3 mL VTM, maintaining the recommended 1 swab per mL of VTM. An aliquot of this media was then transferred into an Xpert Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert instrument in our laboratory following manufacturer’s instructions. Based on analytic laboratory studies of the Cepheid Xpert Express SARS-CoV-2 test,4 we assume a clinical performance comparable to other reverse-transcriptase PCR (RT-PCR) tests, which have so far demonstrated sensitivities of 60% to 80% and specificities of 95% to 99%.5
Validation studies were performed on pools made from samples obtained from admitted patients with previously known positive and negative samples tested at the New York State Department of Health, Wadsworth Center laboratory (Albany, New York). A total of 14 samples were used for the instrument validation study, including three samples for pooled testing. The cycle threshold (Ct) value is defined as the number of PCR cycles required for the signal to be detectable. Ct values for each nucleic acid target of a known positive sample tested singly and in the pool with known negative patients were compared. A small shift in Ct values was noted between single and pooled testing, demonstrating no decrease in analytic sensitivity and suggesting that we would experience no decrease in clinical sensitivity.
We selected the pooling of 3 samples into 1 cartridge for several reasons: (1) 3-sample pools are well within the appropriate pooling size for the percentage positive rate in the population being tested. The use of larger pool sizes results in the need for more repeat testing when a positive result is obtained; (2) Given our supply lines, the projected savings would allow us to continue this strategy; and (3) Holding 3 patients in the ED until a pool was ready was manageable given our rate of admissions and ED volume.
The strategy required patients being held in the ED until a pooled group of 3 could be tested. On select occasions when holding patients in the ED to obtain a pool of 3 was not practical, 2 patients were tested in the pool. These decisions required close coordination between the laboratory, ED, and nursing staff.
RESULTS
This strategy resulted in 530 unique patient tests in 179 cartridges (172 with three swabs and 7 with two swabs). We had 4 positive pooled tests, requiring the use of 11 additional cartridges, for a positive rate of 0.8% (4/530) in this low-risk population (patients without COVID-19–related symptoms). There were no patients from negative pools who developed evidence of COVID-19 disease or tested positive for SARS-CoV-2 during their hospitalization. The total number of cartridges used was 190 and the number saved was 340.
DISCUSSION
The strategy of pooled testing for SARS-CoV-2 in patients admitted to our community hospital allowed us to continue rapid testing of admitted patients at low risk for COVID-19 disease during a period when supplies would otherwise not have been sufficient. We believe this strategy conserved PPE, led to a marked reduction in staff and patient anxiety, and improved patient care. Our impression is that testing all admitted patients has also been reassuring to our community. Like many others, we have observed that public fear of entering the hospital during this pandemic has caused delays in patients seeking care for non–COVID-19 conditions. We believe this strategy will help reduce those fears.
This strategy may require modification as the pandemic progresses. Our ED physicians were able to identify patients who they felt to be low risk for having COVID-19 disease based on signs, symptoms, and clinical impression during a time when we had an 8% positive rate among symptomatic outpatients and an estimated community positive rate in the range of 1% to 2%. If the rate of positive tests in our community rises, the use of pooling may need to be limited or the pool size reduced. If our supply of reagents is further limited or patient testing demand increases, the pool size may need to be increased. This will need to be balanced with our ability to hold patients in the ED while waiting for the pool size to be reached.
CONCLUSION
The strategy of pooled testing for SARS-CoV-2 has allowed us to continue to immediately test all admitted patients, thus improving patient care. It has required close coordination between multiple members of our laboratory and clinical staff and may require adjustment as the pandemic progresses. We believe it is a valuable tool during a time of limited resources that may have application in testing other low-risk groups, including healthcare workers and clients of occupational medicine services.
Acknowledgment
The authors gratefully acknowledge the support of Kirsten St. George, MAppSc, PhD, Director, Virology Laboratory, Wadsworth, NYSDOH, and the services supplied by the Wadsworth laboratory to our region.
1. Corona ‘pool testing’ increases worldwide capacities many times over. January 4, 2020. Accessed April 20, 2020. https://healthcare-in-europe.com/en/news/corona-pool-testing-increases-worldwide-capacities-many-times-over.html
2. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
3. Shani-Narkiss H, Gilday OD, Yayon N, Landau ID. Efficient and practical sample pooling for high-throughput PCR diagnosis of COVID-19. medRxiv. April 6, 2020. https://doi.org/10.1101/2020.04.06.20052159
4. Wolters F, van de Bovenkamp J, van den Bosch B, et al. Multi-center evaluation of Cepheid Xpert® Xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:104426. https://doi.org/10.1016/j.jcv.2020.104426
5. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020. Online first. https://doi.org/10.1056/NEJMp2015897
Viral testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of all patients admitted to the hospital is an appealing objective given the recognition of asymptomatic or minimally symptomatic infections. Yet such testing requires that all admitted patients be classified as persons under investigation (PUIs) until their test results are known. If an outside laboratory is used for the SARS-CoV-2 testing, the delay in obtaining results for these PUIs may cause significant personal protective equipment (PPE) use, postpone some care for non-coronavirus disease 2019 (COVID-19) conditions, block beds, and produce anxiety among staff and other patients. Rapid in-house testing of all admitted patients may resolve these issues but may be limited by the supply of reagents. To address this challenge, we piloted a pooled testing strategy for patients at low risk for SARS-CoV-2 admitted to a community hospital.
METHODS
From April 17, 2020, to May 11, 2020, we implemented a pooled testing strategy using the GeneXpert® System (Cepheid, Sunnyvale, California) at Saratoga Hospital, a 171-bed community hospital in upstate New York. Under normal procedures for this system, a single patient swab is placed in a vial containing viral transport media (VTM). An aliquot of this media is then transferred into a Xpert® Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert® instrument in our laboratory. Obtaining immediate results allowed us to assign admitted patients to either a COVID-19 or a non–COVID-19 unit, improving the issues associated with PUIs. Unfortunately, we did not have enough test cartridges to sustain this strategy of rapid individual testing of all admitted patients, and supply lines have remained uncertain.
We sought to conserve our limited Xpert Xpress SARS CoV-2 test cartridges using the strategy of pooled testing, a technique reported in Germany and by the University of Nebraska.1,2 In this method, variable numbers of tests are pooled for a single analysis. If the test from the pooled vial is negative, these patients are all considered negative. If the pooled test is positive, all those patients need individual testing. This pooling method has been theorized to preserve test cartridges when the expected frequency of positive results is low.3
All patients admitted or placed on observation underwent SARS-CoV-2 PCR testing. The Emergency Department (ED) staff stratified patients into high or low risk to determine if they would be tested in a single send-out test (high risk) or a rapid in-house pooled group (low risk). High-risk patients were those with compatible history, physical exam, laboratory markers, and radiographic studies for COVID-19 disease. This often included increased supplemental oxygen requirement, multiple elevated inflammatory markers (including D-dimer, C-reactive protein, erythrocyte sedimentation rate, and ferritin levels), lymphopenia, and findings on chest radiograph or computed tomography scan including ground glass changes, multifocal pneumonia, or pneumonia. High-risk patients were admitted to the COVID unit or intensive care unit, had a send-out SARS-CoV-2 polymerase chain reaction (PCR) test, and were treated as a PUI until the results of their testing was known and correlated with their clinical course. Low-risk patients were those without complaints suggestive of COVID-19 infection and who may have had negative inflammatory markers, no significant lymphopenia, and negative imaging.
The samples from 3 admitted patients thought to be at low-risk for COVID-19 using the clinical judgement of our ED staff were pooled for testing. All samples were obtained using nasopharyngeal swabs by experienced staff. The swabs from these patients were placed into a single vial of 3 mL VTM, maintaining the recommended 1 swab per mL of VTM. An aliquot of this media was then transferred into an Xpert Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert instrument in our laboratory following manufacturer’s instructions. Based on analytic laboratory studies of the Cepheid Xpert Express SARS-CoV-2 test,4 we assume a clinical performance comparable to other reverse-transcriptase PCR (RT-PCR) tests, which have so far demonstrated sensitivities of 60% to 80% and specificities of 95% to 99%.5
Validation studies were performed on pools made from samples obtained from admitted patients with previously known positive and negative samples tested at the New York State Department of Health, Wadsworth Center laboratory (Albany, New York). A total of 14 samples were used for the instrument validation study, including three samples for pooled testing. The cycle threshold (Ct) value is defined as the number of PCR cycles required for the signal to be detectable. Ct values for each nucleic acid target of a known positive sample tested singly and in the pool with known negative patients were compared. A small shift in Ct values was noted between single and pooled testing, demonstrating no decrease in analytic sensitivity and suggesting that we would experience no decrease in clinical sensitivity.
We selected the pooling of 3 samples into 1 cartridge for several reasons: (1) 3-sample pools are well within the appropriate pooling size for the percentage positive rate in the population being tested. The use of larger pool sizes results in the need for more repeat testing when a positive result is obtained; (2) Given our supply lines, the projected savings would allow us to continue this strategy; and (3) Holding 3 patients in the ED until a pool was ready was manageable given our rate of admissions and ED volume.
The strategy required patients being held in the ED until a pooled group of 3 could be tested. On select occasions when holding patients in the ED to obtain a pool of 3 was not practical, 2 patients were tested in the pool. These decisions required close coordination between the laboratory, ED, and nursing staff.
RESULTS
This strategy resulted in 530 unique patient tests in 179 cartridges (172 with three swabs and 7 with two swabs). We had 4 positive pooled tests, requiring the use of 11 additional cartridges, for a positive rate of 0.8% (4/530) in this low-risk population (patients without COVID-19–related symptoms). There were no patients from negative pools who developed evidence of COVID-19 disease or tested positive for SARS-CoV-2 during their hospitalization. The total number of cartridges used was 190 and the number saved was 340.
DISCUSSION
The strategy of pooled testing for SARS-CoV-2 in patients admitted to our community hospital allowed us to continue rapid testing of admitted patients at low risk for COVID-19 disease during a period when supplies would otherwise not have been sufficient. We believe this strategy conserved PPE, led to a marked reduction in staff and patient anxiety, and improved patient care. Our impression is that testing all admitted patients has also been reassuring to our community. Like many others, we have observed that public fear of entering the hospital during this pandemic has caused delays in patients seeking care for non–COVID-19 conditions. We believe this strategy will help reduce those fears.
This strategy may require modification as the pandemic progresses. Our ED physicians were able to identify patients who they felt to be low risk for having COVID-19 disease based on signs, symptoms, and clinical impression during a time when we had an 8% positive rate among symptomatic outpatients and an estimated community positive rate in the range of 1% to 2%. If the rate of positive tests in our community rises, the use of pooling may need to be limited or the pool size reduced. If our supply of reagents is further limited or patient testing demand increases, the pool size may need to be increased. This will need to be balanced with our ability to hold patients in the ED while waiting for the pool size to be reached.
CONCLUSION
The strategy of pooled testing for SARS-CoV-2 has allowed us to continue to immediately test all admitted patients, thus improving patient care. It has required close coordination between multiple members of our laboratory and clinical staff and may require adjustment as the pandemic progresses. We believe it is a valuable tool during a time of limited resources that may have application in testing other low-risk groups, including healthcare workers and clients of occupational medicine services.
Acknowledgment
The authors gratefully acknowledge the support of Kirsten St. George, MAppSc, PhD, Director, Virology Laboratory, Wadsworth, NYSDOH, and the services supplied by the Wadsworth laboratory to our region.
Viral testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of all patients admitted to the hospital is an appealing objective given the recognition of asymptomatic or minimally symptomatic infections. Yet such testing requires that all admitted patients be classified as persons under investigation (PUIs) until their test results are known. If an outside laboratory is used for the SARS-CoV-2 testing, the delay in obtaining results for these PUIs may cause significant personal protective equipment (PPE) use, postpone some care for non-coronavirus disease 2019 (COVID-19) conditions, block beds, and produce anxiety among staff and other patients. Rapid in-house testing of all admitted patients may resolve these issues but may be limited by the supply of reagents. To address this challenge, we piloted a pooled testing strategy for patients at low risk for SARS-CoV-2 admitted to a community hospital.
METHODS
From April 17, 2020, to May 11, 2020, we implemented a pooled testing strategy using the GeneXpert® System (Cepheid, Sunnyvale, California) at Saratoga Hospital, a 171-bed community hospital in upstate New York. Under normal procedures for this system, a single patient swab is placed in a vial containing viral transport media (VTM). An aliquot of this media is then transferred into a Xpert® Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert® instrument in our laboratory. Obtaining immediate results allowed us to assign admitted patients to either a COVID-19 or a non–COVID-19 unit, improving the issues associated with PUIs. Unfortunately, we did not have enough test cartridges to sustain this strategy of rapid individual testing of all admitted patients, and supply lines have remained uncertain.
We sought to conserve our limited Xpert Xpress SARS CoV-2 test cartridges using the strategy of pooled testing, a technique reported in Germany and by the University of Nebraska.1,2 In this method, variable numbers of tests are pooled for a single analysis. If the test from the pooled vial is negative, these patients are all considered negative. If the pooled test is positive, all those patients need individual testing. This pooling method has been theorized to preserve test cartridges when the expected frequency of positive results is low.3
All patients admitted or placed on observation underwent SARS-CoV-2 PCR testing. The Emergency Department (ED) staff stratified patients into high or low risk to determine if they would be tested in a single send-out test (high risk) or a rapid in-house pooled group (low risk). High-risk patients were those with compatible history, physical exam, laboratory markers, and radiographic studies for COVID-19 disease. This often included increased supplemental oxygen requirement, multiple elevated inflammatory markers (including D-dimer, C-reactive protein, erythrocyte sedimentation rate, and ferritin levels), lymphopenia, and findings on chest radiograph or computed tomography scan including ground glass changes, multifocal pneumonia, or pneumonia. High-risk patients were admitted to the COVID unit or intensive care unit, had a send-out SARS-CoV-2 polymerase chain reaction (PCR) test, and were treated as a PUI until the results of their testing was known and correlated with their clinical course. Low-risk patients were those without complaints suggestive of COVID-19 infection and who may have had negative inflammatory markers, no significant lymphopenia, and negative imaging.
The samples from 3 admitted patients thought to be at low-risk for COVID-19 using the clinical judgement of our ED staff were pooled for testing. All samples were obtained using nasopharyngeal swabs by experienced staff. The swabs from these patients were placed into a single vial of 3 mL VTM, maintaining the recommended 1 swab per mL of VTM. An aliquot of this media was then transferred into an Xpert Xpress SARS CoV-2 test cartridge and assayed on the GeneXpert instrument in our laboratory following manufacturer’s instructions. Based on analytic laboratory studies of the Cepheid Xpert Express SARS-CoV-2 test,4 we assume a clinical performance comparable to other reverse-transcriptase PCR (RT-PCR) tests, which have so far demonstrated sensitivities of 60% to 80% and specificities of 95% to 99%.5
Validation studies were performed on pools made from samples obtained from admitted patients with previously known positive and negative samples tested at the New York State Department of Health, Wadsworth Center laboratory (Albany, New York). A total of 14 samples were used for the instrument validation study, including three samples for pooled testing. The cycle threshold (Ct) value is defined as the number of PCR cycles required for the signal to be detectable. Ct values for each nucleic acid target of a known positive sample tested singly and in the pool with known negative patients were compared. A small shift in Ct values was noted between single and pooled testing, demonstrating no decrease in analytic sensitivity and suggesting that we would experience no decrease in clinical sensitivity.
We selected the pooling of 3 samples into 1 cartridge for several reasons: (1) 3-sample pools are well within the appropriate pooling size for the percentage positive rate in the population being tested. The use of larger pool sizes results in the need for more repeat testing when a positive result is obtained; (2) Given our supply lines, the projected savings would allow us to continue this strategy; and (3) Holding 3 patients in the ED until a pool was ready was manageable given our rate of admissions and ED volume.
The strategy required patients being held in the ED until a pooled group of 3 could be tested. On select occasions when holding patients in the ED to obtain a pool of 3 was not practical, 2 patients were tested in the pool. These decisions required close coordination between the laboratory, ED, and nursing staff.
RESULTS
This strategy resulted in 530 unique patient tests in 179 cartridges (172 with three swabs and 7 with two swabs). We had 4 positive pooled tests, requiring the use of 11 additional cartridges, for a positive rate of 0.8% (4/530) in this low-risk population (patients without COVID-19–related symptoms). There were no patients from negative pools who developed evidence of COVID-19 disease or tested positive for SARS-CoV-2 during their hospitalization. The total number of cartridges used was 190 and the number saved was 340.
DISCUSSION
The strategy of pooled testing for SARS-CoV-2 in patients admitted to our community hospital allowed us to continue rapid testing of admitted patients at low risk for COVID-19 disease during a period when supplies would otherwise not have been sufficient. We believe this strategy conserved PPE, led to a marked reduction in staff and patient anxiety, and improved patient care. Our impression is that testing all admitted patients has also been reassuring to our community. Like many others, we have observed that public fear of entering the hospital during this pandemic has caused delays in patients seeking care for non–COVID-19 conditions. We believe this strategy will help reduce those fears.
This strategy may require modification as the pandemic progresses. Our ED physicians were able to identify patients who they felt to be low risk for having COVID-19 disease based on signs, symptoms, and clinical impression during a time when we had an 8% positive rate among symptomatic outpatients and an estimated community positive rate in the range of 1% to 2%. If the rate of positive tests in our community rises, the use of pooling may need to be limited or the pool size reduced. If our supply of reagents is further limited or patient testing demand increases, the pool size may need to be increased. This will need to be balanced with our ability to hold patients in the ED while waiting for the pool size to be reached.
CONCLUSION
The strategy of pooled testing for SARS-CoV-2 has allowed us to continue to immediately test all admitted patients, thus improving patient care. It has required close coordination between multiple members of our laboratory and clinical staff and may require adjustment as the pandemic progresses. We believe it is a valuable tool during a time of limited resources that may have application in testing other low-risk groups, including healthcare workers and clients of occupational medicine services.
Acknowledgment
The authors gratefully acknowledge the support of Kirsten St. George, MAppSc, PhD, Director, Virology Laboratory, Wadsworth, NYSDOH, and the services supplied by the Wadsworth laboratory to our region.
1. Corona ‘pool testing’ increases worldwide capacities many times over. January 4, 2020. Accessed April 20, 2020. https://healthcare-in-europe.com/en/news/corona-pool-testing-increases-worldwide-capacities-many-times-over.html
2. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
3. Shani-Narkiss H, Gilday OD, Yayon N, Landau ID. Efficient and practical sample pooling for high-throughput PCR diagnosis of COVID-19. medRxiv. April 6, 2020. https://doi.org/10.1101/2020.04.06.20052159
4. Wolters F, van de Bovenkamp J, van den Bosch B, et al. Multi-center evaluation of Cepheid Xpert® Xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:104426. https://doi.org/10.1016/j.jcv.2020.104426
5. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020. Online first. https://doi.org/10.1056/NEJMp2015897
1. Corona ‘pool testing’ increases worldwide capacities many times over. January 4, 2020. Accessed April 20, 2020. https://healthcare-in-europe.com/en/news/corona-pool-testing-increases-worldwide-capacities-many-times-over.html
2. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153(6):715-718. https://doi.org/10.1093/ajcp/aqaa064
3. Shani-Narkiss H, Gilday OD, Yayon N, Landau ID. Efficient and practical sample pooling for high-throughput PCR diagnosis of COVID-19. medRxiv. April 6, 2020. https://doi.org/10.1101/2020.04.06.20052159
4. Wolters F, van de Bovenkamp J, van den Bosch B, et al. Multi-center evaluation of Cepheid Xpert® Xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:104426. https://doi.org/10.1016/j.jcv.2020.104426
5. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020. Online first. https://doi.org/10.1056/NEJMp2015897
© 2020 Society of Hospital Medicine
Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
© 2020 Society of Hospital Medicine
Implementation and Evaluation of a 90-Minute Rituximab Infusion Protocol at the Richard L. Roudebush VA Medical Center
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
Rituximab is a genetically engineered chimeric immunoglobulin G1 monoclonal antibody. It functions by binding to the CD20 antigen on the surface of B-cell lymphocytes, leading to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.1 The US Food and Drug Administration approved this therapy to treat patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, along with other nonmalignant indications, including pemphigus vulgaris and rheumatoid arthritis (RA). Historically, a significant amount of time and labor on behalf of medical personnel has been required to administer rituximab according to the original manufacturer’s labeling due to the boxed warning associated with infusion-related reactions (IRRs).2
Originally, the elongated infusion times that were recommended for rituximab were largely due to the perceived risk of serious infusion-related adverse drug reactions. Slower infusion times should reduce the risk of a reaction and are considered to be a good option for those patients who are at a high risk of having a severe IRRs to rituximab. Examples of high-risk patients from previous studies include those with significant cardiovascular disease, a circulating lymphocyte count ≤ 5,000/µL at the start of infusion, and those who have previously had a reaction to rituximab.3-5 In appropriate patients, research has shown a decreasing incidence of all-grade IRRs for patients who are prescribed rituximab as they receive more doses of the drug.2,6 The ability to identify suitable patients for 90-minute infusions of rituximab and the prospect of better health system resource utilization has led investigators to study the effects of shortened infusion times.
The RATE trial addressed this subject with a phase 3 safety study on the effects of a 90-minute rituximab infusion for patients with previously untreated diffuse large B-cell and follicular lymphoma.3 The patients in this study received their first dose of rituximab using the traditional infusion approach. If it was well-tolerated, they received subsequent rituximab infusions using a 90-minute protocol. Only 1.1% of patients who had previously received a rituximab infusion developed a grade 3 or 4 IRR when receiving a faster infusion of the drug for the first time.3 This result led to the addition of instructions for a 90-minute infusion to the package insert.2
In contrast to the RATE trial, the RATE-RA trial evaluated the incidence of IRRs in patients who received rituximab for nonmalignant indications. This study assessed patients with RA receiving rituximab for > 120 minutes. The authors reported 0.6% of the patients in the study developed a grade 3 or 4 IRR associated with the first 120-minute infusion of the medication.5 The researchers concluded that rituximab can be administered at a faster rate during second and subsequent infusions in patients who have been shown to tolerate traditional infusions without increasing the risk or severity of IRRs.5
The US Department of Veterans Affairs (VA) Richard L. Roudebush VA Medical Center (RLRVAMC) in Indianapolis, Indiana, uses traditional directions for the infusion of rituximab due to perceived tolerability and safety concerns specifically in a veteran population—even while other VA medical centers have implemented shortened infusion protocols. This also is despite the fact that available research shows rapid infusions of the drug are well tolerated in a variety of community settings.7,8 Anticipated benefits of implementing a protocol include savings in chair time at the institution’s infusion clinic along with increased nursing and patient satisfaction. This project was conducted to prepare, implement, and assess the safety of a 90-minute rituximab protocol at the RLRVAMC.
Methods
Proactive measures were required before and during the implementation of the 90-minute protocol to ensure patient safety and staff satisfaction. Updates to the RLRVAMC policy for the management of medical emergencies within the infusion center were reviewed and approved by the acute care committee and nursing leadership. A protocol was developed to identify eligible patients, outline the hypersensitivity protocol, instruct pharmacy personnel on admixture preparation, and provide a titration schedule based on dose. Order sets also were created to assist health care providers (HCPs) with the prescribing of rituximab for nonantineoplastic indications. Educational materials were crafted to assist with order verification, product preparation, labeling, and programming of infusion pumps. Live education was provided for physicians, pharmacists, and nurses to ensure smooth implementation of the protocol and appropriate management of medical emergencies based on the updated policy.
Study Design
Nursing staff in the infusion clinic were surveyed once before a live education session and again after the conclusion of the study. The purpose of the survey was to assess the prior experience and current comfort level of the nursing staff with administering rituximab over 90 minutes. Nurses were asked the following questions: (1) Do you have prior experience administering rituximab via 90-minute infusion; and (2) do you feel comfortable administering rituximab via 90-minute infusion?
A weekly report of patients who received rituximab between November 1, 2018 through April 1, 2019 at the RLRVAMC was generated. HCPs were alerted to eligible patients based on protocol requirements. The HCPs then made the final determination and entered orders accordingly.
This study was a retrospective chart review of all who patients received a rapid infusion of rituximab. Patients who were included if they were aged ≥ 18 years, received rituximab infusions in the RLRVAMC infusion clinic, had an absolute lymphocyte count ≤ 5,000/mm3 at the time of their rapid infusions, had no significant baseline cardiovascular disease or respiratory compromise, and had no prior grade 3 or 4 rituximab IRRs as defined by Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.9 This study was a quality improvement initiative and considered exempt by the institutional review board. All data were deidentified and secured to ensure patient privacy.
The primary endpoint for this study was the incidence of grade 3 or 4 IRRs associated with the rapid infusion of rituximab. Secondary endpoints included the proportion of patients who experienced a grade 3 or 4 infusion reaction, who received proper treatment according to the institution’s hypersensitivity protocol, savings in infusion clinic chair time, and nursing satisfaction with education and implementation of the rapid infusion rituximab protocol.
The following data were collected for all included patients: demographics, lactic acid dehydrogenase level, white blood cell count, and absolute lymphocyte count prior to rituximab infusion, indication for treatment, dose of rituximab for 90-minute infusion, date of infusion, starting time, ending time, number of previous rituximab infusions within the past 3 months, symptoms of infusion reactions during rituximab infusion, and grade of any infusion reactions that occurred.
Estimated savings in infusion clinic chair time was calculated by taking the difference in time between each completed rapid infusion and the estimated amount of time it would have taken for each patient to receive a traditional infusion. The estimated amount of time for traditional infusion was determined by following the institution’s protocol for administering rituximab to patients who previously tolerated their first dose of the drug (eg, 100 mg/h starting rate and increasing by 100 mg/h every 30 minutes to a maximum infusion rate of 400 mg/h). All endpoints were analyzed using descriptive statistics.
Results
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.
Limitations
The small sample size in this study was a limitation. Retrospective data related to the management of infusion reactions and length of infusions were collected from nursing notes. The prospective use of a standardized evaluation tool for adverse drug reactions as well as bar code medication administration technology would improve the data available for this study. Additional studies also would be useful to validate the results.
Conclusions
The proactive measures that were used to implement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. Potential time savings with each infusion was significant. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols. Protocols, education, and order sets are being developed for daratumumab and infliximab.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
1. Feugier P. A review of rituximab, the first anti-CD20 monoclonal antibody used in the treatment of B non-Hodgkin’s lymphomas. Future Oncol. 2015;11(9):1327-1342. doi:10.2217/fon.15.57
2. Rituxan [package insert]. South San Francisco, CA: Genentech; 2016.
3. Dakhil S, Hermann R, Schreeder MT, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma. 2014;55(10):2335-2340. doi:10.3109/10428194.2013.877135
4. Dotson E, Crawford B, Phillips G, Jones J. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer. 2016;24(3):1125-1129. doi:10.1007/s00520-015-2869-4
5. Pritchard CH, Greenwald MW, Kremer JM, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE-RA study. BMC Musculoskelet Disord. 2014;15:177. doi:10.1186/1471-2474-15-177
6. Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21(9):1746-1751. doi:10.1200/JCO.2003.09.027
7. Can M, Alibaz-Öner F, Yılmaz-Öner S, Atagündüz P, Înanç N, Direskeneli H. Accelerated infusion rates of rituximab are well tolerated and safe in rheumatology practice: a single-centre experience. Clin Rheumatol. 2013;32(1):87-90. doi:10.1007/s10067-012-2094-1
8. Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109(10):4171-4173. doi:10.1182/blood-2006-11-059469
9. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Updated March 27 2020. Accessed June 15, 2020.
Assessment of Consolidated Mail Outpatient Pharmacy Utilization in the Indian Health Service
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Beefed up inpatient/outpatient care transition is key to suicide prevention
The care transition period between inpatient psychiatric hospitalization and initiation of outpatient mental health services is a time of extraordinarily heightened suicide risk that has been woefully neglected, according to speakers from the National Action Alliance for Suicide Prevention at the virtual annual meeting of the American Association of Suicidology.
This transition period traditionally has been a time when nobody really takes responsibility for patient care. In an effort to close this potentially deadly gap in services, the alliance recently has issued a report entitled, “Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.” The recommendations focus on specific, innovative, evidence-based strategies that health care systems can use to prevent patients from falling through the cracks in care, mainly by implementing protocols aimed at fostering interorganizational teamwork between inpatient and outpatient behavioral health services.
“I believe that improving care transitions in the United States is the area where we can likely save the most lives. It’s within our grasp if we can just do this better,” declared Richard McKeon, PhD, MPH, chief of the Suicide Prevention Branch at the Center for Mental Health Services within SAMHSA, the Substance Abuse and Mental Health Services Administration.
He cited a recent meta-analysis that concluded that the risk of suicide during the first week post discharge after psychiatric hospitalization is a staggering 300 times greater than in the general population, while in the first month, the risk is increased 200-fold. The meta-analysis included 29 studies encompassing 3,551 suicides during the first month and 24 studies reporting 1,928 suicides during the first week post discharge (BMJ Open. 2019 Mar 23;9[3]:e023883. doi: 10.1136/bmjopen-2018-023883).
Everyone in the mental health field as well as patients and their families should know those statistics, but they don’t.
“I think it’s natural for people to think someone who’s been discharged from an inpatient unit or the emergency department is not at risk, when in reality it’s still a high-risk time. Suicide risk is not like a light switch that you can just switch off,” the clinical psychologist observed.
He cited other harrowing statistics that underscore the vast problem of poor care transitions. Nationally, fully one-third of patients don’t complete a single outpatient visit within the first 30 days after discharge from inpatient behavioral health care. And one in seven people who die by suicide have had contact with inpatient mental health services in the year before they died.
“That doesn’t mean that inpatient care did not do everything that they could do. What it does reflect is the need to make sure that there’s follow-up care after inpatient discharge. Too often, people don’t get the follow-up care that they need. And the research literature is clear that intervention can save lives,” Dr. McKeon said.
Panelist Becky Stoll, LCSW, vice president for crisis and disaster management at Centerstone Health in Nashville, Tenn., noted, “We see a lot of no-shows on the outpatient side, because nobody ever asked the patients if they can actually get to the outpatient appointment that’s been made.
“We have got to figure out this care transition and do better. The road to mental health is paved with Swiss cheese. There are so many holes to fall into, even if you know how to navigate the system – and most of the people we’re serving don’t know how,” observed Ms. Stoll, who, like Dr. McKeon, was among the coauthors of the alliance’s guidelines on best practices in care transitions. Ms. Stoll also serves on the AAS board as crisis services division chair.*
The National Action Alliance for Suicide Prevention is a public/private partnership whose goal is to advance the National Strategy for Suicide Prevention, which was developed by the alliance and the U.S. Surgeon General. The alliance includes mental health professionals as well as influential leaders from the military, journalism, entertainment, railroad, health insurance, law enforcement, defense, education, technology, and other industries.
Dr. McKeon and Ms. Stall were joined by Karen Johnson, MSW, another coauthor of the guidelines. They shared highlights of the report.
Inpatient provider strategies
Discharge and crisis safety planning should begin upon admission, according to Ms. Johnson, senior vice president for clinical services and division compliance at Universal Health Services, which owns and operates more than 200 behavioral health facilities across the United States.
Inpatient and outpatient care providers need to sit down and develop collaborative protocols and negotiate a memorandum of understanding regarding expectations, which absolutely must include procedures to ensure timely electronic delivery of medical records and other key documents to the outpatient care providers. The inpatient providers need to work collaboratively with the patient, family, and community support resources to develop a safety plan – including reduced access to lethal mean – as part of predischarge planning.
Among the strategies routinely employed on the inpatient side at Universal Health Services are advance scheduling of an initial outpatient appointment within 24-72 hours post discharge. Also, someone on the inpatient team is tasked with connecting with the outpatient provider prior to discharge to develop rapport.
“If our outpatient providers are located in our facility, as many of them are, we ask them to come in and attend inpatient team meetings to identify and meet with patients who are appropriate for continuing care in outpatient settings,” she explained. “A soft, warm handoff is critical.”
At these team meetings, the appropriateness of step-down care in the form of partial hospitalization or intensive outpatient care is weighed. Someone from the inpatient side is charged with maintaining contact with the patient until after the first outpatient appointment. Ongoing caring contact in the form of brief, encouraging postcards, emails, or texts that do not require a response from the patient should be maintained for several months.
Strategies for outpatient providers
Ms. Stoll is a big believer in the guideline-recommended practice of notifying the inpatient provider that the patient kept the outpatient appointment, along with having a system for red-flagging no-shows for prompt follow-up by a crisis management team.
She and her colleagues at Centerstone Health have conducted two studies of an intensive patient outreach program designed for the first 30 days of the care transition. The program included many elements of the alliance’s best practices guidelines. The yearlong first study, funded by Blue Cross/Blue Shield of Tennessee, documented zero suicides and 92% freedom from emergency department visits during the care transition period, along with greater than $400,000 savings in health care costs, compared with usual care. The second study, funded by SAMHSA, showed much the same over a 2-year period.
She emphasized that this was not a high-tech, intensive intervention. She characterized it, instead as “high-touch follow-up.
“It’s some staff and a phone and a laptop, nothing fancy, just a person who’s competent and confident and skilled with a laptop. With that, you can do some pretty amazing stuff: Get people what they need, keep them alive, and oh, guess what? You can also save a lot of health care dollars that can be put back into the system,” Ms. Stoll said.
She recognizes that it’s a lot to ask busy outpatient providers to leave their practice during the workday to participate in inpatient team meetings addressing discharge planning, as recommended in the alliance guidelines. But in this regard, she sees a silver lining to the COVID-19 pandemic, in that it forces health professionals to rely upon newly opened channels of telemedicine.
“COVID-19 is giving us an opportunity to do things in a different way. Things don’t just have to be done in person. , where we can do things in a more innovative way,” she said.
Dr. McKeon agreed that reimbursement issues have long impeded efforts to improve the inpatient to outpatient care transition. He added that it will be really important that adequate reimbursement of remote forms of care remain in place after COVID-19 fades.
“This is exactly the kind of thing that’s needed to improve care transitions,” according to Dr. McKeon.
*This story was updated 7/9/2020.
The care transition period between inpatient psychiatric hospitalization and initiation of outpatient mental health services is a time of extraordinarily heightened suicide risk that has been woefully neglected, according to speakers from the National Action Alliance for Suicide Prevention at the virtual annual meeting of the American Association of Suicidology.
This transition period traditionally has been a time when nobody really takes responsibility for patient care. In an effort to close this potentially deadly gap in services, the alliance recently has issued a report entitled, “Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.” The recommendations focus on specific, innovative, evidence-based strategies that health care systems can use to prevent patients from falling through the cracks in care, mainly by implementing protocols aimed at fostering interorganizational teamwork between inpatient and outpatient behavioral health services.
“I believe that improving care transitions in the United States is the area where we can likely save the most lives. It’s within our grasp if we can just do this better,” declared Richard McKeon, PhD, MPH, chief of the Suicide Prevention Branch at the Center for Mental Health Services within SAMHSA, the Substance Abuse and Mental Health Services Administration.
He cited a recent meta-analysis that concluded that the risk of suicide during the first week post discharge after psychiatric hospitalization is a staggering 300 times greater than in the general population, while in the first month, the risk is increased 200-fold. The meta-analysis included 29 studies encompassing 3,551 suicides during the first month and 24 studies reporting 1,928 suicides during the first week post discharge (BMJ Open. 2019 Mar 23;9[3]:e023883. doi: 10.1136/bmjopen-2018-023883).
Everyone in the mental health field as well as patients and their families should know those statistics, but they don’t.
“I think it’s natural for people to think someone who’s been discharged from an inpatient unit or the emergency department is not at risk, when in reality it’s still a high-risk time. Suicide risk is not like a light switch that you can just switch off,” the clinical psychologist observed.
He cited other harrowing statistics that underscore the vast problem of poor care transitions. Nationally, fully one-third of patients don’t complete a single outpatient visit within the first 30 days after discharge from inpatient behavioral health care. And one in seven people who die by suicide have had contact with inpatient mental health services in the year before they died.
“That doesn’t mean that inpatient care did not do everything that they could do. What it does reflect is the need to make sure that there’s follow-up care after inpatient discharge. Too often, people don’t get the follow-up care that they need. And the research literature is clear that intervention can save lives,” Dr. McKeon said.
Panelist Becky Stoll, LCSW, vice president for crisis and disaster management at Centerstone Health in Nashville, Tenn., noted, “We see a lot of no-shows on the outpatient side, because nobody ever asked the patients if they can actually get to the outpatient appointment that’s been made.
“We have got to figure out this care transition and do better. The road to mental health is paved with Swiss cheese. There are so many holes to fall into, even if you know how to navigate the system – and most of the people we’re serving don’t know how,” observed Ms. Stoll, who, like Dr. McKeon, was among the coauthors of the alliance’s guidelines on best practices in care transitions. Ms. Stoll also serves on the AAS board as crisis services division chair.*
The National Action Alliance for Suicide Prevention is a public/private partnership whose goal is to advance the National Strategy for Suicide Prevention, which was developed by the alliance and the U.S. Surgeon General. The alliance includes mental health professionals as well as influential leaders from the military, journalism, entertainment, railroad, health insurance, law enforcement, defense, education, technology, and other industries.
Dr. McKeon and Ms. Stall were joined by Karen Johnson, MSW, another coauthor of the guidelines. They shared highlights of the report.
Inpatient provider strategies
Discharge and crisis safety planning should begin upon admission, according to Ms. Johnson, senior vice president for clinical services and division compliance at Universal Health Services, which owns and operates more than 200 behavioral health facilities across the United States.
Inpatient and outpatient care providers need to sit down and develop collaborative protocols and negotiate a memorandum of understanding regarding expectations, which absolutely must include procedures to ensure timely electronic delivery of medical records and other key documents to the outpatient care providers. The inpatient providers need to work collaboratively with the patient, family, and community support resources to develop a safety plan – including reduced access to lethal mean – as part of predischarge planning.
Among the strategies routinely employed on the inpatient side at Universal Health Services are advance scheduling of an initial outpatient appointment within 24-72 hours post discharge. Also, someone on the inpatient team is tasked with connecting with the outpatient provider prior to discharge to develop rapport.
“If our outpatient providers are located in our facility, as many of them are, we ask them to come in and attend inpatient team meetings to identify and meet with patients who are appropriate for continuing care in outpatient settings,” she explained. “A soft, warm handoff is critical.”
At these team meetings, the appropriateness of step-down care in the form of partial hospitalization or intensive outpatient care is weighed. Someone from the inpatient side is charged with maintaining contact with the patient until after the first outpatient appointment. Ongoing caring contact in the form of brief, encouraging postcards, emails, or texts that do not require a response from the patient should be maintained for several months.
Strategies for outpatient providers
Ms. Stoll is a big believer in the guideline-recommended practice of notifying the inpatient provider that the patient kept the outpatient appointment, along with having a system for red-flagging no-shows for prompt follow-up by a crisis management team.
She and her colleagues at Centerstone Health have conducted two studies of an intensive patient outreach program designed for the first 30 days of the care transition. The program included many elements of the alliance’s best practices guidelines. The yearlong first study, funded by Blue Cross/Blue Shield of Tennessee, documented zero suicides and 92% freedom from emergency department visits during the care transition period, along with greater than $400,000 savings in health care costs, compared with usual care. The second study, funded by SAMHSA, showed much the same over a 2-year period.
She emphasized that this was not a high-tech, intensive intervention. She characterized it, instead as “high-touch follow-up.
“It’s some staff and a phone and a laptop, nothing fancy, just a person who’s competent and confident and skilled with a laptop. With that, you can do some pretty amazing stuff: Get people what they need, keep them alive, and oh, guess what? You can also save a lot of health care dollars that can be put back into the system,” Ms. Stoll said.
She recognizes that it’s a lot to ask busy outpatient providers to leave their practice during the workday to participate in inpatient team meetings addressing discharge planning, as recommended in the alliance guidelines. But in this regard, she sees a silver lining to the COVID-19 pandemic, in that it forces health professionals to rely upon newly opened channels of telemedicine.
“COVID-19 is giving us an opportunity to do things in a different way. Things don’t just have to be done in person. , where we can do things in a more innovative way,” she said.
Dr. McKeon agreed that reimbursement issues have long impeded efforts to improve the inpatient to outpatient care transition. He added that it will be really important that adequate reimbursement of remote forms of care remain in place after COVID-19 fades.
“This is exactly the kind of thing that’s needed to improve care transitions,” according to Dr. McKeon.
*This story was updated 7/9/2020.
The care transition period between inpatient psychiatric hospitalization and initiation of outpatient mental health services is a time of extraordinarily heightened suicide risk that has been woefully neglected, according to speakers from the National Action Alliance for Suicide Prevention at the virtual annual meeting of the American Association of Suicidology.
This transition period traditionally has been a time when nobody really takes responsibility for patient care. In an effort to close this potentially deadly gap in services, the alliance recently has issued a report entitled, “Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.” The recommendations focus on specific, innovative, evidence-based strategies that health care systems can use to prevent patients from falling through the cracks in care, mainly by implementing protocols aimed at fostering interorganizational teamwork between inpatient and outpatient behavioral health services.
“I believe that improving care transitions in the United States is the area where we can likely save the most lives. It’s within our grasp if we can just do this better,” declared Richard McKeon, PhD, MPH, chief of the Suicide Prevention Branch at the Center for Mental Health Services within SAMHSA, the Substance Abuse and Mental Health Services Administration.
He cited a recent meta-analysis that concluded that the risk of suicide during the first week post discharge after psychiatric hospitalization is a staggering 300 times greater than in the general population, while in the first month, the risk is increased 200-fold. The meta-analysis included 29 studies encompassing 3,551 suicides during the first month and 24 studies reporting 1,928 suicides during the first week post discharge (BMJ Open. 2019 Mar 23;9[3]:e023883. doi: 10.1136/bmjopen-2018-023883).
Everyone in the mental health field as well as patients and their families should know those statistics, but they don’t.
“I think it’s natural for people to think someone who’s been discharged from an inpatient unit or the emergency department is not at risk, when in reality it’s still a high-risk time. Suicide risk is not like a light switch that you can just switch off,” the clinical psychologist observed.
He cited other harrowing statistics that underscore the vast problem of poor care transitions. Nationally, fully one-third of patients don’t complete a single outpatient visit within the first 30 days after discharge from inpatient behavioral health care. And one in seven people who die by suicide have had contact with inpatient mental health services in the year before they died.
“That doesn’t mean that inpatient care did not do everything that they could do. What it does reflect is the need to make sure that there’s follow-up care after inpatient discharge. Too often, people don’t get the follow-up care that they need. And the research literature is clear that intervention can save lives,” Dr. McKeon said.
Panelist Becky Stoll, LCSW, vice president for crisis and disaster management at Centerstone Health in Nashville, Tenn., noted, “We see a lot of no-shows on the outpatient side, because nobody ever asked the patients if they can actually get to the outpatient appointment that’s been made.
“We have got to figure out this care transition and do better. The road to mental health is paved with Swiss cheese. There are so many holes to fall into, even if you know how to navigate the system – and most of the people we’re serving don’t know how,” observed Ms. Stoll, who, like Dr. McKeon, was among the coauthors of the alliance’s guidelines on best practices in care transitions. Ms. Stoll also serves on the AAS board as crisis services division chair.*
The National Action Alliance for Suicide Prevention is a public/private partnership whose goal is to advance the National Strategy for Suicide Prevention, which was developed by the alliance and the U.S. Surgeon General. The alliance includes mental health professionals as well as influential leaders from the military, journalism, entertainment, railroad, health insurance, law enforcement, defense, education, technology, and other industries.
Dr. McKeon and Ms. Stall were joined by Karen Johnson, MSW, another coauthor of the guidelines. They shared highlights of the report.
Inpatient provider strategies
Discharge and crisis safety planning should begin upon admission, according to Ms. Johnson, senior vice president for clinical services and division compliance at Universal Health Services, which owns and operates more than 200 behavioral health facilities across the United States.
Inpatient and outpatient care providers need to sit down and develop collaborative protocols and negotiate a memorandum of understanding regarding expectations, which absolutely must include procedures to ensure timely electronic delivery of medical records and other key documents to the outpatient care providers. The inpatient providers need to work collaboratively with the patient, family, and community support resources to develop a safety plan – including reduced access to lethal mean – as part of predischarge planning.
Among the strategies routinely employed on the inpatient side at Universal Health Services are advance scheduling of an initial outpatient appointment within 24-72 hours post discharge. Also, someone on the inpatient team is tasked with connecting with the outpatient provider prior to discharge to develop rapport.
“If our outpatient providers are located in our facility, as many of them are, we ask them to come in and attend inpatient team meetings to identify and meet with patients who are appropriate for continuing care in outpatient settings,” she explained. “A soft, warm handoff is critical.”
At these team meetings, the appropriateness of step-down care in the form of partial hospitalization or intensive outpatient care is weighed. Someone from the inpatient side is charged with maintaining contact with the patient until after the first outpatient appointment. Ongoing caring contact in the form of brief, encouraging postcards, emails, or texts that do not require a response from the patient should be maintained for several months.
Strategies for outpatient providers
Ms. Stoll is a big believer in the guideline-recommended practice of notifying the inpatient provider that the patient kept the outpatient appointment, along with having a system for red-flagging no-shows for prompt follow-up by a crisis management team.
She and her colleagues at Centerstone Health have conducted two studies of an intensive patient outreach program designed for the first 30 days of the care transition. The program included many elements of the alliance’s best practices guidelines. The yearlong first study, funded by Blue Cross/Blue Shield of Tennessee, documented zero suicides and 92% freedom from emergency department visits during the care transition period, along with greater than $400,000 savings in health care costs, compared with usual care. The second study, funded by SAMHSA, showed much the same over a 2-year period.
She emphasized that this was not a high-tech, intensive intervention. She characterized it, instead as “high-touch follow-up.
“It’s some staff and a phone and a laptop, nothing fancy, just a person who’s competent and confident and skilled with a laptop. With that, you can do some pretty amazing stuff: Get people what they need, keep them alive, and oh, guess what? You can also save a lot of health care dollars that can be put back into the system,” Ms. Stoll said.
She recognizes that it’s a lot to ask busy outpatient providers to leave their practice during the workday to participate in inpatient team meetings addressing discharge planning, as recommended in the alliance guidelines. But in this regard, she sees a silver lining to the COVID-19 pandemic, in that it forces health professionals to rely upon newly opened channels of telemedicine.
“COVID-19 is giving us an opportunity to do things in a different way. Things don’t just have to be done in person. , where we can do things in a more innovative way,” she said.
Dr. McKeon agreed that reimbursement issues have long impeded efforts to improve the inpatient to outpatient care transition. He added that it will be really important that adequate reimbursement of remote forms of care remain in place after COVID-19 fades.
“This is exactly the kind of thing that’s needed to improve care transitions,” according to Dr. McKeon.
*This story was updated 7/9/2020.
FROM AAS20
The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C)
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Engaging Patients as Stakeholders
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
© 2020 Society of Hospital Medicine
Myocardial Injury Among Postoperative Patients: Where Is the Wisdom in Our Knowledge?
The ability to detect myocardial injury has never been more advanced. With the availability of high-sensitivity troponin testing, microscopic evidence of myocyte death can now be detected, often within an hour or so of the inciting event. This, in turn, has facilitated quicker and more accurate identification and treatment of affected patients. However, these advances in detection have, in some cases, outstripped our understanding of the etiology and appropriate management of troponin elevation.
This dilemma is particularly apparent among patients undergoing noncardiac surgery. Annually, over 200 million of these surgeries occur worldwide, many in patients with elevated cardiac risk or overt cardiac disease. Naturally, physicians treating these patients are concerned that the stress of surgery will provoke myocardial injury. Since symptoms are often masked in the immediate postoperative period because of sedating or analgesic medications, many physicians rely on troponin testing to detect signs of myocardial injury. With the increased sensitivity of these assays, the prevalence of troponin elevation has increased, which currently affects nearly one in five postoperative patients. This knowledge, however, doesn’t lend itself to a clear management strategy, particularly in those patients with no other objective evidence of infarction. To paraphrase T.S. Eliot, have we lost the wisdom in our knowledge?
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI). An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
The authors found that MINS is highly prevalent (19.6%) and associated with both cardiac disease and perioperative hemodynamic stress. Between 2.9% and 13.5% of MINS patients experienced 30-day adverse cardiac events, with higher rates in patients with higher troponin elevations and/or accompanying ischemic symptoms. The authors suggested MINS management with standard cardio-protective medications, such as statins, beta-blockers, and angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. For those patients at low bleeding risk, they also suggested dabigatran based on the recent MANAGE trial. Finally, they noted that US cardiac society guidelines suggested no screening for MINS, while the European and Canadian guidelines advocated for screening in patients at high risk for cardiac complications.
The authors are to be congratulated for highlighting an important and vexing area of postoperative management. To date, it has been difficult to chart the best path forward for these patients because we could “see” the issue, thanks to increasingly sensitive troponin assays, but we didn’t know what to do once we found it.
So what rationale exists to justify screening? Some advocate that the presence of MINS suggests a need for further imaging and closer monitoring of these patients to identify those with an MI. Indeed, several recent MINS registry studies have found that 20% to 40% of MINS patients had definitive evidence of MI.2-4 But what about those patients with troponin elevation and no evidence of MI? A small, propensity-matched, observational study of MINS patients, including those without MI, noted positive associations between cardioprotective medications, such as aspirin and statins, and cardiac outcomes.5 In addition, the MANAGE trial suggested that MINS patients, with or without evidence of an MI, receiving dabigatran had reduced vascular events without increased bleeding complications.6 With this growing base of evidence, the rationale for systematic screening for MINS appears to be standing on stronger footing.
As noted by the authors, the recommendations for MINS screening differ across three major cardiovascular societies. How does the practicing clinician make sense of this discordant advice? Differences often occur when the evidence is of moderate or low quality, which means guideline committees must make their own interpretations of equivocal findings. Another driver of discordant recommendations is the timing of the guidelines. Both the US and European guidelines were published in 2014, while the Canadian guidelines were published in 2017. Over time, experience with postoperative troponin testing increased, which may have influenced the Canadian guidelines. Finally, many members of the Canadian guideline writing committee were the ones conducting the various studies identifying management options for MINS patients, which may have guided their ultimate recommendation. Regardless, practicing physicians should collectively view the guidelines as acceptable “guardrails” to guide their practice. Selection of the appropriate strategy can then be tailored to the individual patient’s risks and benefits, as well as available management options.
In this era of high-sensitivity troponin testing, we now possess an exquisite opportunity to “see” minute levels of myocardial injury among postoperative patients. Our growing ability to effectively act on this knowledge will enable us to make wise decisions with our patients to optimize their cardiac outcomes during the vulnerable postoperative period.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
2. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
3. Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113
4. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360
5. Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053-1063. https://doi.org/10.1213/ane.0000000000000302
6. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8
The ability to detect myocardial injury has never been more advanced. With the availability of high-sensitivity troponin testing, microscopic evidence of myocyte death can now be detected, often within an hour or so of the inciting event. This, in turn, has facilitated quicker and more accurate identification and treatment of affected patients. However, these advances in detection have, in some cases, outstripped our understanding of the etiology and appropriate management of troponin elevation.
This dilemma is particularly apparent among patients undergoing noncardiac surgery. Annually, over 200 million of these surgeries occur worldwide, many in patients with elevated cardiac risk or overt cardiac disease. Naturally, physicians treating these patients are concerned that the stress of surgery will provoke myocardial injury. Since symptoms are often masked in the immediate postoperative period because of sedating or analgesic medications, many physicians rely on troponin testing to detect signs of myocardial injury. With the increased sensitivity of these assays, the prevalence of troponin elevation has increased, which currently affects nearly one in five postoperative patients. This knowledge, however, doesn’t lend itself to a clear management strategy, particularly in those patients with no other objective evidence of infarction. To paraphrase T.S. Eliot, have we lost the wisdom in our knowledge?
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI). An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
The authors found that MINS is highly prevalent (19.6%) and associated with both cardiac disease and perioperative hemodynamic stress. Between 2.9% and 13.5% of MINS patients experienced 30-day adverse cardiac events, with higher rates in patients with higher troponin elevations and/or accompanying ischemic symptoms. The authors suggested MINS management with standard cardio-protective medications, such as statins, beta-blockers, and angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. For those patients at low bleeding risk, they also suggested dabigatran based on the recent MANAGE trial. Finally, they noted that US cardiac society guidelines suggested no screening for MINS, while the European and Canadian guidelines advocated for screening in patients at high risk for cardiac complications.
The authors are to be congratulated for highlighting an important and vexing area of postoperative management. To date, it has been difficult to chart the best path forward for these patients because we could “see” the issue, thanks to increasingly sensitive troponin assays, but we didn’t know what to do once we found it.
So what rationale exists to justify screening? Some advocate that the presence of MINS suggests a need for further imaging and closer monitoring of these patients to identify those with an MI. Indeed, several recent MINS registry studies have found that 20% to 40% of MINS patients had definitive evidence of MI.2-4 But what about those patients with troponin elevation and no evidence of MI? A small, propensity-matched, observational study of MINS patients, including those without MI, noted positive associations between cardioprotective medications, such as aspirin and statins, and cardiac outcomes.5 In addition, the MANAGE trial suggested that MINS patients, with or without evidence of an MI, receiving dabigatran had reduced vascular events without increased bleeding complications.6 With this growing base of evidence, the rationale for systematic screening for MINS appears to be standing on stronger footing.
As noted by the authors, the recommendations for MINS screening differ across three major cardiovascular societies. How does the practicing clinician make sense of this discordant advice? Differences often occur when the evidence is of moderate or low quality, which means guideline committees must make their own interpretations of equivocal findings. Another driver of discordant recommendations is the timing of the guidelines. Both the US and European guidelines were published in 2014, while the Canadian guidelines were published in 2017. Over time, experience with postoperative troponin testing increased, which may have influenced the Canadian guidelines. Finally, many members of the Canadian guideline writing committee were the ones conducting the various studies identifying management options for MINS patients, which may have guided their ultimate recommendation. Regardless, practicing physicians should collectively view the guidelines as acceptable “guardrails” to guide their practice. Selection of the appropriate strategy can then be tailored to the individual patient’s risks and benefits, as well as available management options.
In this era of high-sensitivity troponin testing, we now possess an exquisite opportunity to “see” minute levels of myocardial injury among postoperative patients. Our growing ability to effectively act on this knowledge will enable us to make wise decisions with our patients to optimize their cardiac outcomes during the vulnerable postoperative period.
The ability to detect myocardial injury has never been more advanced. With the availability of high-sensitivity troponin testing, microscopic evidence of myocyte death can now be detected, often within an hour or so of the inciting event. This, in turn, has facilitated quicker and more accurate identification and treatment of affected patients. However, these advances in detection have, in some cases, outstripped our understanding of the etiology and appropriate management of troponin elevation.
This dilemma is particularly apparent among patients undergoing noncardiac surgery. Annually, over 200 million of these surgeries occur worldwide, many in patients with elevated cardiac risk or overt cardiac disease. Naturally, physicians treating these patients are concerned that the stress of surgery will provoke myocardial injury. Since symptoms are often masked in the immediate postoperative period because of sedating or analgesic medications, many physicians rely on troponin testing to detect signs of myocardial injury. With the increased sensitivity of these assays, the prevalence of troponin elevation has increased, which currently affects nearly one in five postoperative patients. This knowledge, however, doesn’t lend itself to a clear management strategy, particularly in those patients with no other objective evidence of infarction. To paraphrase T.S. Eliot, have we lost the wisdom in our knowledge?
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI). An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
The authors found that MINS is highly prevalent (19.6%) and associated with both cardiac disease and perioperative hemodynamic stress. Between 2.9% and 13.5% of MINS patients experienced 30-day adverse cardiac events, with higher rates in patients with higher troponin elevations and/or accompanying ischemic symptoms. The authors suggested MINS management with standard cardio-protective medications, such as statins, beta-blockers, and angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. For those patients at low bleeding risk, they also suggested dabigatran based on the recent MANAGE trial. Finally, they noted that US cardiac society guidelines suggested no screening for MINS, while the European and Canadian guidelines advocated for screening in patients at high risk for cardiac complications.
The authors are to be congratulated for highlighting an important and vexing area of postoperative management. To date, it has been difficult to chart the best path forward for these patients because we could “see” the issue, thanks to increasingly sensitive troponin assays, but we didn’t know what to do once we found it.
So what rationale exists to justify screening? Some advocate that the presence of MINS suggests a need for further imaging and closer monitoring of these patients to identify those with an MI. Indeed, several recent MINS registry studies have found that 20% to 40% of MINS patients had definitive evidence of MI.2-4 But what about those patients with troponin elevation and no evidence of MI? A small, propensity-matched, observational study of MINS patients, including those without MI, noted positive associations between cardioprotective medications, such as aspirin and statins, and cardiac outcomes.5 In addition, the MANAGE trial suggested that MINS patients, with or without evidence of an MI, receiving dabigatran had reduced vascular events without increased bleeding complications.6 With this growing base of evidence, the rationale for systematic screening for MINS appears to be standing on stronger footing.
As noted by the authors, the recommendations for MINS screening differ across three major cardiovascular societies. How does the practicing clinician make sense of this discordant advice? Differences often occur when the evidence is of moderate or low quality, which means guideline committees must make their own interpretations of equivocal findings. Another driver of discordant recommendations is the timing of the guidelines. Both the US and European guidelines were published in 2014, while the Canadian guidelines were published in 2017. Over time, experience with postoperative troponin testing increased, which may have influenced the Canadian guidelines. Finally, many members of the Canadian guideline writing committee were the ones conducting the various studies identifying management options for MINS patients, which may have guided their ultimate recommendation. Regardless, practicing physicians should collectively view the guidelines as acceptable “guardrails” to guide their practice. Selection of the appropriate strategy can then be tailored to the individual patient’s risks and benefits, as well as available management options.
In this era of high-sensitivity troponin testing, we now possess an exquisite opportunity to “see” minute levels of myocardial injury among postoperative patients. Our growing ability to effectively act on this knowledge will enable us to make wise decisions with our patients to optimize their cardiac outcomes during the vulnerable postoperative period.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
2. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
3. Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113
4. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360
5. Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053-1063. https://doi.org/10.1213/ane.0000000000000302
6. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
2. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
3. Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113
4. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360
5. Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053-1063. https://doi.org/10.1213/ane.0000000000000302
6. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8
© 2020 Society of Hospital Medicine